
Antibacterial Evaluation of the Rutaceae and Rosaceae Plant
Collection from Cibodas Botanical Garden, Indonesia
Intani Quarta Lailaty
1
a
, Ihsanti Fairuz Anatasya
2
, Agus Budiawan Naro Putra
3
b
,
Suluh Normasiwi
1
c
, Lily Ismaini
1
d
and Urip Perwitasari
4
e
1
Research Center for Applied Botany, National Research and Innovation Agency (BRIN), Cibinong, Bogor 16911, Indonesia
2
Faculty of Pharmacy, Universitas Gadjah Mada (UGM), Sekip Utara, Yogyakarta 55281, Indonesia
3
Research Center for Pharmaceutical Ingredients and Traditional Medicine, National Research and Innovation Agency
(BRIN), Cibinong, Bogor 16911, Indonesia
4
Research Center for Applied Microbiology, National Research and Innovation Agency (BRIN),
Cibinong, Bogor 16911, Indonesia
Keywords: Antibacterial Activity, Bioprospecting, Cibodas Botanical Gardens, Rosaceae, Rutaceae.
Abstract: Plants produce a variety of bioactive compounds with numerous biological activities, including antibacterial
properties. The potential of plant extracts possessing antibacterial properties can be further developed as raw
materials for drugs and cosmetics. In this study, antibacterial screening was conducted on three species of
plants (Rubus fraxinifolius, R. rosifolius, and Prunus cerasoides) from the Rosaceae family and two species
of plants (Acronychia pedunculata and Zanthoxylum acanthopodium) from the Rutaceae family, sourced from
the Cibodas Botanic Gardens collection in West Java. The antibacterial assay was carried out utilizing the
disc diffusion method. The bacterial isolates tested included Pseudomonas aeruginosa, Staphylococcus
aureus, and S. epidermidis. The findings demonstrated that the leaf ethanolic extract of A. pedunculata
(Rutaceae) exhibited the highest antibacterial activity compared to other species, followed by R. rosifolius
(Rosaceae). Conversely, R. fraxinifolius leaf shoot extract demonstrated the lowest antibacterial activity based
on tests against the three bacteria. Overall, all extracts produced the largest inhibition zone diameter against
S. epidermidis. Further research is necessary to develop plant bioprospecting with antibacterial properties for
pharmaceutical raw materials.
1 INTRODUCTION
Herbal medicines have been used for many
generations to treat various medical conditions,
including infectious diseases. Several natural
chemical compounds have been clinically proven to
function as medicinal raw materials, and many
studies have been conducted on their use as
antimicrobial agents (Mahady et al., 2008).
Antibiotics from natural ingredients, although
historically important, have not received the same
level of investment in research and development,
standardization, and marketing as synthetic
a
https://orcid.org/0000-0002-9744-9458
b
https://orcid.org/0000-0002-3226-3767
c
https://orcid.org/0000-0003-3885-0230
d
https://orcid.org/0000-0002-0853-183X
e
https://orcid.org/0000-0001-5964-4474
antibiotics. Synthetic antibacterial agents have also
been widely used in cosmetics to prevent microbial
contamination and ensure product safety (Halla et al.,
2018). Conversely, there has been a major trend
toward the application of natural resources for
therapeutic purposes. This shift is driven by consumer
concerns about the safety of synthetic ingredients and
preference for natural alternatives (Varvaresou et al.,
2009). Regulatory aspects and consumer safety
remain paramount, as evidenced by the presence of
hazardous ingredients in cosmetics in some markets
and strict laws regulating cosmetic ingredients
(Manent and Abellán, 2007).
Lailaty, I. Q., Anatasya, I. F., Putra, A. B. N., Normasiwi, S., Ismaini, L. and Perwitasari, U.
Antibacterial Evaluation of the Rutaceae and Rosaceae Plant Collection from Cibodas Botanical Garden, Indonesia.
DOI: 10.5220/0013474200004612
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of BRIN’s 2nd International Conference for Health Research (ICHR 2024), pages 9-18
ISBN: 978-989-758-755-9
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
9

The Rosaceae and Rutaceae plant families exhibit
significant antibacterial activity as demonstrated in
various studies. Extracts from Rosaceae fruits like
hawthorn and dog rose show potent antibacterial
properties against uropathogenic Escherichia coli
strains, reducing bacterial adhesion and biofilm
formation (Andrzej et al., 2020). Additionally, the
essential oil from rose, a member of the Rosaceae
family, exhibits antimicrobial activity against various
microorganisms, with notable effectiveness against
Staphylococcus aureus, E. coli, and Candida
albicans, showcasing its potential as a natural
antibacterial agent (Li et al., 2009). In contrast, the
ethanolic extracts from Rutaceae leaves such as
Acronychia pedunculata and Glycosmis pentaphylla,
display antibacterial effects against a range of
bacterial strains, including E. coli, S. aureus, and P.
aeruginosa, indicating their potential medicinal
applications (Hong et al., 2020).
Discovering potent antibacterial substances from
sources of nature, such as the Rutaceae and Rosaceae
plant families, holds immense promise for various
applications in healthcare and pharmaceutical
industries. These plant-derived compounds may serve
as valuable alternatives or complementary treatments
to conventional antibiotics, potentially addressing the
growing challenge of antimicrobial resistance
(Barbieri et al., 2017).
A key area of interest is the development of novel
antimicrobial therapies for treating various bacterial
infections, especially skin infections. Pseudomonas
aeruginosa InaCC B52 and Staphylococcus
epidermidis FNCC 0048 were used as gram-negative
bacteria and Staphylococcus aureus ATCC 25923
was used as gram-positive bacteria. These three
bacterias were common bacteria causing chronic skin
wound infections. The identification of potent
antibacterial compounds from Rutaceae and
Rosaceae plants could lead to the creation of new
drug candidates or the enhancement of existing
antimicrobial formulations (Chintaluri et al., 2015;
Zazharsky et al., 2020; Garcia-Oliviera et al., 2020).
