
A Skill based Educational Program for Future Regulatory Affairs
Professionals in the Medical Devices Industry: A Top down Approach
at Polytech Lyon University, France
Norbert Noury
1a
, Emmanuel Perrin
1b
and Claire Gaillard
2c
1
Université de Lyon, Polytech Engineer School, Villeurbanne, France
2
Université de Lyon, Université Claude Bernard Lyon I, INSA Lyon, CNRS, MATEIS, UMR5510, 69008 Lyon, France
Keywords: Medical Devices, Regulatory Affairs, Education.
Abstract: The Medical devices industry is facing a shortage in the professionals in Regulation Affairs who are in charge
with the regulation steps to bring their MD to the market. The Master ATRDM at Polytech Lyon 1 University,
France, was elaborated following a top-down approach based on the development of competencies of the
learners, with an agile iterative process to update the contents.
1 INTRODUCTION
The industry of Medical Devices (MD) is facing a
shortage in Regulatory Affairs (RA) professionals.
Medical Devices need professionals to fulfil each step
of the agreements, in order to obtain the CE Marking
and to deliver their MD in the market. The transition
from the directive on MDs (MDD 93/42) to the
regulation on MDs (MDR 2017/745) challenges
manufacturers because new requirements require an
added budget and human resources for manufacturers
and other actors in the medical device supply chain
(
Bayrak, 2022
). Therefore, the MD industry is seeking
for professionals in charge of RA for qualifying their
MD. The notified body are also seeking for specialists
to run the audits of MD devices organisations.
Although in the past, the persons in charge with
RA were partially the professionals in charge of the
Quality Affairs (QA), the RA of MD has strongly
specialised with the MDR and needs the professionals
to go through a specific training.
Furthermore, the person in charge with RA of MD
needs to be able to drive the project of bringing the
MD on the market, involving various actors from
Marketing, from RTD, from QA, etc. Therefore, the
MD-RA professional needs to develop transversal
skills in communication and project leadership.
a
https://orcid.org/0000-0002-0037-2256
b
https://orcid.org/0009-0002-4501-4863
c
https://orcid.org/0000-0002-0630-510X
In the global context of the impact of human
activity, the MD-RA professional must consider the
sustainability of the production of their MD and its
carbon impact.
Actually, there is a plethora of training companies
- universities and private operators - offering MD-RA
contents. At Polytech Lyon, the School of
Engineering of University Lyon 1, we have
developped a 'top-down' approach starting with from
the identification of the various skills needed by the
future MD-RA professionals.This approach is at the
heart of the Master Degree “Affaires Techniques et
Réglementaires du Dispositif Medical” (Regulatory
and Technical Affairs for MD) labeled ATRDM.
2 CONTEXT OF THE MEDICAL
DEVICES REGULATION
The MDR 2017/745 (MDR, 2017) comes with
stronger requirements and is more demanding that the
MDD 93-42-CEE (MDD, 1993).
First the MDR adds more rules to MD than the
MDD for considering the development of software,
nanomaterials, and combined MD at the frontier
between MD and Medicaments.
858
Noury, N., Perrin, E. and Gaillard, C.
A Skill based Educational Program for Future Regulatory Affairs Professionals in the Medical Devices Industry: A Top down Approach at Polytech Lyon University, France.
DOI: 10.5220/0012616100003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 858-863
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.

Second, the classification of MD, in Annex VIII
of the MDR, now includes 22 rules and 80 criteria
whereas Annex IX of the MDD contained only 18
rules and 56 criteria. It directly impacts the strategy
of classification of the MD. The MDs at higher risks
suffer stronger costly controls, but therefore are not
accessible to smaller, less competitive, industrial
structures.
Eventually, a Post Market Surveillance policy
must be settled in order to gather, correct and
timestamp each malfunction and non-conformities.
The MDR was not deployed until May 2021 and
should be definitively implemented in May 2024 - for
MD concerned by a notified body – and 2028 for most
MD. But the notified bodies are not numerous
enough, and they also lack skilled professionals to
face the deadline.
A recent survey by France's leading medical
device trade union, SNITEM (Syndicat National des
Industriels des Technologies Médicales), showed that
3 out 4 companies in MD are struggling to recruit
such regulatory executives.
