
Use of a Digital Positioning and Categorisation Aid for Clinical
Investigations on Medical Devices: Questioning the Complexity of the
Field and Harmonizing Stakeholders' Understanding
Jean-Baptiste Pretalli
1a
, Stéphanie Py
1
, Fatimata Seydou Sall
1b
, Magali Nicolier
1
,
Karine Charrière
1c
, Thierry Chevallier
2,4,5 d
and Thomas Lihoreau
1,2,3 e
1
Centre Hospitalier Universitaire de Besançon, Centre d'Investigation Clinique, INSERM CIC 1431, 25030,
Besançon, France
2
Tech4Health network - FCRIN, France
3
Université de Franche-Comté, SINERGIES, F-25000 Besançon, France
4
Department of Biostatistics, Clinical Epidemiology, Public Health, and Innovation in Methodology,
CHU of Nimes, University of Montpellier, Nimes, France
5
Desbrest Institute of Epidemiology and Public Health UMR, INSERM - University of Montpellier, Montpellier, France
kcharriere@chu-besancon.fr, thierry.chevallier@chu-nimes.fr, tlihoreau@chu-besancon.fr
Keywords: Medical Devices, Clinical Research, Regulation.
Abstract: Medical devices must comply with the safety and performance requirements of the European Medical Device
Regulation. For clinical investigations, regulatory approval from competent authorities is required.
ICTROUVE is a digital tool designed to help identify the clinical investigation’s category when applying to
the French competent authority, the Agence Nationale de Sécurité du Médicament et des produits de santé
(ANSM). We aimed to evaluate ICTROUVE and to prepare a larger-scale study.
This pilot study was divided in two sequences. The aim of the first was to recruit experts and to collect study
synopses for which the clinical investigation’s category issued by the ANSM was known. To achieve this
aim, we created and sent a questionnaire to researchers and regulatory managers via the Tech4Health network.
During the second sequence, the experts had to read the synopses and assign them a clinical investigation’s
category, first without and then with the help of ICTROUVE. A satisfaction questionnaire was then
completed.
We found a low decision agreement between experts and ANSM (39% without ICTROUVE, 51.7% with).
ICTROUVE was perceived as useful, easy and quick to use. Information was gathered to facilitate a larger-
scale evaluation, notably on the collection of synopses and the search for experts..
1 INTRODUCTION
Medical devices (MDs) offer a wide range of
innovative healthcare solutions. They enable
pathological conditions to be diagnosed, monitored,
treated or alleviated. They influence patient longevity
and quality of life while relieving pressure on the
healthcare system (‘Medical Devices Must Be
Carefully Validated’, 2018).
a
https://orcid.org/0000-0003-1035-3566
b
https://orcid.org/0000-0002-8351-0406
c
https://orcid.org/0000-0003-4542-8003
d
https://orcid.org/0000-0002-5110-6273
e
https://orcid.org/0000-0001-8417-6609
Clinical investigation are related to medical
devices and fall within the scope of the European
Regulation 2017/745 (MDR) (HAS, 2017;
Regulation (UE) 2017/745, 2017).
The MDR brings many necessary advances but it
also implies a significant increase in the requirements
expected from manufacturers and from notified
bodies which must adapt to the new regulations. This
has a major impact in terms of cost and time that
864
Pretalli, J., Py, S., Sall, F., Nicolier, M., Charrière, K., Chevallier, T. and Lihoreau, T.
Use of a Digital Positioning and Categorisation Aid for Clinical Investigations on Medical Devices: Questioning the Complexity of the Field and Harmonizing Stakeholders’ Understanding.
DOI: 10.5220/0012617600003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 864-871
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.

could be difficult to absorb for manufacturers,
especially small and medium-sized enterprises, which
account for 95% of the total (SNITEM, 2020), and
those with a portfolio of old, low-risk products
(SNITEM, 2022). Shortages are also envisaged in
hospitals (Académie nationale de médecine, 2022;
Sayin et al., 2022). Conversely, any reduction in time
expended can confer a competitive edge upon a
manufacturer in relation to its competitors.