These natural-based solutions may offer unique
mechanisms of action, reduced side effects, and
improved efficacy compared to synthetic antibiotics,
making them attractive options for clinicians and
patients (Fadilah et al., 2020; Mota et al., 2020).
Furthermore, the exploration of plant-derived
antibacterial agents from Rutaceae and Rosaceae
could have broader applications in the fields of food
preservation, personal care, and environmental
remediation. The antimicrobial properties of these
plant compounds could be harnessed to develop
natural preservatives for food and cosmetic products,
thereby reducing their reliance on synthetic
antimicrobials. Additionally, plant-based
antimicrobials could be explored for their potential in
water treatment and soil remediation, contributing to
more sustainable and eco-friendly solutions to
environmental challenges (van Vuuren & Viljoen,
2011; Vaou et al., 2021).
This study aimed to identify and analyze three
species of Rosaceae and two species of Rutaceae with
antibacterial activity from a collection of Cibodas
Botanic Gardens, West Java, Indonesia. Thus, it can
provide information on new antibacterial sources,
which can aid in the development of more effective
and safer medicines for treating bacterial infections
based on local natural resources. The discovery of
effective antibacterial compounds in these plant
sources could lead to the creation of new
antimicrobial medications, food preservatives, and
environmental remediation strategies, ultimately
contributing to the global efforts to combat the threat
of antimicrobial resistance.
2 METHODS
2.1 Sample Preparation
Samples from 5 plant species were acquired from
Cibodas Botanic Garden plant collections in simplisia
forms. All samples were the leaves of the plants
unless otherwise stated (fruits). They are Rubus
fraxinifolius, R. rosifolius, and Prunus cerasoides
from the Rosaceae family and Acronychia
pedunculata and Zanthoxylum acanthopodium from
the Rutaceae family. Five grams of each sample were
macerated in 70% ethanol solution at ratio of 1:5
(v/v). The sample was shaken using an incubator
shaker (Taitec BR-43FL) at 25°C, 170 rpm for 24 h.
After that, the sample was centrifuged (Tomy
KITMAN-T24) at 4°C, 5000 ×g, for 10 min to
separate the supernatant from the unsoluble
substances. Subsequently, the sample was re-
extracted twice with the same procedure and then the
filtrate was collected. Afterward, the extract was
evaporated by a rotary evaporator (IKA RV 10
Digital) to remove the solvent, followed by
lyophilization (Alpha 1-2 LD Plus Christ). The
lyophilized ethanol extract was dissolved in a 10%
dimethyl sulfoxide - phosphate-buffered saline
(DMSO-PBS) solution for antibacterial evaluation.
ICHR 2024 - BRIN’s International Conference for Health Research (ICHR)
10

2.2 Bacterial Isolate
The bacterial cultures used in this research were
Pseudomonas aeruginosa InaCC B52 and
Staphylococcus epidermidis FNCC 0048 as gram-
negative bacteria and Staphylococcus aureus ATCC
25923 as gram-positive bacteria. Mueller-Hinton
Agar (MHA) and Mueller-Hinton Broth (MHB)
(HiMedia, Mumbai, India) were used as culture
media and prepared according to the manufacturer's
instructions.
2.3 Antibacterial Assay
The clear zone method was employed for a semi-
quantitative antibacterial activity test. The suspension
of each bacterial isolate of P. aeruginosa, S. aureus,
or S. epidermidis was poured and spread on Mueller-
Hinton Agar (MHA) in petri dishes. A 0.5 cm
diameter Whatman filter paper (paper disk) was then
positioned on the agar plate, followed by adding 10
μL of sample (lyophilized ethanol extract dissolved in
10% DMSO-PBS) onto the paper disk. Each sample
was prepared at a concentration of 1000 µg/mL.
Additionally, the positive and negative controls used
were 12.5 µg/mL of commercial chloramphenicol
(Novapharin, Gresik, Indonesia) and 10% DMSO-
PBS, respectively. After incubating bacterial cultures
at 37°C for 24 hours, the zone of inhibition diameter
was measured using a caliper. Triplication was
performed for each sample tested. The inhibition
zones formed in each test were observed and
measured as the appearance of antibacterial activity.
2.4 Data Analysis
The statistical significance of the difference was
tested by the one-way ANOVA method completed by
the Tukey-Kramer test. Values with *p < 0.05 or
**p < 0.01 were considered statistically significant
against negative control (10% DMSO-PBS). In
addition, plant descriptions were explained by works
of literature. Plant origin was based on the Cibodas
Botanic Gardens data collection database, plant
distribution by POWO database, and antibacterial
compounds were collected from related journal
articles.
3 RESULTS AND DISCUSSION
3.1 Plant Characteristics of Rutaceae
and Rosaceae
The Rutaceae and Rosaceae families are well-known
for their diverse and often economically important
plant species. The Rutaceae family, commonly
known as the rue or citrus family, encompasses
various plants, including familiar citrus fruits, such as
oranges, lemons, and limes. These plants are
renowned for their aromatic compounds and have
long been used in traditional medicine because of
their medicinal properties. On the other hand, often
called the "rose family", the Rosaceae family is a
broad group of plants that includes a range of fruits,
including strawberries, pears, and apples, as well as
ornamental species like roses. Like the Rutaceae
family, Rosaceae plants have a rich history of
traditional medicinal use, with many species
exhibiting a range of bioactivities, including
antimicrobial properties. Plant descriptions with
antibacterial properties from Rutaceae and Rosaceae
species from the Cibodas Botanic Gardens collection
were described in Table 1.
Approximately 1800 species and 156 genera belong
to the Rutaceae family, which is widely distributed
throughout tropical and subtropical climates, especially
Southeast Asia. Many species of Rutaceae, such as A.
pedunculata and Z. acanthopodium, have many
biologically active compounds. Numerous beneficial
goods, including medicines, food, spices, and essential
oils, are made from those natural resources (Van et al.,
2020).
