
Genetic Aspects of Arterial Hypertension: How Gene Polymorphisms
Determine the Risk of Developing Hypertension
Abidova Dilorom Ergashevna
1
, Muhamedova Muyassar Gafurjanovna
2
and Mullabayeva Guzal Uchkunona
3
1
Republican Specialized Scientific Practical Medical Center of Cardiology, Tashkent, Uzbekistan
2
Research Institute of Military Medicine of the Military Medical Academy of the Armed Forces of the
Republic of Uzbekistan, Uzbekistan
3
Republican Specialized Scientific Practical Medical Center of Cardiology, Tashkent, Uzbekistan
Keywords: Arterial, Premature, Special, Genetic.
Abstract: Arterial hypertension (AH) is one of the leading causes of cardiovascular diseases and premature death.
Recently, special attention has been paid to genetic markers that may predispose to the development of AH
and its complications. The presented study, conducted with the participation of 392 people (100 without AH
and 292 with AH), was focused on the analysis of genetic factors influencing the development of
hypertension, myocardial hypertrophy and obesity. Genetic markers, such as polymorphisms of the AGTR1,
AGTR2, AGT, ADD1, CYP11B2, NOS3 and GNB3 genes, were studied using PCR and other molecular
methods. The results showed that the AGTR1 and AGTR2 genes did not have statistically significant
differences between the groups. However, the ADD1 gene was associated with a family history of
hypertension, and the CYP11B2 mutation was associated with obesity, as confirmed by the WC/HR indicator.
The GNB3 gene showed a direct relationship with the presence of left ventricular hypertrophy and the WC/HR
level. These data confirm the importance of genetic testing for assessing the risks of developing hypertension
and its complications, as well as the need for further research to develop personalized approaches to the
treatment and prevention of hypertension.
1 INTRODUCTION
Arterial hypertension (AH) remains one of the main
causes of cardiovascular diseases and premature
death worldwide. In particular, more than 1.1 billion
people suffer from hypertension, which is associated
with lifestyle changes, population aging and an
increase in risk factors such as obesity, stress and lack
of physical activity (Shabalov et al., 2019). Genetic
predisposition to hypertension has become an
important area of research in recent years, as it can
significantly affect the development of the disease
and its complications. The influence of genetic
factors on the development of hypertension,
myocardial hypertrophy and obesity has been
confirmed by a number of international and Russian
studies. For example, Sato et al. (2008) note the
association of gene polymorphisms, such as AGT and
CYP11B2, with the risk of hypertension and its
progression (Sato et al., 2008). In Russia, genetic
markers associated with predisposition to
hypertension are being studied. In the works of
Shabalov V.M. (2019) emphasizes the importance of
genetic testing for the early diagnosis of
hypertension. Also, as shown in the studies of
Kawarazaki et al. (2016), certain mutations in genes
regulating sodium balance may be associated with the
development of hypertension and obesity. An
important topic is also a genetic predisposition to left
ventricular hypertrophy (LVH), which is confirmed
by the works of Weiss et al. and Nazarov N.I. et al..
Thus, the study of genetic markers such as ADD1
G1378T, CYP11B2 C344T and GNB3 C825T
remains an urgent task for improving the methods of
prognosis, early diagnosis and creating individualized
approaches to the treatment of arterial hypertension
and its complications, which served as the goal of our
scientific work.
762
Ergashevna, A. D., Gafurjanovna, M. M. and Uchkunona, M. G.
Genetic Aspects of Arterial Hypertension: How Gene Polymorphisms Determine the Risk of Developing Hypertension.
DOI: 10.5220/0013424600004654
In Proceedings of the 4th International Conference on Humanities Education, Law, and Social Science (ICHELS 2024), pages 762-769
ISBN: 978-989-758-752-8
Copyright © 2025 by Paper published under CC license (CC BY-NC-ND 4.0)

2 MATERIAL AND METHODS
The study included 392 people, of whom 100 were
individuals without hypertension (control group) and
292 had arterial hypertension (AH) of varying
severity (main group). The ratio of men to women
was 266/126, i.e. the number of men was 2.1 times
greater than the number of women. The gender ratio
in the groups was as follows: in the main group
198/94 (i.e. 2.1/1.0) and in the control group – 68/32
(i.e. 2.1/1.0). The average time from the diagnosis of
hypertension to inclusion in the study was 2.2 years.
The clinical parameters of the examined patients
included: measurement of systolic and diastolic blood
pressure (SBP and DBP, mmHg) of the maximum,
usual and at the time of examination, as well as
measurement of heart rate (HR, bpm).