There is also a huge need for educating the
competent professionals in most MD industries to
meet the new profile of “person in charge with
Regulatory affairs” set out in the MDR.
2.1 QA Versus RA?
Historically, the first professionals in charge with
MD-RA were integrated with the Quality
Management System (QMS). Therefore, they were
mostly located in the manufacturing departments. But
the QMS professional is a manufacturing generalist,
not specifically MD oriented.
Nowadays, the MD-RA manager must be
involved in each step of the life-cycle of the MD. And
because the Regulatory requirements are
continuously evolving, he must stay informed and be
dedicated to the MD-RA. Of course, in small
organisations (SMEs, start-ups), there is a strong
temptation for the “Swiss knife approach” with
professionals acting in various domains. Therefore,
MD-RA certified professionals are needed.
2.2 Executives Versus Managers?
Although the management of MD-RA may look like a
“filing-forms” tasks, actually the person in charge with
MD-RA has a wider field of action, interacting with all
the department of companies from research and
development to the marketing department. The MD-
RA professional impacts the strategy of the MD
company. He must have a global vision of the life cycle
of the MD, starting with the marketing, preclinic and
clinic studies, supplies, manufacturing, controls,
marking, packaging, logistic, reconditioning…
To bring a MD to the market is a project.
Therefore, the person in charge with MD-RA must be
a project leader, interacting with various stakeholders
from various departments in the company, e.g.
commercials, buyers, quality management and
control. The function of MD-RA can be seen in terms
of entrepreneurship as he is involved in creating
value. Nevertheless, there is still a need for MD-RA
officers assisting the MD-RA entrepreneurs. The later
can be internal officers, becoming operational after a
short training.
2.3 Cross-Functional vs Longitudinal
Approach to the MD Life Cycle
The MD specialist must have a global vision of the
life cycle of the MD. Starting with the knowledge of
the regulatory texts, the MD-RA professional must
also understand the various technologies involved in
the large domain of the MD (physics, electronics,
chemistry, materials, informatics) and their principle
of actions on the pathology, in order to be able to
perform the analysis and mitigation of the risks
associated with the use of the MD.
The MD professional must be aware of the
economic aspects of the MD market and the health
ecosystem economy, which obviously follows
specific rules.
He must know the manufacturing processes,
including the supply chains, and the QMS, in order to
interact smoothly with them professionals.
Some additional knowledges concern the MD
marking technics, the logistic for maintaining the
integrity of the MD and the sterilization if needed.
Also, the end of life of the MD with reconditioning.
On top of the knowledges, the person in charge
with MD-RA must behave as a project leader. He
needs to be familiar with management tools and
methods. He needs communication skills, in
particular oral presentation skills. He needs the skills
for piloting the project meetings. He also needs to
know basics of crisis management.
3 METHODOLOGY
3.1 Identification of the Skills for
Future RA Executives?
When designing a new curriculum – leading to a
A Skill based Educational Program for Future Regulatory Affairs Professionals in the Medical Devices Industry: A Top down Approach at
Polytech Lyon University, France
859

professional qualification - the architect of the
curriculum must start with considering the objective
of acquisitions in terms of skills and competencies
(
Makulova, 2015). Only then should he consider the
knowledges (contents) that learners will need to
acquire.
The need for training, anticipated in France in a
proposal from CSIS (Conseil stratégique des industries
de santé), was defined in 2020 by a national working
group led by the MESRI (Ministère de l’Enseignement
Supérieur, de la Recherche et de l’Inovation) with
numerous stakeholders (SNITEM, Conférence des
doyens de Pharmacie, Conférence des écoles
d'Ingénieurs en biomédical, EUROPHARMAT,
Tech4Health network, Inserm F-CRIN clinical
research infrastructure, Association des étudiants en
pharmacie, etc.), and has been endorsed by both the
French Ministries of Health and Industry.
From these recommendations, we propose the
following list of skills which we consider essential for
future RA executives in the MD industry.
Firstly, the learners should be able to understand
and mobilise a wide range of scientific and technical
knowledge in the field of MD, to identify and
translate regulatory requirements into product
requirements, compliance actions and marketing
strategies. To achieve this, they will need to be able
to keep abreast of scientific, technological, and
regulatory developments, in order to acquire and
apply new knowledge and know-how, including in
the field of medical practices.