Often referred to as claw-flowered Laural or Lake,
Kayu Semidra, or Jejerukan (in Indonesia), Acronychia
pedunculata L. (Rutaceae) is a tiny tree with glabrous
branches and pale, smooth bark. This plant's leaves are
oval, 7.5-12.5 cm long, and can be placed simply,
oppositely, or alternately. The flowers are arranged
loosely in pyramidal or divaricate patterns on long,
straight axillary peduncles and are small, regular,
polygamous, and pale yellowish green in color.
Usually, flowering takes place from February to April.
The spherical, indehiscent fruits measure between 1.2
and 1.8 centimeters in length. It's interesting to note
that fruits have four chambers, with one seed in each
chamber (Jayaweera, 1982). One species of the
Rutaceae family that is widespread in Indonesia is A.
pedunculata (Figure 1a). Traditional medicine has
utilized stem bark to treat rheumatism, diarrhea, fever,
and asthma (Tanjung et al., 2018).
Antibacterial Evaluation of the Rutaceae and Rosaceae Plant Collection from Cibodas Botanical Garden, Indonesia
11
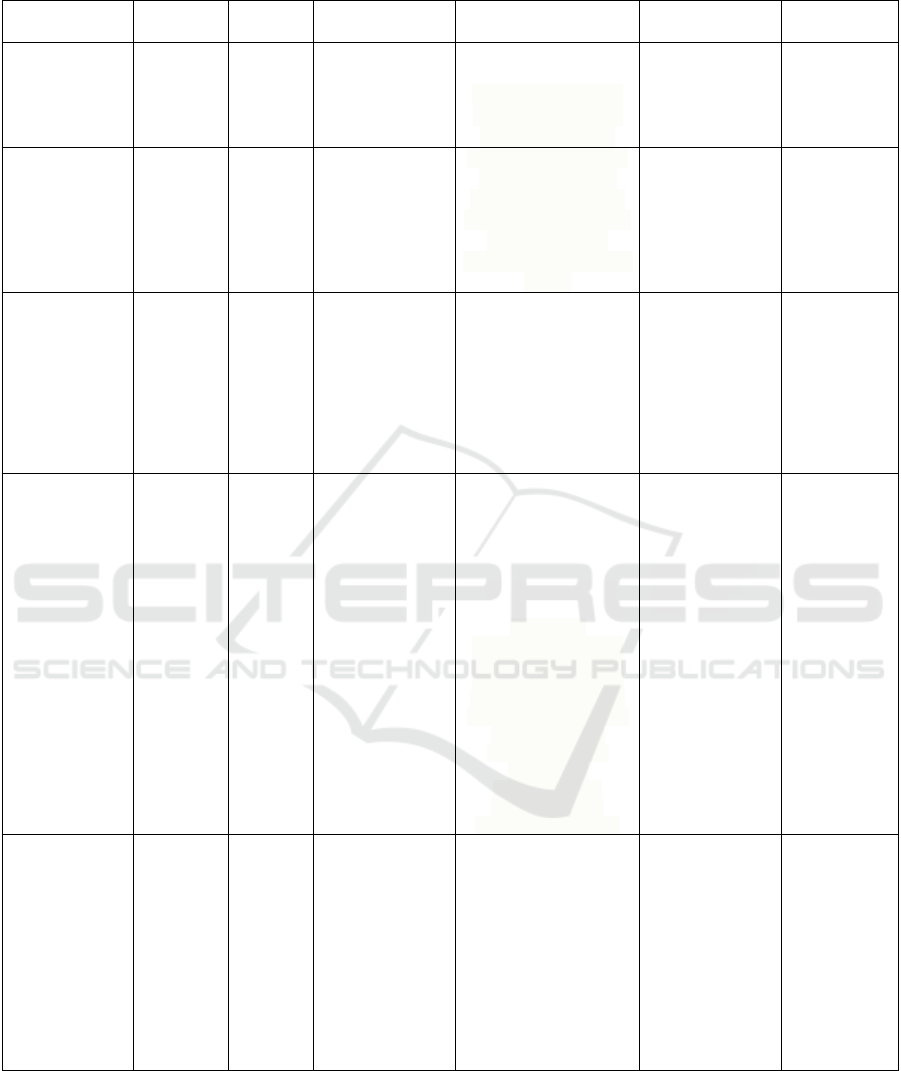
Table 1: Plant descriptions with antibacterial properties.
Scientific
names
Family Origin* Distribution** Antibacterial activity Compounds References
Rubus
fraxinifolius
Poir.
Rosaceae West
Java
Lesser Sunda Is.,
Taiwan,
Mauritius,
Rodrigues,
Réunion
Leaf extract from R.
fraxinifolius had
antibacterial potency
against B. subtilis, S.
aureus, and
E
. coli
Flavonoids,
quercetin,
naringenin
Dewi et al.
(2019)
Rubus rosifolius
Sm.
Rosaceae West
Java
Central & S.
China to Tropical
Asia
The fruit crude extract
of R. rosifolius had
antimicrobial activity
against a wide range of
microorganisms,
including S. aureus and
E
. coli
The phenolic
compounds,
alkaloids,
anthraquinones,
and alcohols
Alvares et
al. (2013)
Prunus
cerasoides
Buch.-Ham. ex
D.Don
Rosaceae Himalaya NE. Pakistan to
Indo-China
Assam, Myanmar,
East Himalaya,
Pakistan, Laos,
Nepal, Vietnam,
Sri Lanka, West
Himalaya,
Thailan
d
Ethyl acetate stem bark
extract P. cerasoides
inhibits the growth of
Staphylococcus
aureus and Klebsiella
pneumoniae
Phytoconstituent
, flavonoids,
diterpenes, and
cardiac
glycosides
Mahajan &
Arora (2019)
Acronychia
pedunculata
(L.) Miq.
Rutaceae Java Tropical &
Subtropical Asia
Methanol extract from
a root, seed, flower,
leaves, and stem bark
extract gave the
maximum inhibition on
test pathogenic bacteria
(S. typhi, E. coli, B.
subtilis, and S. aureus).