From the anamnestic data, the presence of
concomitant pathologies was analyzed: chronic
obstructive pulmonary disease (COPD),
cerebrovascular pathology, anemic syndrome (with a
blood Hb level < 100 g / l), oncology, previous covid-
19, chronic kidney disease (CKD), visual impairment.
In addition to a general clinical examination, their
taste sensitivity to table salt was studied using a
modified method of R.J. Henkin. Salt sensitivity was
determined using the method of M.H. Weinberger.
Genetic studies were performed using peripheral
blood collected on ethylenediaminetetraacetic acid
(EDTA). Genomic DNA was isolated using the
QIAamp DNA Blood Mini Kit (QIAGEN,
Germantown, MD, USA). We investigated the
A1166C polymorphism of the AGTR1 gene and the
G1675A polymorphism of the AGTR2 gene using
real-time polymerase chain reaction (RT PCR)
performed using the ViiA 7 Real Time PCR System
(Life Technologies, USA). We used the TaqMan Pre-
Designed SNP Genotyping Assay. The following
candidate genes were analyzed:
angiotensin I receptor type 1 gene AGTR1
(A1166C),
angiotensin II receptor type 2 gene AGTR2
(G1675A),
angiotensinogen gene AGT (C521T and
T704C),
ADD1 gene G1378T was determined to
determine the genetic predisposition to salt-sensitive
hypertension,
aldosterone synthase gene CYP11B2
C344T,
GNB3 gene C825T,
as well as NOS3 genes G894T and NOS3
T786C.
Standard chi-square (x2) and Student's t-test
methods were used for statistical analysis;
calculations were performed using the Statistica 6.0
statistical program to determine the probability of
differences in the distribution of genotypes during the
development of hypertension. Differences were
considered reliable at p<0.05.
3 RESEARCH RESULTS
We studied in detail the frequency of certain
candidate genes and their genotypes in the analyzed
sample (Table 1). As can be seen from Table 1, the
studied genetic markers AGTR2 G1675A, AGTR1
A1166C, AGT C521T, AGT T704C, as well as NOS3
G894T and NOS3 T786C did not differ significantly
between the groups (all p>0.05).
Direct analysis of the isolated genotypes
established that the homozygous AA genotype of the
genetic marker AGTR1 A1166C, the CC genotype of
the marker AGT C521T and the TT genotype of the
marker AGT T704C were comparable in frequency of
occurrence and turned out to be the predominant
genotypes in general in all individuals of the studied
sample, regardless of the presence/absence of AH
(Table 1).
A similar situation was observed for the
homozygous GG genotype of the genetic marker
NOS3 G894T and the TT genotype of the marker
NOS3 T786C, which made up the majority of these
genes, both in patients with and without AH (Table
1).
In the cohort examined by us, the prevalence of
the homozygous GG genotype was revealed for the
ADD1 G1378T gene in both groups of patients, both
in the control group (87.0%) and in patients with AH
(56.5%). However, in the main group, this advantage
was not so pronounced and was 30.5% less in
comparison with the control group (p < 0.00001). In
the group of patients with AH, in comparison with the
control group, the heterozygous GT genotype
prevailed (32.9% versus 9.0%, respectively, in the
main and control groups; p < 0.00001). Also, among
patients with AH, the homozygous TT genotype was
noted 6.6% more often than in patients without AH.
That is, the last two genotypes are heterozygote GT
and homozygote TT, although they did not constitute
the majority of genotypes, nevertheless, their
frequency prevailed among individuals with AH
(about 43.5%), while in the control group these
genotypes constituted, in total, 13.0%.