They will need to collect and interpret data to
identify and solve problems. To do this, they will need
to know how to gather information, analyse a complex
situation and update the regulatory text database.
Because the accreditation process of a MD is a
project that involves a wide range of professionals,
they will need to know how to manage a team. To do
this, they will have to implement a project
methodology. They will have to manage risks,
uncertainties and regulatory constraints. In addition,
they will have to implement a continuous
improvement approach.
They must be able to communicate orally and in
writing in French and other languages - including
English - both face-to-face and remotely. They will
be required to practise interpersonal communication
adapted to each professional context, and to know
how to interact in a group.
3.2 How to Bring the Skills to Future
RA Executives?
It comes naturally with bringing the theory from
experts in the main domains of Medical Devices and
Regulatory Affairs. Then the learners will have to do
practical exercises on selected academic cases, under
the direction of experienced professionals. And on
top, the learners must alternate with a professional
practise in the RA department of a MD company
during 12 months of their education.
3.3 How to Evaluate the Acquisition of
the Skills?
The question of evaluation is central in the approach of
competences because educators need to deliver
degrees to the right person. It is of major importance
that the graduates be involved in their own evaluation
so as to feel involved in their education process, rather
than only lean back on their experienced teachers.
Formative evaluations must be provided so that
learners can practice in autonomy along their learning
paths to evaluate their own progression (e.g. MCQS -
Multiple Choice Questions and Solutions).
A useful method to evaluate the acquisition of
knowledge, is solving professional situations through
their students' projects. Their productions are usually
evaluated from a written report and oral defense in
front of their peers.
Eventually, individual terminal examinations
must be set to evaluate both the knowledge
acquisition (contents) and their capacity of synthesis.
At the end of their 12 months apprenticeship, a
final oral defense in front of professionals of the
domain of MD-RA, is compulsory to evaluate their
implication and their ability to communicate
efficiently. It is useful that their peers will be invited
to listen to other’s presentations to learn by example,
and also to develop their own skills through the
evaluation of each others’ production.
3.4 How to Maintain a Sustainable
Employability?
A main pillar of professional education at University,
is to prepare the future graduates with a solid
background of knowledge, to be prepared to face the
constant technical evolutions of their professional
domain. Also, to bring to the future graduate the soft
skills which will guarantee him with flexibility and
adaptation to the evolutions of his professional
activities.
Actually, the engineering pedagogy for
professional domains is facing 2 contradictory
challenges:
• To produce professionals in order to face
immediate needs in industry
ClinMed 2024 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
860

• guaranteeing the future of graduates in a rapidly
evolving professional world
The Methodical approach that we put in practice
consists in:
• Bring a wider range of knowledge (than needed
for graduation)
• Bring information on the latest technologies
developed in research labs, by researchers who
show the orientations to follow.
• Take a step back on the needs
• Help the learners to draw perspectives on their
own skills
• familiarize the learners with the concepts of Soft-
skills vs Hard-skills
3.5 Recruit the Right Learners?
Obviously, the learners shall be recruited from their
academic skills (biography, academic results) and
their motivations (written letter, personal professional
project).
Furthermore, after a selection of the best profiles
from their written elements, it is essential to conduct
individual interviews to address the non-technical
skills of future learners.
In the particular field of MD-RA, a key point is to
mix the backgrounds of learners (engineers,
pharmacists and biologists). Also, the diversity of
profiles gives usually some good results when
involving both young learners and retraining
professionals.
The building of a team of learners is of utmost
importance from the beginning. Each member
playing a role in his own education but also acting on
the learning paths of their peers. The students projects
are good opportunities to challenge the students.
To strengthen the team spirit (team building),
social events must be organized, such as after-work
parties and visits to industrial establishments. The
alumni network is also a structuring tool. Newcomers
should be encouraged to join the network quickly, so
that they can experience what it's like to belong.
4 THE MASTER ATRDM AT
POLYTECH LYON SCHOOL OF
ENGINEERING
4.1 The Polytech Lyon School of
Engineering
Polytech is a network of 16 public schools of
engineering from 16 French universities. It graduates
annually 4,200 students and already has 90,000 active
graduates. The Polytech Network covers 12
engineering domains (energy, electronics, electrical,
applied mathematics, Information Technologies,
biology, Biomedical, Civil, material, environment,
industry) with 100 specialties (6 specialties at
Polytech Lyon: Biomedical, MD-RA, IT, Materials,
Mathematics, Mechanics, Robotics).