It has been
demonstrated that the
essential oil extracted
from the plant's aerial
parts has a wide range
of antibacterial activity
against different
microorganisms.,
particularly
Staphylococcus
epidermidis and
Salmonella enterica
Sterols,
flavonoids,
terpenoids,
resins, saponins,
carbohydrates,
tannins, and
glycosides
Lesueur et al.
(2008),
Gireesha &
Raju (2016),
Ratnasooriya
et al. (2016),
Muthukuda
& Jayakody
(2021)
Zanthoxylum
acanthopodium
DC.
Rutaceae North
Sumatera
Himalaya to S.
China and W.
Malesia
The antibacterial
activity of the
Andaliman fruit's ethyl
acetate extract was
stronger response S.
aureus and S.
typhimurium than E.
Coli. 50 - 75% extracts
fruits also against
Salmonella typhi,
although did not against
all aquaculture
p
atho
g
ens
Alkaloids,
steroids, tannins
and saponins
Muzafri et al.
(2018),
Sihombing et
al. (2019),
Sibero et al.
(2021), Adrian
et al. (2023)
In North Sumatera Utara, Indonesia, andaliman (Zanthoxylum acanthopodium DC.) is a common wild plant.
Many members of the Batak ethnic group use Andaliman for food processing, particularly fish and meat. It is
known as "Batak pepper" for its hot flavor and peculiar scent. Andaliman is used by the Batak population as a
traditional medicine in addition to being used in food preparation (Adrian et al., 2023).
ICHR 2024 - BRIN’s International Conference for Health Research (ICHR)
12

Figure 1: a) A. pedunculata, b) Z. acanthopodium.
Figure 2: a) R. fraxinifolius, b) R. rosifolius, and c) P. cerasoides.
With low, prickly branches, trunks, and twigs, the
Andaliman is a shrub or small tree that can grow up
to 6 meters in height. Its odd-pinnate, dispersed,
stemmed compound leaves, which are 5–20 cm long
and 3–15 cm broad, contain oil glands. Three to
eleven thorny, oblong-shaped leaflets with tapering
ends and coarsely serrated edges, reaching 1 to 7 cm
in length and 0.5 to 2.0 cm in width, are affixed to the
winged rachis. Some leaves have a green top surface
and a reddish-green underside, while young or light
leaves have a sparkling green top surface and a green
bottom. The petals are pale yellow, androgynous, and
measure 5-7 cm long (1-2 cm). The flower has three
to four pistils, an apocarpous ovule, reddish anthers,
and around five to six stamens at the base. Andaliman
produces actual box fruits or capsules that are round,
2-3 mm in diameter, shiny black, contain one seed,
have a hard skin, and are brilliant green when young
or dark red when old. After ten days at room
temperature, the black seeds will sprout from the old
fruit (Rahmawaty et al., 2019; Sonangda et al., 2019).
The Andaliman plant is presented in Figure 1b.
Genus Rubus belongs to the Rosaceae family,
Rosoideae subfamily. With more than 1,350 species,
it is a sizable and varied genus. In high-altitude
woods, such as those in the Himalayas and the
Nilgiris, rubus is extensively distributed (Schulz and
Chim, 2019; Sharma et al., 2021). The ragimot berry
(Rubus spp.) is an erect shrub that grows to a height
of 2-3 meters and has stems with up to 6 mm prickles.
Its pinnate leaves have a terminal leaflet and four
pairs of opposing leaflets. These elliptic leaflets are
2-9 × 1.4 cm, with 7-10(-15) vein pairs, scant hair
coating, and serrated edges (Lamb, 2019). Grayish
yellow-green flowers with bulging shapes grow on
inflorescence panicles that measure 6-20 cm long
(Normasiwi et al., 2021).
R. rosifolius J. Sm. and Rubus fraxinifolius Poir.
are Indonesian Raspberry species native to Indonesia
(Figure 2a dan 2b). In West Java (Sundanese), the
fruit of R. rosifolius and R. fraxinifolius is known as
"Beberetean" or "Arben". Both fruits are edible, have
a similar appearance (small, red), and possess a sweet
instead of sour taste (Desmiaty et al., 2018).
Prunus is a member of the Rosaceae, specifically
the Amygdalaceae subfamily. It consists of around
430 deciduous and evergreen shrub and tree species
that are mostly found in the northern hemisphere and
Antibacterial Evaluation of the Rutaceae and Rosaceae Plant Collection from Cibodas Botanical Garden, Indonesia
13
temperate zones (Agrawal et al., 2024). Prunus has
simple, alternate, generally lanceolate, unlobed
leaves with nectarines on the stem. These flowers
have five petals, five sepals, and many stamens. They
are often white to pink but can also be crimson.
Flowers bloom singly, in umbels of two to six, or
even more, on racemes. Fruits are fleshy drupes with
a single rather big, hard-coated seed, popularly called
the stone fruit (Joseph et al., 2018). In India, Prunus
grows widely, and the majority of them have
significant therapeutic and commercial value, one of
which is P. cerasoides or Himalayan Cherry Blossom
(Agrawal et al., 2024), see Figure 2.