In France, to conduct a clinical investigation (CI)
on medical devices, authorisation from the Agence
nationale de sécurité du médicament et des produits
de santé (ANSM) is essential. However, the
acceptance rate in the first round is very low. A study
of the 284 dossiers submitted for CI between May 26,
2021, and February 28, 2022, found that only 30
(10.5%) had been accepted outright. In addition, 34
(12%) dossiers for which ANSM had requested
additional information were not resubmitted by the
manufacturers (ANSM, 2022). Identifying the CI
category and compiling the application for
authorisation appear thus to be a complex task. Any
time-saving assistance would clearly be beneficial to
patients and manufacturers alike.
There are several reasons why identifying the
right CI category is so difficult. First, not every
research using an MD fall under the scope of the
MDR. Research using an MD but with a main
objective not related to the evaluation of its safety,
performance and/or effectiveness may fall under the
scope of the French Loi Jardè n°2012-300. This law
concerns all Research Involving the Human Person
(RIPH in French) with a view to furthering biological
or medical knowledge. The approach is based on risk
in three types of study. RIPH category 1 is a research
implying an intervention on the patient which is not
justified by their usual treatment. RIPH category 2
concerns interventional research with minor
obligations and risk. RIPH category 3 concerns
observational research.
Moreover, for research falling under the scope of
the MDR, seven CI’s categories exist. The number of
decision nodes required to identify the correct one is
very large, and the definitions of CI categories are
very close to each other. In addition, the definitions
are difficult to interpret. Finally, if the personnel
responsible for identifying CI categories are
qualified, they may be insufficient in number to cope
with the required workload (SNITEM, 2020).
Category 1 and 2 (CI1 and CI2) concerns clinical
investigations on a MD when CE conformity is
sought. CI3 concerns a CE-marked DM used in its
intended purpose with any additional
burdensome/invasive procedure. CI4.1 concerns a
CE-marked DM used in its intended purpose with no
additional burdensome/invasive procedure. CI4.2
concerns a CE-marked MD (any class), used in its
intended purpose without the conformity assessment
and including additional procedures. CI4.3 concerns
a CE-marked MD (of any class) used outside its
intended purpose without the purpose of CE marking
or conformity assessment. CI4.4 concerns non-CE-
marked MD (all classes) without a CE marking
objective.
ICTROUVE is a digital tool designed to help
identify the CI’s category to which an MD must be
subjected. It is a questionnaire produced online, based
on the requirements of the MDR and the adaptations
made at national level by the ANSM (Chevallier et
al.). This tool could save a considerable amount of
time in CI authorisation applications. It could also
facilitate a more relevant orientation of the
investigations and offer a way for developers and
evaluators to question their project strategy before
submission.
ICTROUVE's efficiency in correctly identifying
CI categories compared with the standard method (i.e.
as the experts usually do, without ICTROUVE) needs
to be evaluated. It means testing the concordance
between the CI categories identified with
ICTROUVE and the CI categories identified by
ANSM. This involves collecting a sufficient number
of use cases for which ANSM has issued an opinion.
It also implies the participation of a sufficient number
of representative experts to carry out the various tests.
The aim of this pilot work was to initiate this
evaluation. We present the results of a survey testing
the methods for collecting the use cases and recruiting
the experts. Another objective of the survey was to
obtain feedback on the use of ICTROUVE by
researchers and regulatory managers, and thus to
identify possible improvements to be made to
ICTROUVE. We also intended to obtain an initial
assessment of ICTROUVE's ability to identify the CI
category.
2 MATERIAL AND METHODS
2.1 Study Design
Survey using a questionnaire and interviews.
2.2 Objectives and Outcomes
The main objective was to prepare a large-scale
evaluation of ICTROUVE's ability to correctly
identify CI categories.
Use of a Digital Positioning and Categorisation Aid for Clinical Investigations on Medical Devices: Questioning the Complexity of the Field
and Harmonizing Stakeholders’ Understanding
865

Secondary objectives were to:
1. obtain information on the actual working
methods of the experts responsible for
identifying CI categories in French
university hospitals;
2. describe the need for assistance in
identifying CI’s categories;
3. evaluate the use case identification and
collection method used to evaluate
ICTROUVE;
4. appraise the method used to identify and
invite potential experts to participate in the
ICTROUVE evaluation;
5. assess the expert’s capacity to recognise a
clinical investigation compared to a
‘classical’ RIPH study (studies involving
human subjects);
6. compare the performance of ICTROUVE
with that of the standard method for
identifying the correct CI category;
7. evaluate ICTROUVE usability in terms of
ease of use, clarity of questions and user
satisfaction;
8. obtain suggestions for improving
ICTROUVE by questioning the experts
taking part in the study;
9. describe potential failures in the use of
ICTROUVE in order to implement
corrective measures.