Genetic Aspects of Arterial Hypertension: How Gene Polymorphisms Determine the Risk of Developing Hypertension
763
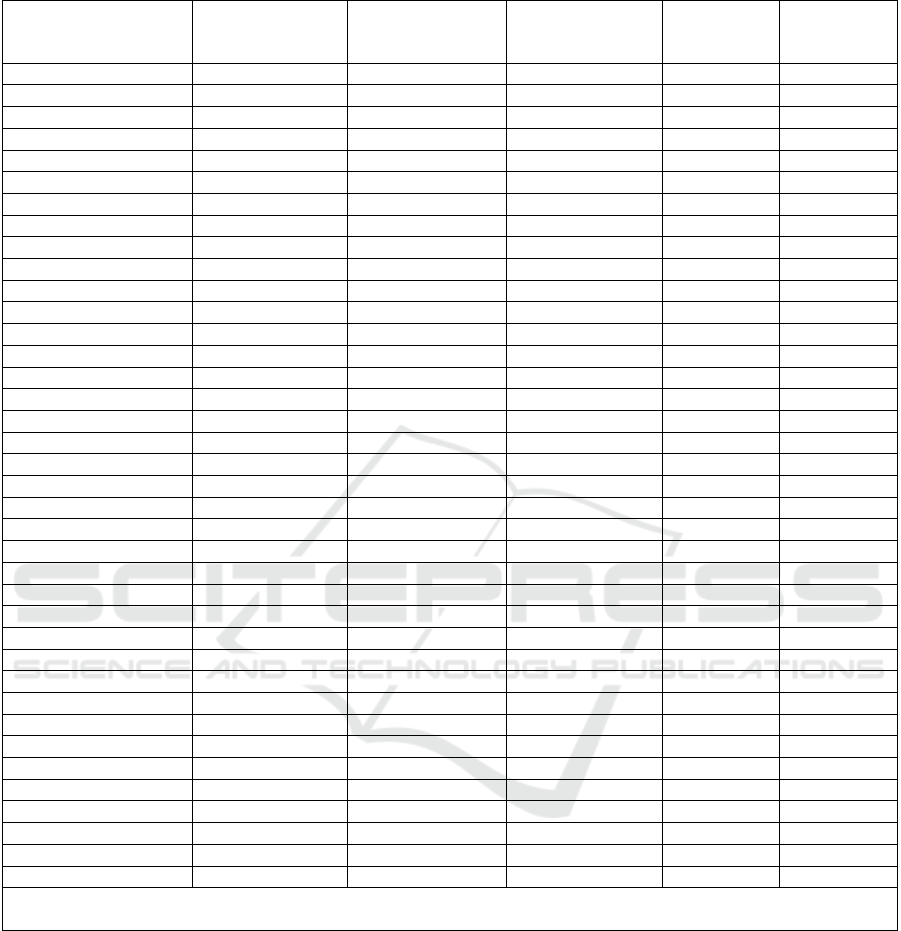
Table 1: Frequency of the candidate genes in question in the analyzed sample and depending on the presence/absence of AH.
Genes, genetic
markers and their
g
enot
yp
es
The entire
sample (n=392)
Without AH
(n=100)
With AH
(n=292)
p χ2
AGTR2 G1675A
А 129
(
32,9%
)
32
(
32,0%
)
97
(
33,2%
)
0,920 0,010
G 137 (34,9%) 36 (36,0%) 101 (34,6%) 0,894 0,018
АА 38 (9,7%) 10 (10,0%) 28 (9,6%) 0,940 0,006
GA 40 (10,2%) 10 (10,0%) 30 (10,3%) 0,910 0,013
GG 48
(
12,2%
)
12
(
12,0%
)
36
(
12,3%
)
0,929 0,008
AGTR1 A1166C 392
АА 266
(
67,9%
)
69
(
69,0%
)
197
(
67,5%
)
0,874 0,025
АС 85 (21,7%) 20 (20,0%) 65 (22,3%) 0,740 0,111
СС 41 (10,5%) 11 (11,0%) 30 (10,3%) 0,988 0,000
AGT C521T
СС 309
(
78,8%
)
82
(
82,0%
)
227
(
77,7%
)
0,449 0,575
СТ 62
(
15,8%
)
14
(
14,0%
)
48
(
16,4%
)
0,676 0,175
ТТ 21 (5,4%) 4 (4,0%) 17 (5,8%) 0,660 0,195
AGT T704C
СС 40 (10,2%) 9 (9,0%) 31 (10,6%) 0,778 0,073
ТС 84
(
21,4%
)
22
(
22,0%
)
62
(
21,2%
)
0,984 0,000
ТТ 268
(
68,4%
)
69
(
69,0%
)
199
(
68,2%
)
0,974 0,001
ADD1 G1378T
GG 252 (64,3%) 87 (87,0%) 165 (56,5%) 0,000 28,854
GT 105 (26,8%) 9 (9,0%) 96 (32,9%) 0,000 20,454
TT 35
(
8,9%
)
4
(
4,0%
)
31
(
10,6%
)
0,072
#
3,238
CYP11B2 C344T
СС 269
(
68,6%
)
83
(
83,0%
)
186
(
63,7%
)
0,000 12,007
СТ 69 (17,6%) 12 (12,0%) 57 (19,5%) 0,121 2,409
ТТ 54 (13,8%) 5 (5,0%) 49 (16,8%) 0,006 7,740
GNB3 C825T
СС 241
(
61,5%
)
71
(
71,0%
)
170
(
58,2%
)
0,032 4,612
СТ 114
(
29,1%
)
20
(
20,0%
)
94
(
32,2%
)
0,029 4,794
ТТ 37
(
9,4%
)
9
(
9,0%
)
28
(
9,6%
)
0,981 0,001
NOS3 G894T
GG 259 (66,1%) 69 (69,0%) 190 (65,1%) 0,553 0,353
GT 92
(
23,5%
)
20
(
20,0%
)
72
(
24,7%
)
0,417 0,659
TT 41
(
10,5%
)
11
(
11,0%
)
30
(
10,3%
)
0,988 0,000
NOS3 T786C
CC 45 (11,5%) 11 (11,0%) 34 (11,6%) 0,995 0,000
TC 89 (22,7%) 24 (24,0%) 65 (22,3%) 0,826 0,048
TT 258 (65,8%) 65 (65,0%) 193 (66,1%) 0,939 0,006
Note: n – number of patients; AH – arterial hypertension; p – significance of differences between groups with/without
A
H at p<0.05; #
–
tendency to significance
Since the ADD1 gene encodes the alpha subunit
of the adducin protein, one of the regulators of
sodium-potassium adenosine triphosphatase (Na+,
K+-ATPase), the latter, in turn, is involved in the
transport of these ions through the membrane of the
renal epithelium, this gene is used in assessing the
genetic predisposition to salt-sensitive hypertension.