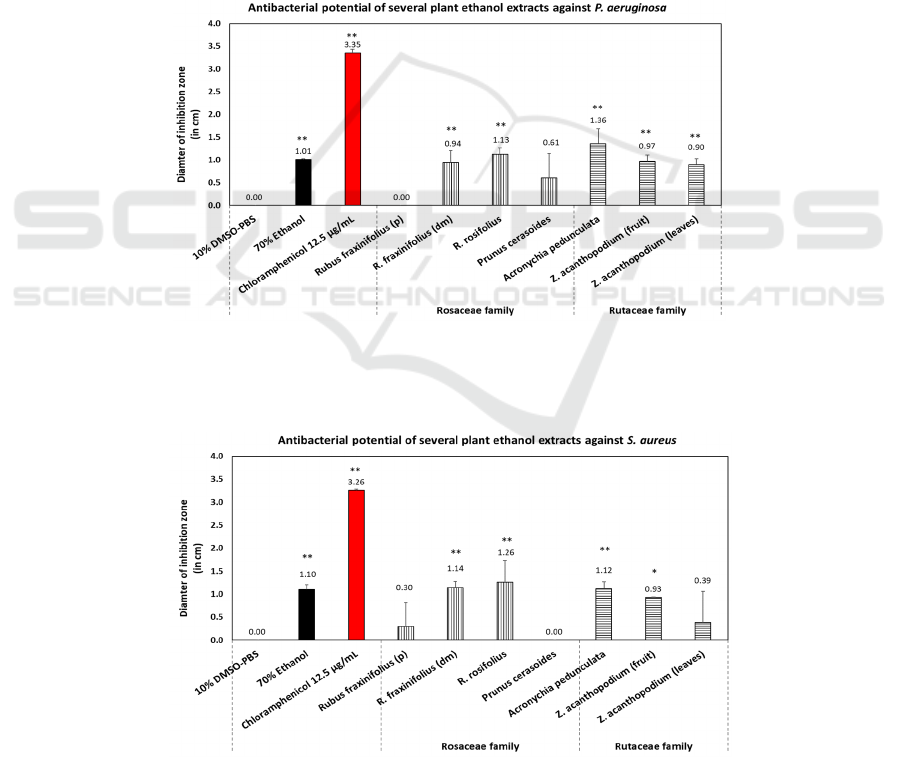
3.1 Antibacterial Activity
The diameter of the inhibition zones against different
bacteria was used to measure the antibacterial activity
of ethanolic extracts from the leaves and fruits of
Rosaceae and Rutaceae species (see Figure 3, 4, and
5). The ethanol extracts of the leaves of R. rosifolius,
A. pedunculata, and R. fraxinifolius (young leaves)
were considered as the top three that significantly
inhibited the growth of all three bacterial strains, i.e:
P. aeruginosa, S. aureus, and S. epidermidis.
However, results revealed that ethanol extract of A.
pedunculata leaves (from the Rutaceae family)
exhibited the highest antibacterial activity compared
to other species, followed by R. rosifolius (Rosaceae).
Conversely, R. fraxinifolius (p) leaf bud extract
demonstrated the lowest antibacterial activity based
on tests against the three bacteria. Overall, all extracts
produced the largest inhibition zone diameter against
S. epidermidis.
Figure 3: Antibacterial activity of plant collections from the families of Rutaceae and Rosaceae against Pseudomonas
aeruginosa. Values with *p < 0.05 or **p < 0.01 were considered statistically significant against negative control (10%
DMSO-PBS). Data were represented as mean ± SD. All samples were the leaves of the plants unless otherwise stated (fruits).
Notes: p: leaf bud, dm: young leaf.
Figure 4: Antibacterial activity of plant collections from the families of Rutaceae and Rosaceae against Staphylococcus
aureus. Values with *p < 0.05 or **p < 0.01 were considered statistically significant against negative control (10% DMSO-
PBS). Data were represented as mean ± SD. All samples were the leaves of the plants unless otherwise stated (fruits). Notes:
p: leaf bud, dm: young leaf.
ICHR 2024 - BRIN’s International Conference for Health Research (ICHR)
14
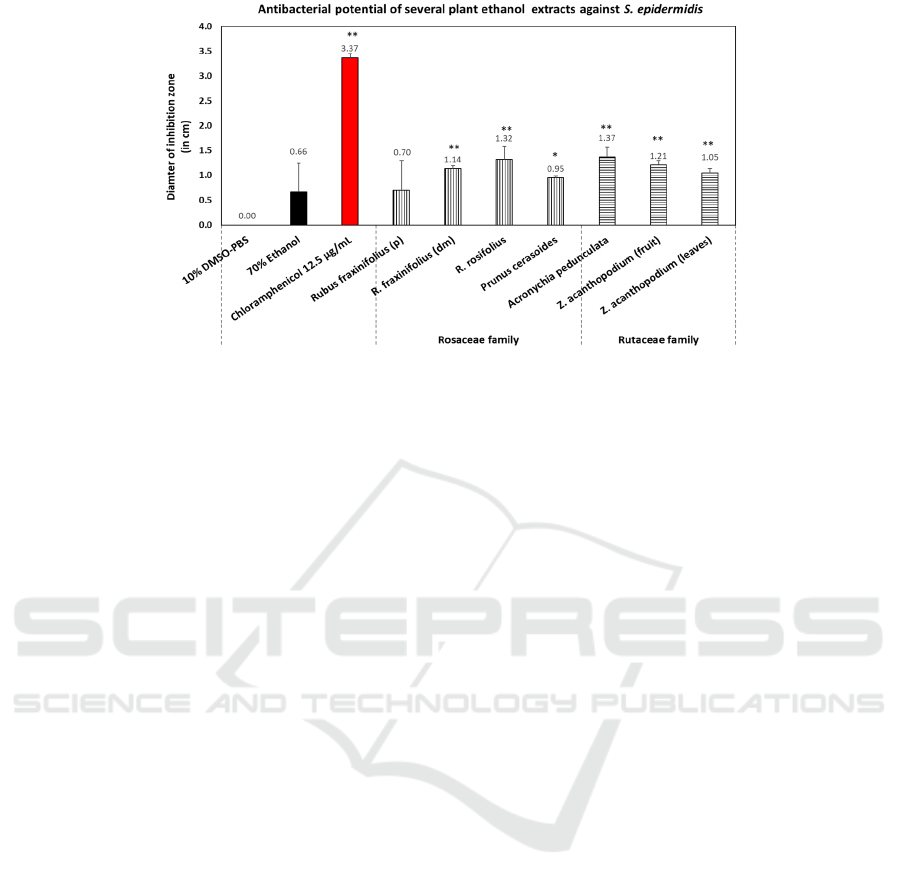
Figure 5: Antibacterial activity of plant collections from the families of Rutaceae and Rosaceae against Staphylococcus
epidermidis FNCC 0048. Values with *p < 0.05 or **p < 0.01 were considered statistically significant against negative control
(10% DMSO-PBS). Data were represented as mean ± SD. All samples were the leaves of the plants unless otherwise stated
(fruits). Notes: p: leaf bud, dm: young leaf.