For secondary objectives 1, 2, 7 and 8,
assessments were carried out using Likert scales
completed at the end of the test series. Likert scales
consisted of propositions for which the respondent
expressed a degree of agreement or disagreement
(‘strongly disagree’, ‘somewhat agree’, ‘neither agree
nor disagree’, ‘somewhat agree’, ‘strongly agree’).
For secondary objective 9, potential ICTROUVE
failures were characterised by the inability to
complete all the questions and arriving at a usable
result.
2.3 ICTROUVE
ICTROUVE is a free online application developed
under REDCap (Research Electronic Data Capture)
by Louise Bastide, Hugo Potier and Thierry
Chevallier of Nîmes’ University Hospital.
2.4 Study Population
Firstly, a questionnaire was sent to researchers and
regulatory managers in several French university
hospitals (via the Tech4Health network). The purpose
of this questionnaire was to assess the usefulness of a
tool to help identify CI categories, to recruit experts
and to collect use cases for the second phase of the
study.
‘Phase 2’ was carried out on volunteers referred
to in this report as ‘experts’. The set of use cases was
presented to all participants in the same order
(random order). Experts had to classify the use cases
collected in phase 1 in ‘CI’ or ‘RIPH’. Then, the
experts identified the RIPH category (RIPH 1, 2 or 3)
or the CI category (CI1, CI2, CI3, CI4.1, CI4.2, CI4.3
or CI4.4) first without ICTROUVE, then with
ICTROUVE. Finally, each participant completed a
questionnaire on usability, ease of use and
satisfaction with ICTROUVE.
The category identified by ANSM remained
secret until the end of the evaluation.
2.5 Statistical Analyses
A description of all participants was drawn up for the
following parameters: profession, number of years'
experience, and workplaces.
Categorical variables were presented in the form
of numbers and percentages. They were compared
using the Chi2 test or Fisher's exact test.
3 RESULTS
3.1 Identification and Collection of the
Use Cases
Seven use cases were collected. Three concerned
RIPH studies and 4 CI studies. For each, we had the
study category issued by the ANSM. They were
obtained from four University Hospital Centres.
3.2 Identification and Invitation of the
Experts
Twelve people replied to our contact e-mail. Nine
agreed to take part as experts: 4 researchers and 5
regulatory managers. All worked at Besançon
University Hospital, except for experts 7 (researcher)
and 9 (regulatory manager), who worked at Nancy
University Hospital.
Seven (78%) had at least 10 years of experience
in their positions, while 2 (22%) had between 1 and 5
years of experience. None had used ICTROUVE
prior to this study. Table 1 presents these experts.
ClinMed 2024 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
866
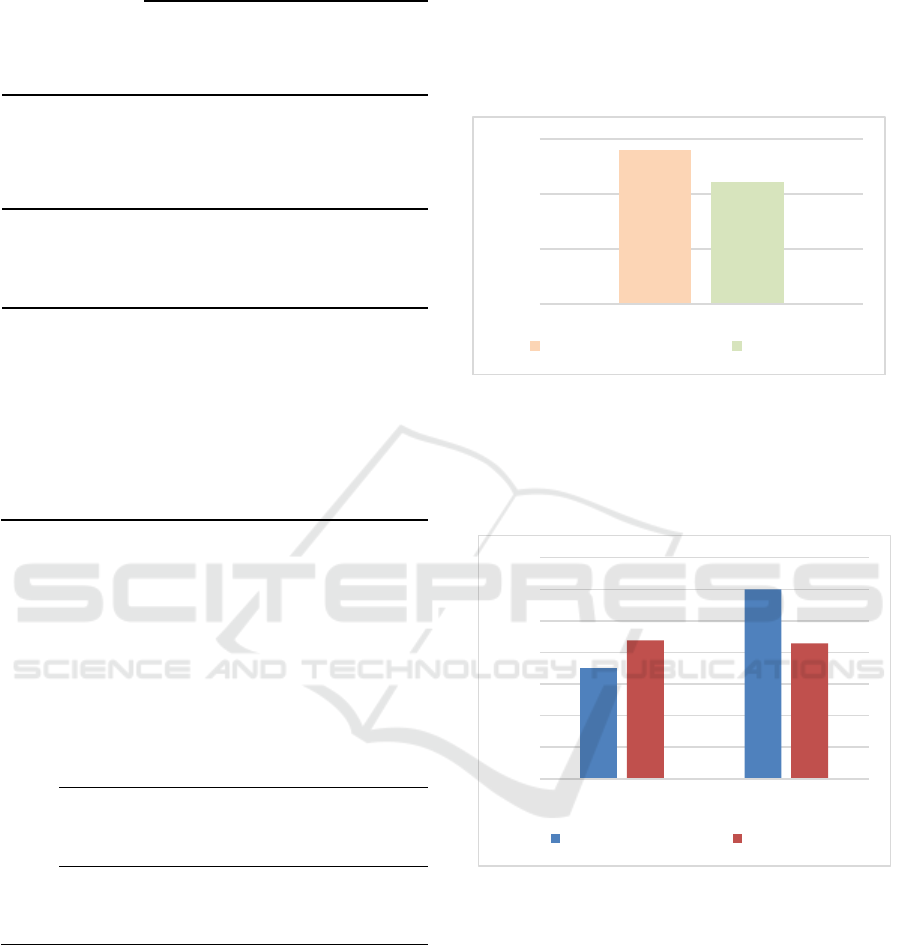
Table 1: Experts’ participating in study phase 2.