Based on this fact, we conducted a parallel
analysis with family history data. In this aspect, it was
found that in general, 201 (51.3%) people in the entire
sample of subjects indicated a burdened heredity for
AH, and in most cases (175 people) these were
patients from the main group, i.e. with the presence
of AH, the remaining 26 were from the control group.
In percentage terms of the number of groups, this
indicator also prevailed among people with AH,
amounting to 59.9% versus 26.0% in the control
group (p = 0.0000 and χ2 = 32.983). When
conducting a correlation analysis between the
frequency of the isolated genotypes of the ADD1
G1378T gene (GG, GT and TT) and family history,
the following dependencies were revealed (Fig. 1A
ICHELS 2024 - The International Conference on Humanities Education, Law, and Social Science
764
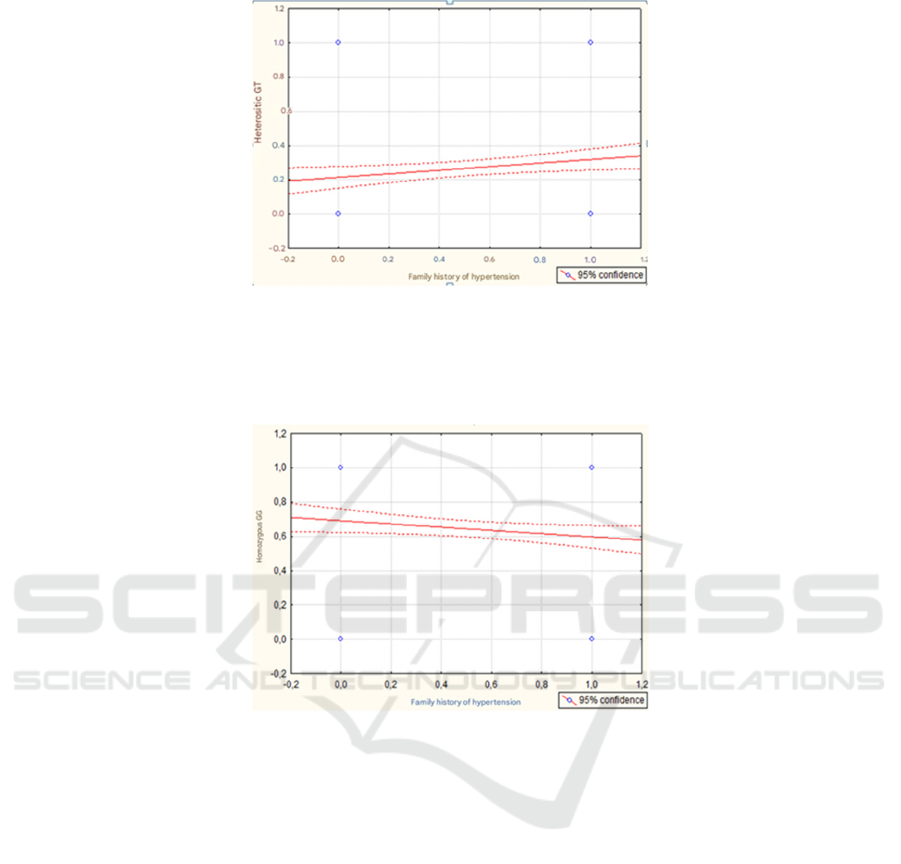
Note: On the X-axis, under the number “0” - the absence of an aggravated family history and under the number “1” - the
presence of an aggravated heredity for AH; on the Y-axis, under the number “0” - the absence of GT heterozygote and
under the number “1” - the presence of GT heterozygote of the ADD1 G1378T gene.