The once-considered harmless bacterium S.
epidermidis, which thrives on human skin, has
developed into a significant opportunistic pathogen.
Numerous infections have been linked to these
bacteria, and treatment has been impeded by its
resistance to several drugs. New strains of multidrug-
resistant bacteria may arise as a result of S.
epidermidis acting as a reservoir for antibiotic
resistance genes that can be passed from one
Staphylococci species to another, including S. aureus
(Ahmadunissah et al., 2022).
In this study, A. pedunculata had the highest
antibacterial activity against the three test bacteria.
This is in line with the research of Van et al. (2020)
that all six bacterial strains—Bacillus cereus,
Staphylococcus aureus, Escherichia coli,
Pseudomonas aeruginosa, Salmonella enteritidis,
and Salmonella typhimurium—were able to
withstand the ethanolic extracts made from A.
pedunculata leaves.
Further, the extract of Acronychia pedunculata
contained polyphenols, triterpene alcohols, and
acetophenones, which were effective in inhibiting the
activity of Staphylococcus epidermidis and
Salmonella enterica (Kumar et al., 1989; Su et al.,
2003).
In addition, as shown in Figure 3, 4, and 5, the
ethanol extract of the fruit of Z. acanthopodium
slightly exhibited a wider inhibition zone compared
to that of its leaf ethanol extract. Majumder et al.
(2014) reported that traditionally the fruits of
Andaliman have been used as a spice, to treat fish
poisoning, to alleviate toothaches, and to treat
stomach colic, etc. The essential oil analysis of
Andaliman fruit identified 21 components, with
major components i.e. δ-3-carene (13.525%),
Limonine (16.903%), Eucalyptol (36.563%), and
Methyl-cinnamate (9.366%). With a broader zone of
inhibition, andaliman exhibits encouraging
antibacterial action, particularly against S. aureus.
Furthermore, the ethanol extract of the young leaf
of R. fraxinifolius showed higher antibacterial activity
than that of its leaf bud. Based on Shamsudin et al.
(2019), In addition to other phytochemicals including
alkaloids, phytosterols, tannins, and terpenoids, R.
fraxinifolius has been shown to possess flavonoids
and phenolic compounds. Free radical-related
illnesses have been treated and prevented with these
chemical substances. These substances exhibit
promise for use as natural antioxidants for human
well-being.
4 CONCLUSIONS
Natural materials have become increasingly popular
as traditional remedies in Indonesia. Traditional
treatments have been reported to have fewer negative
effects than chemical-based therapies. Many plant
species are widely used by the community as
traditional medicinal ingredients in daily life and have
antibacterial properties. Hence, according to our
results, plants from the Rutaceae and Rosaceae
families, especially the ethanol extracts of the leaves
of R. rosifolius (Indonesian Raspberry) and A.
pedunculata (Kayu Semidra) have the potential to be
further utilized as an active ingredient for
antibacterial products, especially against skin bacteria
Antibacterial Evaluation of the Rutaceae and Rosaceae Plant Collection from Cibodas Botanical Garden, Indonesia
15

(Pseudomonas aeruginosa, Staphylococcus aureus,
and S. epidermidis) which are safer and more
comfortable. However, a more specific antibacterial
mechanism needs further investigation.
ACKNOWLEDGEMENTS
This study was supported by the Indonesian Institute
of Sciences (LIPI) through “Program Prioritas
Nasional Gelombang Ke-2 di Lingkungan Deputi
Bidang Ilmu Pengetahuan Teknik LIPI” Year 2021.
REFERENCES
Agrawal, S., Kumar, A., Singh, A.K., Singh, H., Kumar, P.,
2024. Antioxidant and antibacterial evaluation of leaf
extracts of Prunus cerasoides Buch.-Ham. ex D.Don;
An In Vitro and In Silico study. Pharmacological
Research - Natural Products 3: 100037
Ahmadunissah, A., Aazmi, S., Abd Hamid, U.M., Aziyah,
A.A., 2022. Multidrug resistance of Staphylococcus
epidermidis: An emerging threat to global health. J
Appl Pharm Sci, 2022; 12(06):001–010.
Adrian, Syahputra, R.A., Juwita, N.A., Astyka, R., Lubis,
M.F., 2023. Andaliman (Zanthoxylum acanthopodium
DC.) a herbal medicine from North Sumatera,
Indonesia: Phytochemical and pharmacological review.
Heliyon. 9(5): e16159. https://doi.org/10.1016/j.heli
yon.2023.e16159.
Alvares, L.V., Caunca, E.S., Nolido, J.G., 2013.
Antimicrobial activity of Rubus rosifolius J.E. SM.
(Rosaceae) Fruit Crude Extract. PUP Journal of
Science and Technology. 6(1):1-9
Barbieri, R., Coppo, E., Marchese, A., Daglia, M., Sobarzo-
Sánchez, E., Nabavi, S.F., Nabavi, S.M., 2017.
Phytochemicals for human disease: An update on plant-
derived compounds antibacterial activity. Micro
biological research. 196: 44-68.
Chang, Y., Xia, S., Fei, P., Feng, H., Fan, F., Liu, Y., Qin,
L., Ma, L., Song, Q., Liu, Y., 2023. Houttuynia cordata
Thunb. crude extract inactivates Cronobacter sakazakii:
Antibacterial components, antibacterial mechanism,
and application as a natural disinfectant. Food Control.
145: 109467. https://doi.org/10.1016/j.foodcont.2022
.109467.
Chintaluri, A.K., Komarraju, A.L., Chintaluri, V.K.,
Vemulapalli, B., 2015. Comparative study of
antimicrobial activity of essential oils of selected plants
of Rutaceae and TLC bioautographic studies for
detection of bioactive compounds. Journal of essential
oil research. 27(1): 9-16.