Regulatory
managers
n=5 (55%)
Researchers
n=4 (45%)
Total
n=9
(100%)
Years of
ex
p
erience
>10 years 4 (80%) 3 (75%) 7 (78%)
1 to 5 years 1 (20%) 1 (25%) 2 (22%)
University
Hos
p
ital
Besançon 4 (80%) 3 (75%) 7 (78%)
Nancy 1 (20%) 1 (25%) 2 (22%)
When you have to identify a CI's category, do you
usually work :
- alone? 0 (0%) 1 (25%) 1 (11%)
- in a group? 0 (0%) 0 (0%) 0 (0%)
- alone and then
in a group?
4 (80%) 3 (75%) 7 (78%)
- alone, then in a
group for
difficult cases?
1 (20%) 0 (0%) 1 (11%)
3.3 Results Regarding the Need for
Assistance in Identifying CI
Categories
The need for help was unanimously reported (table
2).
Table 2: “Do you think a tool to help you identify the
clinical investigation category of a medical device would be
useful?”
Regulatory
manager
n=8 (67%)
Researchers
n=4 (33%)
Total
n=12 (100%)
Yes
8 (100%) 4 (100%) 12 (100%)
No
0 (0%) 0 (0%) 0 (0%)
Total 8 (100%) 4 (100%) 12 (100%)
3.4 Identification of the RIPH and CI’s
Category
It took between 45 and 75 minutes for the experts to
analyse the 7 use cases and answer the ICTROUVE
evaluation questionnaire.
The experts’ first task was to recognise which
synopses corresponded to RIPH studies (falling under
the scope of the Jardè law) and which corresponded
to clinical investigations (falling under the scope of
the MDR). This task was to be carried out without the
help of ICTROUVE, leaving the experts to proceed
as usual. The 9 experts analysed 7 synopses each (63
tests have been performed in total). Figure 1 presents
the number of synopses adequately recognised as
RIPH studies or clinical investigations by regulatory
managers and researchers.
Figure 1: Correct identification of clinical investigations.
Regarding IC’s categories, the 9 experts analysed
4 synopses first without and then with the assistance
of ICTROUVE (36 tests have been performed in
total). Results are presented in Figure 2.
Figure 2: Correct identification of clinical investigations’
categories without and with ICTROUVE.
Without ICTROUVE, the correct overall response
rate averaged 39% (14 out of 36). For researchers, the
correct response rate was 43.8% (7/16). For
regulatory managers, it was 35% (7/20) (p=0.734).
With ICTROUVE, the correct overall response
rate averaged 42.8% (15/35). For researchers, the
correct response rate was 42.9% (6/14). For project
managers, the rate was 60% (9/15) (p=0.466).
Out of the 29 CI studies recognised as such by the
experts, the application of ICTROUVE yielded
divergent results compared to the method without
ICTROUVE in 7 (23%) cases. Of these 7 cases, the
n=24
(55,8%)
n=19
(44,2%)
0%
20%
40%
60%
Regulatory manager Researcher
35,0%
60,0%
43,8%
42,9%
0%
10%
20%
30%
40%
50%
60%
70%
Without ICTROUVE With ICTROUVE
Regulatory manager Researcher
Use of a Digital Positioning and Categorisation Aid for Clinical Investigations on Medical Devices: Questioning the Complexity of the Field
and Harmonizing Stakeholders’ Understanding
867

use of ICTROUVE resulted in the identification of
the correct CI category on 4 (57%) occasions.