Figure 1A: Correlation graph between the heterozygous GT genotype of the ADD1 gene and the presence of a burdened
family history of AH. р=0,020; r=0,117 и t=2,328.
p=0.052#; r= -0.098 and t= -1.947
Note: On the X-axis, under the number “0” - the absence of an aggravated family history and under the number
“1” - the presence of an aggravated heredity for AH; on the Y-axis, under the number “0” - the absence of a GG homozygote
and under the number “1” - the presence of a GG homozygote of the ADD1 G1378T gene.
Figure 1B: Correlation graph between the homozygous GG genotype of the ADD1 gene and the presence of a burdened
family history of AH.
and 1B). Namely, the prevalence of the heterozygous
GT genotype was characterized by a direct
relationship with an aggravated family history of AH,
and the identified relationship reached the level of
reliability (Fig. 1A). No significant interdependence
was found between a family predisposition to AH and
the homozygous TT genotype (p=0.738; r= -0.016
and t= -0.334). On the contrary, an inverse correlation
with a tendency toward reliability was established
between the homozygous GG genotype and an
indication of an aggravated family history (Fig. 1B).
That is, a heredity burdened by AH, or a family
predisposition to the development of AH, had a clear
association with the heterozygous GT genotype of the
ADD1 gene, and given its connection with a
predisposition to salt-sensitive hypertension, we can
conclude that the patients we examined had, one way
or another, a nutritional disorder. On the contrary, the
homozygous GG genotype of the ADD1 gene,
according to the results of our analysis, was more
associated with healthy (free from AH) individuals.
Analysis of the genetic marker C344T of the
CYP11B2 gene also established the prevalence of the
homozygous CC genotype. Namely, in the sample as
a whole, its frequency of occurrence was 68.6%, and
in the compared groups 83.0% in the control group,
i.e. in individuals without AH and 63.7% in patients
with AH.
The heterozygous CT genotype accounted for
17.6% of cases in the entire sample (Table 1). In the
Genetic Aspects of Arterial Hypertension: How Gene Polymorphisms Determine the Risk of Developing Hypertension
765
group of patients with AH, this indicator was 7.5%
higher than in the control group (p=0.121 and
χ2=2.409).
A similar trend was observed for the homozygous
TT genotype. In particular, in the sample as a whole,
this genotype was recorded in 13.8% of cases, and in
the compared groups in 5.0% and 16.8% of cases
(p<0.05), respectively, in the control group and in the
main group of patients (Table 1).
It is widely known that the CYP11B2 gene
encodes aldosterone synthetase, which ensures the
synthesis of aldosterone from deoxycorticosterone.
Detection of the C344T mutation in the regulatory
region of the gene is associated with an increase in
aldosterone synthesis. The latter is known to be
responsible for the functioning of the renal sodium-
potassium pump and maintenance of water-
electrolyte balance. In this regard, we conducted a
correlation analysis between the isolated genotypes
(CC, CT and TT) of the CYP11B2 gene and the
presence of CKD in the examined patients. In this
aspect, no significant relationships were revealed.
Namely, the presence of renal pathology was
characterized by an inverse relationship with the
heterozygous CT genotype (p = 0.329; r = -0.057 and
t = -0.976) and a positive correlation with
homozygous CC (p = 0.900; r = 0.006 and t = 0.000)
and TT genotypes (p = 0.854; r = 0.010 and t = 0.183),
however, the identified trends did not reach the level
of reliability. The obtained results were probably due
to the small number of patients with CKD: 28.1% of
the total sample, and in the selected groups their
number was 12.0% in the control group and 33.6% in
the group of patients with AH (p=0.0000 and
χ2=16.104). According to the literature [3], some
variants of genes associated with sensitivity to salt
affect the risk of obesity, and together with salt
consumption, their combination may be associated
with the development of hypertension in obese
people. In this regard, we tried to study the
relationship of the isolated genetic marker CYP11B2
C344T with obesity, or rather its indirect indicator -
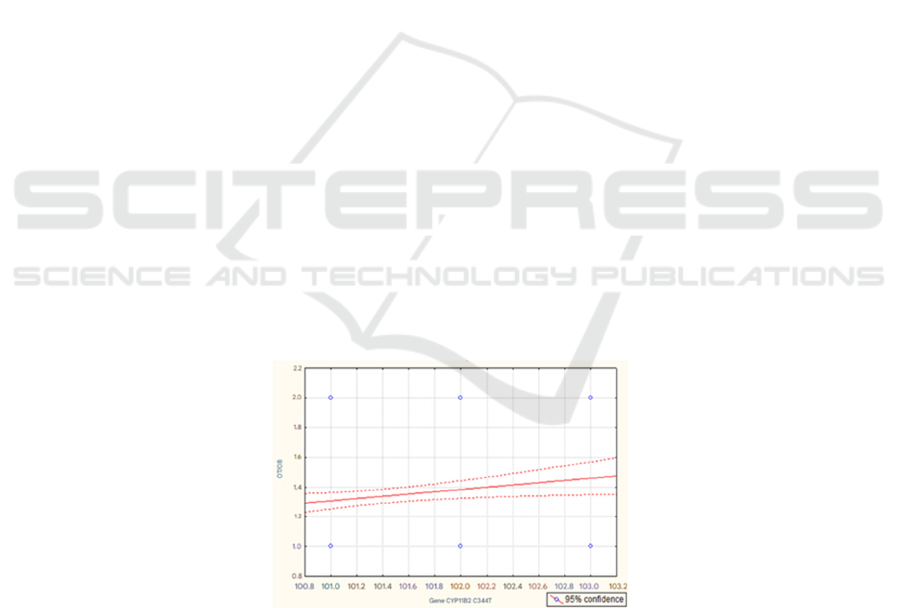
the waist-to-hip ratio (WHR). We found a positive
correlation (Fig. 2) between the studied genetic
marker and the WHR (p<0.05).