Desmiaty, Y., Elya, B., Saputri, F.C., Hanafi, M., Prastiwi,
R., 2018. Antioxidant activity of Rubus fraxinifolius
Poir. and Rubus rosifolius J. Sm. Leaves. J
YoungPharm. 10(2): s93-s96
Dewi, R.T, Fitria, I., Sundowo, A., Agustian, E., Ismaini,
L., Normasiwi, S., Noviady, I., Destri, Surya, M.I.,
2019. Phytochemical constituent’s comparison using
various drying effects on Rubus fraxinifolius Pour
Leaves. Curr Agri Res. 7(3). http://dx.doi
.org/10.12944/CARJ.7.3.06
Fadilah, N.I.M., Maarof, M., Motta, A., Tabata, Y., Fauzi,
M.B., 2022. The discovery and development of natural-
based biomaterials with demonstrated wound healing
properties: a reliable approach in clinical trials.
Biomedicines. 10(9): 2226.
Fu, J., Dai, L., Lin, Z., Lu, H., 2013. Houttuynia cordata
Thunb: A Review of Phytochemistry and
Pharmacology and Quality Control. Chinese Medicine
04(03): 101-123.
García-Oliveira, P., Fraga-Corral, M., Pereira, A.G.,
Lourenço-Lopes, C., Jimenez-Lopez, C., Prieto, M.A.,
Simal-Gandara, J., 2020. Scientific basis for the
industrialization of traditionally used plants of the
Rosaceae family. Food chemistry. 330: 127197.
Gireesha, J., Raju, N.S., 2016. Phytochemical analysis,
antibacterial and antioxidant potential of Acronychia
pedunculata (L.) Miq. Annals of Phythomedicine. 5(2):
147-151.
Iszkuło, G., Kosiński, P., Hajnos, M., 2013. Sex influences
the taxanes content in Taxus baccata. Acta Physiologiae
Plantarum. 35(1): 147–152.
Halla, N., Fernandes, I.P., Heleno, S.A., Costa, P.,
Boucherit-Otmani, Z., Boucherit, K., ... & Barreiro,
M.F., 2018. Cosmetics preservation: a review on
present strategies. Molecules. 23(7): 1571.
Hendrich, A.B., Strugała, P., Dudra, A., Kucharska, A.Z.,
Sokół-Łętowska, A., Wojnicz D., Cisowska, A., Sroka,
Z., Gabrielska, J., 2020. Microbiological, antioxidant
and lipoxygenase-1 inhibitory activities of fruit extracts
of chosen Rosaceae family species. Advances in
Clinical and Experimental Medicine. 29(2): 215–224
Jayaweera, D.M.M., 1982. Medicinal plants (Indigenous
and Exotic) used in Ceylon Part V National Science
Foundation, Colombo, Sri Lanka. pp 3-4.
Joseph, N., Anjum, N., Tripathi, Y.C., 2018. Prunus
cerasoides D. Don: A Review on its ethnomedicinal
uses, phytochemistry and pharmacology. Int. J. Pharm.
Sci. Rev. Res. 48(1): 62-69
Kurniawan, R., Sukrasno, Ashari, A., Suhartati, T., 2024.
Diving into paclitaxel: isolation and screening content
from Taxus sumatrana at Singgalang Conservation
Center, West Sumatra. Natural Product Research.
Lamb, A., 2019. A guide to wild fruits of Borneo (1st ed.),
pp. 226–227. Natural History Publications.
Lesueur, D., Serra, D.D.R, Bighelli, A., Hoi, T.M., Thai,
T.H., Casanova, J., 2008. Composition and
antimicrobial activity of the essential oil of Acronychia
pedunculata (L.) Miq. from Vietnam. Nat Prod Res.
22(5): 393-8. doi:10.1080/14786410701475636.
Li, Yujie., Liu, Xiaolei., Liu, Xia., Wu, Nan., Peng, Xiao.,
Liang, Lu., Fu, Yujie., 2009. Chemical composition and
antimicrobial activity of the essential oil from Rosa
rugosa Thunb. Bulletin of Botanical Research, doi:
10.7525/J.ISSN.1673-5102.2009.04.018
ICHR 2024 - BRIN’s International Conference for Health Research (ICHR)
16

Mahady, G.B., Huang, Y., Doyle, B.J., Locklear, T., 2008.
Natural products as antibacterial agents. Studies in
natural products chemistry. 35: 423-444.
Mahajan, H., Arora, D.S., 2019. Major Phytoconstituents of
Prunus cerasoides Responsible for Antimicrobial and
Antibiofilm Potential Against Some Reference Strains
of Pathogenic Bacteria and Clinical Isolates of MRSA.
Appl Biochem Biotechnol. 188(4): 1185-1204. doi:
10.1007/s12010-019-02985-4.
Majumder, M., Sharma, H.K., Zaman, K., Lyngdoh, W.,
2014. Evaluation of physico-chemical properties and
antibacterial activity of the essential oil obtained from
the fruits of Zanthoxyllum acanthopodium DC.
collected from Meghalaya, India. Int J Pharm Pharm
Sci. 6(5): 543-546
Manent, B.F., Abellán, E.G., 2007. Quality Control of
Cosmetic Products. Specific Legislation on Ingredients.
Analysis of Cosmetic Products, 29.
Mota, A.H., Sousa, A., Figueira, M., Amaral, M., Sousa, B.,
Rocha, J., ... & Reis, C.P., 2020. Natural-based
consumer health nanoproducts: Medicines, cosmetics,
and food supplements. In Handbook of functionalized
nanomaterials for industrial applications (pp. 527-
578). Elsevier.
Muthukuda, C.L., Jayakody, T.R., 2021. Antibacterial
effect of Acronychia pedunculata fresh extract against
Staphylococcus aureus: A study in vitro. International
Journal of Trend in Scientific Research and
Development. 5(4): 1678-1682
Muzafri, A., Julianti, E., Rusmarilin, H., 2018. The
extraction of antimicrobials component of andaliman
(Zanthoxylum acanthopodium DC.) and its application
on catfish (Pangasius sutchi) fillet. IOP Conf. Ser.