3.5 Results of the ICTROUVE
Usability Survey
The results of the ICTROUVE usability survey are
presented in Table 3.
Table 3: Usability survey.
Regulatory
mana
g
ers
Researchers Total
n=4 (50%) n=4 (50%) n=8 (100%)
ICTROUVE is easy to use
totally agree 4 (80%) 2 (50%) 6 (67%)
agree 0 (0%) 1 (25%) 1 (11%)
neither agree nor disagree 1 (20%) 1 (25%) 2 (22%)
disagree 0 (0%) 0 (0%) 0 (0%)
strongly disagree 0 (0%) 0 (0%) 0 (0%)
ICTROUVE questions are clea
r
totally agree 1 (20%) 1 (25%) 2 (22%)
agree 3 (60%) 2 (50%) 5 (56%)
neither agree nor disagree 1 (20%) 1 (25%) 2 (22%)
disagree 0 (0%) 0 (0%) 0 (0%)
strongly disagree 0 (0%) 0 (0%) 0 (0%)
Compared with the standard method, ICTROUVE is more satisfactor
y
totally agree 0 (0%) 0 (0%) 0 (0%)
agree 3 (60%) 2 (50%) 5 (56%)
neither agree nor disagree 2 (40%) 2 (50%) 4 (44%)
disagree 0 (0%) 0 (0%) 0 (0%)
strongly disagree 0 (0%) 0 (0%) 0 (0%)
Compared with the standard method, ICTROUVE meant that I didn't forget any important criteria when identifying the
right CI category.
totally agree 3 (60%) 0 (0%) 3 (33%)
agree 1 (20%) 2 (50%) 3 (33%)
neither agree nor disagree 1 (20%) 1 (25%) 2 (22%)
disagree 0 (0%) 1 (25%) 1 (11%)
strongly disagree 0 (0%) 0 (0%) 0 (0%)
Compared with the standard method, I'm more confident in my ability to identify the right CI category with ICTROUVE.
totally agree 1 (20%) 0 (0%) 1 (11%)
agree 1 (20%) 1 (25%) 2 (22%)
neither agree nor disagree 3 (60%) 3 (75%) 6 (67%)
disagree 0 (0%) 0 (0%) 0 (0%)
strongly disagree 0 (0%) 0 (0%) 0 (0%)
Additional information (definitions, etc.) should be added to facilitate completion.
totally agree 2 (40%) 1 (25%) 3 (34%)
agree 3 (60%) 1 (25%) 4 (44%)
neither agree nor disagree 0 (0%) 1 (25%) 1 (11%)
disagree 0 (0%) 1 (25%) 1 (11%)
strongly disagree 0 (0%) 0 (0%) 0 (0%)
If ICTROUVE's ability to identify the category of a CI was equivalent to that of the usual method, I would prefer to use
ICTROUVE
totally agree 1 (20%) 3 (75%) 4 (44%)
agree 3 (60%) 0 (0%) 3 (33%)
neither agree nor disagree 0 (0%) 1 (25%) 1 (11%)
disagree 1 (20%) 0 (0%) 1 (11%)
strongly disagree 0 (0%) 0 (0%) 0 (0%)
ClinMed 2024 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
868

4 DISCUSSION
The European Medical Device Regulation adopted in
2017 strengthens the safety and performance
requirements imposed on medical devices (MD). This
reinforcement has an impact particularly on clinical
investigations (CI), which, in themselves, are already
time-consuming and costly.
Our survey suggests that the preparation of CI
application dossiers to the ANSM, the French
competent authority, is complex. We found little
agreement between the CI categories identified by the
experts and those finally assigned by ANSM.
Concordance was found in only 14 of the 36 cases
(39%), even if we could argue that knowledge of the
detailed projects could enhance this result.