That is, this fragment of our work indicates a
relationship between the genetic marker CYP11B2
C344T and obesity (p<0.05), mediated by the
presence of a mutation in the regulatory region of the
gene responsible for the synthesis of aldosterone. At
the same time, its relationship with the potassium-
sodium renal pump and water-electrolyte balance did
not reach the level of reliability (all p>0.05), which
was probably due to the small number of diagnosed
renal pathology in the analyzed sample of patients. In
the sample of patients examined by us, another
genetic marker attracts attention - this is GNB3
C825T. Detection of the C825T mutation in the
GNB3 gene indicates changes in the differentiation of
lymphoblasts, fibroblasts and proliferative activity. In
scientific research, the study of this genetic marker is
carried out with the aim of identifying a genetic
predisposition to AH, as well as assessing the
relationship with LV myocardial hypertrophy (LVH),
the development of obesity and diabetes mellitus.
p=0.022; r=0.115 and t=2.290
Notes: on the X-axis – the selected genotypes of the CYP11B2 C344T marker and on the Y-axis – under the number “1” –
WC/HR < 1 and under the number “2” – WC/HR > 1.
Figure 2: Graph of the correlation between the genetic marker CYP11B2 C344T and the WC/HR ratio.
ICHELS 2024 - The International Conference on Humanities Education, Law, and Social Science
766
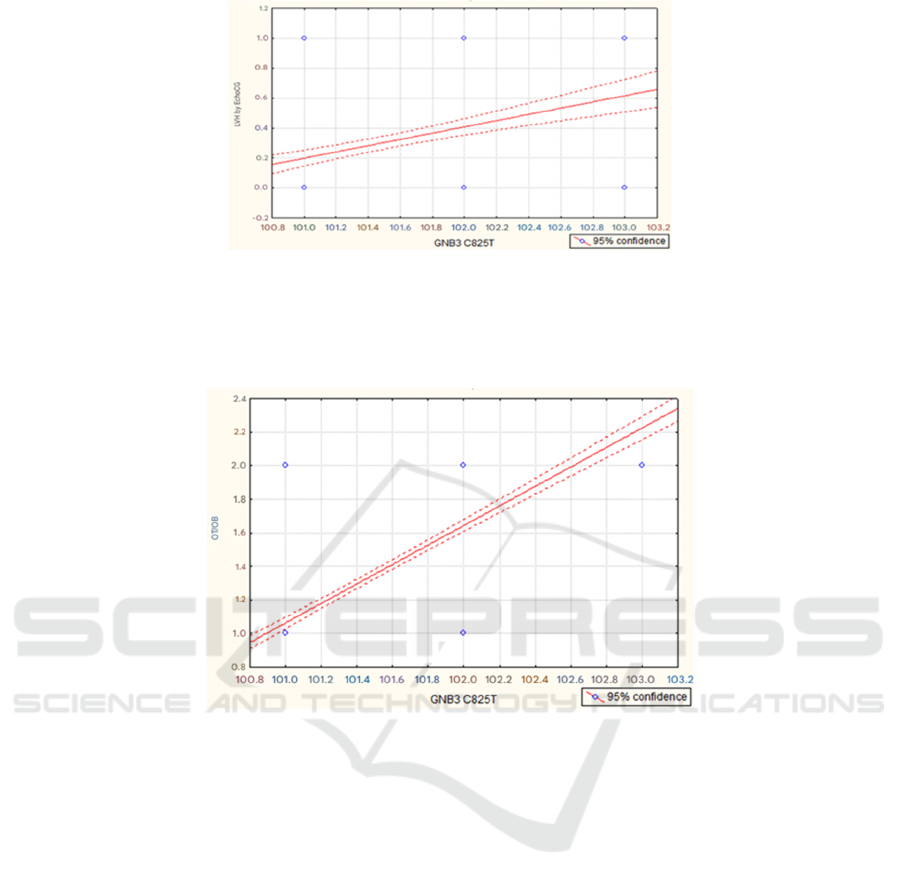
p=0.000; r=0.302 and t=6.260
Notes: X-axis – isolated genotypes of the marker GNB3 C825T and Y-axis – left ventricular hypertrophy (LVH) detected
by echocardiography.