Earth Environ. Sci. 122(1): 012089. Doi:10.1088/1755-
1315/122/1/012089
Normasiwi, S., Salamah, A., Surya, M.I., 2021.
Morphological characteristics of Indonesian Rubus
flowers. Biodiversitas. 22(3): 1441-1447
Rahmawaty, Samosir, J.B., Batubara, R., Rauf, A., 2019.
Diversity and distribution of medicinal plants in the
Universitas Sumatera Utara arboretum of deli serdang,
North Sumatra, Indonesia. Biodiversitas. 20(5): 1457–
1465. https://doi.org/10.13057/biodiv/d200539.
Ranaweera, C.B., Karunathilaka, N., Silva, A.R.N.,
Karunarathna, S., Pathirana, R., Ratnasooriya, D.,
2021. Antibacterial activity of aqueous Root, Seed,
Flower and Stem Bark Extracts of Acronychia
pedunculata Grown in Sri Lanka. International Journal
of Pharmaceutical Research & Allied Sciences. 5(2):
21-25.
Rodrigo, S.K., Jayasinghe, U.L.B., Bandara, B.M.R., 2007.
Antifungal, antioxidant and cytotoxic activity of
Acronychia pedunculata and Adenanthera povinina.
Proceedings of Peradeniya University Research
Sessions, Sri Lanka. 12: 94-95.
Schulz, M., Chim, J.F., 2019. Nutritional and bioactive
value of Rubus berries. Food Biosci. 31: 100438
Shamsudin, N.A, Matawali, A., Gansau, J.A., 2019.
Comparison of Antioxidant Activity and
Phytochemical Content of Borneo Wild Berry, Rubus
fraxinifolius (Rogimot). Transactions on Science and
Technology. 6(1): 36 - 41
Sharma, S., Kaur, R., Kumar, K., Kumar, D., Solanke,
A.K., 2021. Genetic variability in Rubus ellipticus
collections assessed by morphological traits and EST-
SSR markers. J Plant Biochem Biotech. 30: 37–55
Sibero, M.T., Siswanto, A.P., Murwani, R., Frederick, E.H.,
Wijaya, A.P., Syafitri, E., Farabi, K., Saito, S., Igarashi,
Y., 2020. Antibacterial, cytotoxicity and metabolite
profiling of crude methanolic extract from andaliman
(Zanthoxylum acanthopodium) fruit. Biodiversitas. 21
(9): 4147-4154
Sihombing, N., Sihombing, F., Juwitaningsih, T., Pasaribu,
D.M., 2019. Antibacterial Activity Analysis of
Zanthoxylum Acanthopodium DC Extract on Bacteria
of Bacillus subtilis, and Salmonella typhi. Proceedings
of the 5th Annual International Seminar on Trends in
Science and Science Education, AISTSSE 2018, 18-19
October 2018, Medan, Indonesia. doi: 10.4108/eai.18-
10-2018.2287303
Sonangda, M., Sinaga, H., Limbong, L.N., 2019. Effect of
ratio of andaliman (Zanthoxylum acanthopodium) with
garlic and aging time on the quality of sambal tuk tuk.
IOP Conf. Ser. Earth Environ. Sci. 260(1).
https://doi.org/10.1088/1755-1315/260/1/012084.
Tanjung, M., Nurmalasari, I., Wilujeng, A.K., Saputri, R.
D., Rachmadiarti, F., Tjahjandarie, T.S., 2018.
Acronyculatin P, A New Isoprenylated Acetophenone
from the Stem Bark of Acronychia pedunculata.
Natural Product Sciences. 24(4): 284-287
Ugwoke, C.E.C., Nzekwe, U., Ameh, G.I., 2010.
Phytochemical constituents and ethnobotany of the leaf
extract of bitter leaf (Vernonia amygdalina). Journal of
Pharmaceutical and Allied Sciences. 7(3)
Usunobun, U, Ngozi, O., 2016. Phytochemical analysis and
proximate composition of Vernonia amygdalina.
International Journal of Scientific World. 4(1)
Van, H.T., Nguyen, D.G.M., Huynh, N.T.A, Le, V.S., 2020.
Antibacterial activities of ethanolic extract of four
species of Rutaceae family. Plant Science Today. 7(3):
463–468. https://doi.org/10.14719/pst.2020.7.3.784
Van Vuuren, S., Viljoen, A., 2011. Plant-based
antimicrobial studies–methods and approaches to study
the interaction between natural products. Planta
medica. 77(11): 1168-1182.
Vaidyanathan, L., Lokeswari, T.S., 2021. Compounds from
Vernonia arborea Buch.Ham. Inhibit Microbes that
Impair Wound Healing. Journal of Pharmaceutical
Research International. 33(44B): 103-113.
Vaou, N., Stavropoulou, E., Voidarou, C., Tsigalou, C.,
Bezirtzoglou, E., 2021. Towards advances in medicinal
plant antimicrobial activity: A review study on
challenges and future perspectives. Microorganisms.
9(10): 2041.
Varvaresou, A., Papageorgiou, S., Tsirivas, E., Protopapa,
E., Kintziou, H., Kefala, V., Demetzos, C., 2009. Self‐
preserving cosmetics. International Journal of cosmetic
science. 31(3): 163-175.
Wu, Z., Deng, X., Hu, Q., Xiao, X., Jiang, J., Ma, X., Wu,
M., 2021. Houttuynia cordata Thunb: An
Antibacterial Evaluation of the Rutaceae and Rosaceae Plant Collection from Cibodas Botanical Garden, Indonesia
17

Ethnopharmacological Review. Front. Pharmacol. 12:
714694. doi: 10.3389/fphar.2021.714694
Zazharskyi, V.V., Davydenko, P., Kulishenko, O., Borovik,
I.V., Zazharska, N.M., Brygadyrenko, V.V., 2020.
Antibacterial and fungicidal activities of ethanol
extracts of 38 species of plants. Biosystems Diversity.
28(3): 281-289.
ICHR 2024 - BRIN’s International Conference for Health Research (ICHR)
18