ICTROUVE could serve as a facilitator for this
task. Although some modifications could be
considered, the usability survey showed that
ICTROUVE did indeed appear to be a good guide,
easy and quick to use according to the majority of the
participants. The concordance between the CI
categories identified with ICTROUVE and those
issued by the ANSM was 51.7%. However, its
performance needs to be validated by a larger-scale
study. This future confirmation will require the
participation of experts, such as researchers and
regulatory managers, in charge of preparing
submission dossiers to the ANSM, and in a sufficient
number. It will also require a large number of use
cases to be tested. The survey we conducted provided
information that could increase the feasibility of these
two key aspects of the next stages of this research.
During a webinar on the theme of clinical
investigations under Regulation 2017/745, the
ANSM presented the results of an analysis examining
the 284 CI authorisation applications submitted
between May 26, 2021, and February 28, 2022.
Within this pool of applications, 46 (16.2%) were in
the IC1 category, 47 (16.5%) in the IC2 category, 4
(1.4%) in the IC3 category, 113 (39.8%) in the IC4.1
category, 50 (17.6%) in the IC4.2 category, 11 (3.9%)
in the IC4.3 category and 13 (4.6%) in the IC4.4
category. It appeared that 216 (76%) applications
were validated, but only 30 (10.5%) in the first round.
The very low rate of acceptance in the first round
implies additional costs and delays for setting up the
CI, or even the abandonment of the project due to the
impossibility for manufacturers to respond to
ANSM's requests and complete their dossier (in 34
(12%) cases) (ANSM, 2022).
There are several potential reasons for ANSM's
refusal at this stage, such as an incomplete application
or a request that doesn't align with a CI but rather to
a RIPH study. It is important to identify the causes of
errors in order to propose appropriate solutions.
A first cause of error might stem from the
distinction between CI and RIPH. Indeed, it may be
difficult to know whether the research project
concerns a clinical investigation of a medical device.
In our tests, errors at this stage could concern up to
one third of the cases. An additional error could arise
from misidentifying the CI category. Participants in
our study reported a strong need for help on this
particular point. To the question, "Do you think a tool
to help identify the clinical investigation category of
a medical device would be useful to you?" the 12
experts questioned in phase 1 answered in the
affirmative. In addition, several participants
expressed a strong lack of confidence in their ability
to carry out the CI category identification exercise.
This lack of confidence seems to reflect a real
difficulty. Our results highlight a significant
discrepancy between the categories identified by the
experts, with or without the help of ICTROUVE, and
the categories validated by the ANSM. Without
ICTROUVE, the experts correctly identified the CI
category in 39% of cases. With ICTROUVE, this
success rate rose to 51.7%.
The number of decision nodes required to identify
the correct one is very large, and the definitions of CI
categories are very close to each other. In addition,
the definitions are difficult to interpret. For example,
the sponsor must assess whether the additional
procedures provided for in the clinical investigation
plan should be considered burdensome and/or
invasive. Burdensome additional procedures can
include a wide variety of different interventions,
including procedures that may cause pain,
discomfort, fear or potential risks or side effects,
disruption of life and personal activities, or other
unpleasant experiences. Burdensomeness is primarily
determined from the point of view of the person
bearing the burden. Invasive procedures include, but
are not limited to, penetration inside the body,
including through the mucous membranes of body
orifices, or penetration through a body orifice
(Medical Device Coordination Group, 2021).
Regarding the use of ICTROUVE, the feedback
from our 9 experts was positive, suggesting that it was
easily learned, user-friendly and that the questions
were clearly formulated. Finally, if ICTROUVE's
ability to identify the category of a CI was equivalent
to that of the standard method, 7 (77%) participants
said they would prefer to use ICTROUVE rather than
the standard method.
However, several improvements could be
envisaged. After ICTROUVE has been used, a button
Use of a Digital Positioning and Categorisation Aid for Clinical Investigations on Medical Devices: Questioning the Complexity of the Field
and Harmonizing Stakeholders’ Understanding
869

to submit a new application would be useful. A
majority of participants indicated a need for additional
information to facilitate filling in ICTROUVE. The
most frequently requested information concerned
definitions of MD characteristics (implantable, etc.).
One expert would have liked concrete examples to
illustrate the questions. Finally, ICTROUVE failed on
one occasion in which it proposed no new questions
and no results.
The use cases we provided to participants did not
mention the class of the MD. This information is
essential for identifying the CI category and is
complex to determine. This adds a further source of
error that can reduce performance with and without
ICTROUVE. Participants reported not having to
identify the class of the MD in their real working
lives. Furthermore, if the purpose of the study was
mentioned in the use cases, most participants would
have preferred it to be presented unambiguously. In
real life, these ambiguities are typically resolved
through direct communication with the manufacturer
or principal investigator.