Figure 3A: Correlation graph between the isolated genetic marker GNB3 C825T and the presence of LV myocardial
hypertrophy.
p=0.000; r=0.810 and t=27.292
Notes: on the X-axis – isolated genotypes of the marker GNB3 C825T and on the Y-axis – under the number “1” - WC/HR
< 1 and under the number “2” - WC/HR > 1.
Figure 3B: Correlation graph between the isolated genetic marker GNB3 C825T and the WC/HR ratio.
Based on the above, we conducted a correlation
analysis between the selected genotypes (CC, CT and
TT) of the genetic marker GNB3 C825T and the
presence of LVH according to echocardiography, an
indirect indicator of obesity - WC/OB and
postprandial blood glucose values. From this
position, a direct positive correlation was revealed
between the genetic marker GNB3 C825T and the
presence of LVH according to echocardiography - on
the one hand (Fig. 3A) and the level of WC/OB - on
the other hand (Fig. 3B), while both correlations were
highly reliable (both p < 0.0000). A direct
relationship was also established between the genetic
marker GNB3 C825T and the level of postprandial
blood glucose, although it did not reach the level of
reliability (p=0.082; r=0.087 and t=1.738).
A study of the frequency of occurrence of homo-
and heterozygous genotypes for the GNB3 C825T
gene revealed a picture that, in the sample as a whole,
regardless of the presence/absence of AH, the
homozygous CC genotype prevailed (61.5%), while
in patients with AH it was recorded in 58.2%, and in
individuals without AH - in 71.0% of cases. The
presence of the heterozygous CT genotype was
present in 114 (29.1%) patients, of which 94 (82.5%
of 114 people with this genotype or 32.2% of the main
group n=292) were people with AH and 20 people
(17.5% of 114 or 20.0% of the control group n=100)
were from the control group (p<0.05). The
homozygous TT genotype was found in the smallest
number of cases, amounting to 9.4% of the total
sample, and 9.0% and 9.6% in the analyzed control
groups and in people with AH, respectively (Table 1).
Thus, a detailed analysis of the genetic marker
CYP11B2 C344T established its relationship with the
presence of LVH (according to echocardiography)
Genetic Aspects of Arterial Hypertension: How Gene Polymorphisms Determine the Risk of Developing Hypertension
767

and obesity, or rather with its indirect indicator – the
WC/OB ratio, while both dependencies were highly
reliable (p<0.0001). There was also a positive
correlation with the level of postprandial blood
glucose, but this trend did not reach the level of
reliability. The genotypic picture of the GNB3 gene
revealed the prevalence of the homozygous CC
genotype, regardless of the presence/absence of AH,
but the heterozygous CT genotype was predominant
among patients with AH (82.5%), while the
homozygous TT genotype occurred with almost the
same frequency in the compared groups (9.0% and
9.6%).
4 DISCUSSION
The study found that among the genetic markers
studied (AGT, CYP11B2, GNB3, ADD1 and NOS3),
only some of them demonstrate a significant
association with the development of arterial
hypertension (AH) and its complications, such as
myocardial hypertrophy and obesity. This is
consistent with the results of many international
studies that emphasize the role of genetic
predisposition in the pathogenesis of AH.
The AGT gene (angiotensin I converting enzyme
gene) and its polymorphisms, such as AGT C521T,
AGT T704C, have long attracted the attention of
researchers as key markers of AH risk. The works of
Curb et al. (2007) and Gagliardi et al. (2009) show
that variations in genes encoding components of the
renin-angiotensin-aldosterone system (RAAS)
significantly affect the development of hypertension,
including the difficulty in its treatment [8,9]. Our
results, which did not show statistically significant
differences in these markers in the sample with AH
and the control group, are generally consistent with
similar studies, where polymorphisms in these genes
do not always have a clear effect on the severity of
the disease. The CYP11B2 gene, encoding
aldosterone synthetase, is also of interest as a factor
influencing the development of hypertension. The
works of Kawarazaki and Fujita (2016), as well as
Wang et al. (2018) demonstrated an association of
mutations in this gene with increased sensitivity to
salt and excessive secretion of aldosterone, which in
turn contributes to an increase in blood pressure. Our
findings of the prevalence of the homozygous CC
genotype in the control group and the prevalence of
the heterozygous CT genotype in the AH group
confirm the results of similar studies. However, as in
some studies, for example, Yang et al. (2015), we did
not observe a significant correlation with the
development of chronic kidney disease, which may
be due to differences in the sample or a lower
incidence of kidney disease in the study group. The
GNB3 C825T gene has been actively studied in
recent years in the context of hypertension and its
complications. Studies by Cohen et al. (2005) and
Weiss et al. (2003) show that a mutation in this gene
may be associated with myocardial hypertrophy,
obesity, and diabetes. Our findings on the high
prevalence of the heterozygous CT genotype among
patients with AH are also confirmed by international
data, which may indicate the importance of this
marker for predicting the risk of developing
hypertension and its cardiovascular complications.
The ADD1 gene, involved in the regulation of
sodium-potassium adenosine triphosphatase
(Na+,K+-ATPase), showed a significant association
with salt-sensitive hypertension, which is consistent
with the opinion of other researchers. According to
the work of Sowers et al. (2013), mutations in this
gene can increase the body's sensitivity to excess salt,
which leads to an increase in blood pressure. The
results of our study, where the heterozygous GT
genotype was more common among patients with AH
and was associated with a family predisposition, are
fully consistent with these findings. Overall, our
findings confirming the role of genetic markers such
as AGT, CYP11B2, ADD1 and GNB3 in the
development of AH are consistent with international
data and highlight the need for further research to
identify the exact mechanisms of their action. At the
same time, the lack of significant association with
chronic kidney disease in the case of CYP11B2 and
AGT requires additional studies aimed at clarifying
their role in the pathogenesis of AH.
5 CONCLUSION
The prevalence of certain genotypes in the group of
patients with AH (e.g., homozygous AGTR1
A1166C, AGT C521T, and NOS3 G894T genotypes)
suggests that these markers do not have a significant
difference in frequency between the groups with and
without AH. However, the ADD1 G1378T gene
demonstrated an association with a familial
predisposition to AH, especially in the case of the
heterozygous GT genotype, which is associated with
malnutrition and salt sensitivity.
The results of the CYP11B2 C344T marker
analysis showed a positive correlation with an
indirect indicator of obesity (WC/HC), which
confirms its possible role in the regulation of
metabolism and the development of obesity.
ICHELS 2024 - The International Conference on Humanities Education, Law, and Social Science
768

However, its association with renal pathology in this
sample was not statistically significant, which is
probably due to the small number of patients with
CKD. The GNB3 C825T marker showed a strong
positive correlation with left ventricular hypertrophy
(LVH) and obesity level, confirming its potential role
in the development of metabolic disorders and
hypertension. At the same time, the dependence on
the level of postprandial blood glucose was not
significant, which may indicate more complex
interactions between genes and metabolic processes
.
REFERENCES
Cohen, J. D., et al. (2005). American Journal of
Hypertension, 18(3), 429-437.
Curb, J. D., et al. (2007). Hypertension, 49(6), 1200-1206.
Gagliardi, L., et al. (2009). Journal of Hypertension, 27(10),
2024-2032.
Henkin, R. J., Gill, L. P., & Bartter, F. C. (1963). J. Clin
Invest, 42, 727-735.
Kawarazaki, W., & Fujita, T. (2016). American Journal of
Hypertension, 29(4), 415-423.
Назарова, Н. И., Морозова, И. В., & Иванова, А. Г.
(2020). Кардиология, 60(9), 74-81.
Sato, M., et al. (2008). Journal of Hypertension, 26(5), 934-
944.
Shabalov, V. M., Krylov, M. Yu., Panov, D. A., et al.
(2019). Журнал кардиологии, 59(6), 452-461.
Sowers, J. R., et al. (2013). Hypertension, 62(5), 897-905.
Wang, W., et al. (2018). Journal of Human Hypertension,
32(4), 255-262.
Weinberger, M. H. (1996). Hypertension, 27, 481-490.
Weiss, J., et al. (2003). Hypertension Research, 26(1), 35-
42.
Weiss, J., et al. (2003). Hypertension Research, 26(1), 35-
42.
Yang, X., et al. (2015). Kidney International, 88(5), 1049-
1057.
Genetic Aspects of Arterial Hypertension: How Gene Polymorphisms Determine the Risk of Developing Hypertension
769