This may explain why ICTROUVE, while not
judged less satisfactory than the standard method,
was not judged more satisfactory either, with 5 (56%)
experts "agreeing" and 4 (44%) "neither agreeing nor
disagreeing". Similarly, only 3 (33%) participants
answered "strongly agree" or "agree" to the question
"Compared to the standard method, I'm more
confident in my ability to identify the right CI
category with ICTROUVE". The remaining 6 (67%)
answered "neither agree nor disagree".
There are a number of limitations to this survey.
The first relates to the questionnaire used to collect
part of the results (Stratton, 2012, 2015). The
response rate remains unknown, and the potential for
significant differences between respondents and non-
respondents has yet to be established. Respondents
may not be representative, as a voluntary effect is
always possible. It is also possible that the people
who responded are precisely those individuals who
strongly felt the most important need for assistance in
identifying the CIs categories. However, even if, in
the worst-case scenario, the study population were
not representative of the target population, and even
if only some of the experts were to declare a need for
help, ICTROUVE's existence would still be justified,
provided that this number of people was sufficiently
large. The unanimous expression of the need for
assistance from all participating experts indicates that
such a necessity is widespread.
An additional constraint that could impact the
representativeness of the study population is the fact
that all the experts belonged to public institutions. The
experts involved (in industry, in contract research
organisations) may have specific functions and
encounter specific difficulties which would be
interesting to study. Nevertheless, even if ICTROUVE
were to be evaluated and judged as performing well
only in the academic arena, this would be sufficient
justification for having developed and disseminated it.
It is also possible that the questions were worded
in such a way that the opinions and prejudices of the
researchers influenced the people who responded to
the survey. The questions to be asked in the next steps
of our work will have to be carefully worked out and
tested to prevent such bias.
Our questionnaire offered the opportunity to
participate voluntarily and without obligation but did
not give the option of remaining anonymous. These
choices may explain the low number of responses
obtained, a number which confers reduced statistical
power to our study. However, the purpose of this
survey was to determine the feasibility of a larger
survey, and it therefore did not require significant
statistical power (Brooks & Stratford, 2009). This
larger study will enable the hypotheses formulated to
be tested more rigorously.
In light of the small number of experts recruited
for phase 2, we chose to ask them all to identify the
CI categories with and without ICTROUVE. It cannot
be ruled out that the identification without
ICTROUVE had an influence on the identification
with ICTROUVE. However, all the experts worked in
the same order, first without and then with
ICTROUVE, which appeared to us to be the least
biased. The use of ICTROUVE is highly
standardised, leaving little room for interpretation.
Within the framework of the larger study to be
conducted, a way to eliminate this bias could be found
in the randomisation of experts into "STANDARD
method" versus "ICTROUVE method" clusters.
Another limitation is that we were unable to carry
out the study under real-life conditions. One of the
aims of the survey was to gain a better understanding
of how the experts responsible for identifying CI
categories work. The information obtained in our
survey suggests that, while the identification of CI
categories is initially carried out individually, it is
often followed by collegial work, which we did not
replicate. The next steps will need to consider the
possibility of allowing experts to work in groups.
Some of the experts' comments also indicated that
they often turn to the manufacturers or principal
investigators for further clarification. This mainly
concerns technical information on the MD, and
information on the purpose and methodology of the
study. In the context of the next steps we intend to
ClinMed 2024 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
870

follow in our study, this information will have to be
taken into account and a solution found to make it
available to the experts.
Our search for use cases indicates that they are not
yet widely available and are very difficult to collect.
As the EUDAMED database is not yet operational on
all aspects, synopses have been obtained via
university hospitals directly. This data collection
method frequently necessitates acquiring
authorisation from the principal investigator to utilise
information pertaining to their studies. Furthermore,
the individuals interviewed during phase 1 indicated
a scarcity of use cases available for sharing. An
approach involving the ANSM directly would make
it possible to obtain a larger number of cases through
a single contact, as well as involving the authority for
its opinion and input on the ICTROUVE solution.
Collecting a sufficient number of use cases is an
essential point. An insufficient number would reduce
statistical power, limit the choice of the most
appropriate methodology, and make it impossible to
work on all existing CI categories. According to
ANSM, certain categories are poorly represented (e.g.
IC3 and IC4.3). However, it is possible that some CI
categories are more difficult to identify.
Finally, we considered that the CI categories
identified by ANSM were correct. It is indeed
important that ICTROUVE arrives at the same CI
categories as the ANSM, since it is the latter that does
or does not authorise CIs. However, there could be
discrepancies between the ANSM and the new MD
regulations. Verifying this hypothesis could be one of
the objectives of the next steps.
5 CONCLUSIONS
Our work identified a real difficulty for experts and
researchers to identify the CI category in France.
Inherent difficulties arising from the text issued from
the ANSM could constitute an explanation. Another
one is that the task is highly complex and requires a
great deal of interpretation. It is not unlikely that such
a subjective process will be observed in any EU
country. A validated computer aid like ICTROUVE
could remove the need for interpretation and improve
the concordance between competent authorities,
researchers and regulatory managers.
Our study points out that a larger-scale study
would be useful and feasible. ICTROUVE appears to
be well designed, and the few suggestions for
improvement put forward by expert users seem
straightforward to implement. Finally, the survey
gathered information that could prove relevant to the
prospective implementation of a larger-scale study,
particularly with regard to the process of collecting
use cases and finding experts.
It might be worth extending this work to the
European level in order to identify possible needs for
assistance and to develop a continental tool that
would not only simplify national research but also
facilitate and harmonise international research.
REFERENCES
Académie nationale de médecine. (2022). Un risque réel de
pénurie de dispositifs médicaux. Bulletin de l’Académie
Nationale de Médecine, 206(8), 921‑922.
ANSM. (2022). Les investigations cliniques DM selon le
règlement 2017/745—Webinaire #5 (accessed 12.28.23).
https://www.youtube.com/watch?v=wOEsXkeJDPY
Brooks, D., & Stratford, P. (2009). Les études pilotes :
Déterminer si elles peuvent être publiées dans
Physiotherapy Canada. Physiother Can, 61(2), 67.
Chevallier, T., Bastide, L., & Potier, H. ICTROUVE.
(accessed 12.28.23). https://rc.chu-nimes.fr/surveys/?s=J
E39NHHC9L7WY8KW .
HAS. (2017). Parcours du dispositif médical en France.
(accessed 12.28.23). https://www.has-sante.fr/upload/
docs/application/pdf/2009-12/guide_pratique_dm.pdf
Medical Device Coordination Group (MDCG). (2021).
Regulation (EU) 2017/745 – Questions & Answers
regarding clinical investigation. (accessed 12.28.23).
https://health.ec.europa.eu/medical-devices-sector/new-
regulations/guidance-mdcg-endorsed-documents-and-
other-guidance_en
Medical devices must be carefully validated. (2018). Nature
Biomedical Engineering, 2(9), 625‑626.
Regulation UE 2017/745. (2017). Règlement 2017/ 745 du
parlement européen et du conseil relatif aux dispositifs
médicaux. (p. 175).
Sayin, H., Gaillard, C., Henry, A., Cabelguenne, D., &
Armoiry, X. (2022). Impact du nouveau règlement
européen 2017/745 relatif aux dispositifs médicaux sur
l’activité des pharmacies hospitalières : Exemple de la
fonction d’approvisionnement pharmaceutique au sein
d’un centre hospitalo-universitaire français. Annales
Pharmaceutiques Françaises, 80(5), 730‑737.
SNITEM. (2022). Panorama 2021 et analyse qualitative de
la filière industrielle des dispositifs médicaux en France.
(accessed 12.28.23). https://www.snitem.fr/wp-content/
uploads/2022/02/Snitem-Panorama-DM-2022.pdf
SNITEM. (2020). Nouveau panorama de la filière
industrielle des dispositifs médicaux en France.
(accessed 12.28.23) https://www.snitem.fr/presse/nouve
au-panorama-de-la-filiere-industrielle-des-dispositifs-
medicaux-en-france/.
Stratton, S. J. (2012). Publishing Survey Research. Prehosp
Disaster Med, 27(4), 305‑305.
Stratton, S. J. (2015). Assessing the Accuracy of Survey
Research. Prehosp Disaster Med, 30(3), 225‑226.
Use of a Digital Positioning and Categorisation Aid for Clinical Investigations on Medical Devices: Questioning the Complexity of the Field
and Harmonizing Stakeholders’ Understanding
871