Polytech Lyon is the School of Engineering of
Claude Bernard Lyon 1 University and member of the
Polytech Network. Polytech Lyon, as a full part
faculty of Lyon 1 university, also proposes Master
Degree as a specialization of its Engineer Diploma.
The Polytech Lyon Biomedical Engineering
Department graduates Engineers and Master (Perrin,
2007). Considering the wide spectrum of MD, the
need for MD-RA is to train Engineers, Scientists and
Pharmacist, and this is naturally achieved with a
strong collaboration with Lyon 1 Faculty of
Pharmacy (ISPB).
4.2 A Time-Based Organisation
The Master ATRDM (Noury, 2022) covers a 12-
months training period alternating between a
professional activity in a private company and the
academic training in university. The later consists in
300 hours of face-to-face education and 150 hours of
projects. The projects playing an important role in
skills acquisition and evaluation. During the Fall
Semester (September to February), the rhythm of
alternation is 3 days of training each week and 2 days
in the company. After the academic evaluations in
February, the students start the Spring semester which
consists in a full-time professional education in their
companies. In this last period, only 3 days each month
are dedicated to projects of student in groups in the
University.
4.3 An Elaborated Curriculum
The curriculum is based on a core education on the
MD-RA and side trainings.
The core education on MD-RA (140h) is
composed of 3 parts:
• The generalities of the ecosystem: industrialists,
organizations, stakeholders, notified bodies,
understanding requirements,
• Regulatory Aspects: MD classification,
European standards (MDR 2017/745,
93/42/CEE), Risk Analysis, clinical evaluations,
UDI, PMS…
• Technical aspects of various MD: materials, active
electronics, implantable, software, combined
A Skill based Educational Program for Future Regulatory Affairs Professionals in the Medical Devices Industry: A Top down Approach at
Polytech Lyon University, France
861
The side (corollary) trainings (160h) covers
complementary aspects:
• Project management
• Quality management (ISO 13485)
• Legal aspects: Contract laws, Competition law,
Best practices, Sunshine Act
• Security aspects: clinical evaluations, materio-
vigilance, radio-vigilance, reacto-vigilance,
traceability, PSUR, Risk management (ISO
14971), electro-devices (IEC60601-2-62),
software (IEC62304), GDPR, Biocompatibility
(ISO 10993)
• Economy of MD: the French Health system,
Hospital Purchasing, pricing of MD (Market
price), reimbursement, Market Access
• Data strategy: Stake and approach of the
company's strategic information management,
patents, lobbying,
• MD Regulatory in other countries (US, China, ,
Canada, Brazil, Japan, South Korea, Australia,
…)
• And of course, Professional English practice.
4.4 Evaluation of the Skills
Acquisitions
Following the methodology introduced in section 3.3,
the evaluations are done through formative
evaluations, individual examinations and
professional situations during the students’ projects.
The first “individual project” focuses on the study
of the Technical and Regulatory aspects of a selected
Medical Device. The learners must demonstrate they
were able to gather relevant scientific and technical
information to explain how the MD operates and what
is its “principle of action” on the patient, with the
targeted population, and to produce an analysis of the
risks associated with the use of the MD. Then they
must collect the complete book of regulations
associated with the lifecycle of their MD, including
the classification strategy.
The second one is a “Group project”, namely
“Bring an innovative MD on the Market”. On top of
the technical aspects of the technical file, the group of
students must address the European Regulatory
Aspects and the regulations in one or several other
countries (USA, Middle East, Asia, Russia,
Africa…). This project also includes
“entrepreneurial” aspects such as a market analysis.
In addition, the group is evaluated specifically on the
elaboration of his project management strategy, and
his QMS plan.
4.5 Master ATRDM in a Few Figures
The Curriculum was first elaborated in
collaboration with SNITEM, which is the main union
of MD industrialists in France (snitem, 2023). The
first opening was in September 2013. The average
capacity is 24 students each year, in order to maintain
constructive interactions between the classmates and
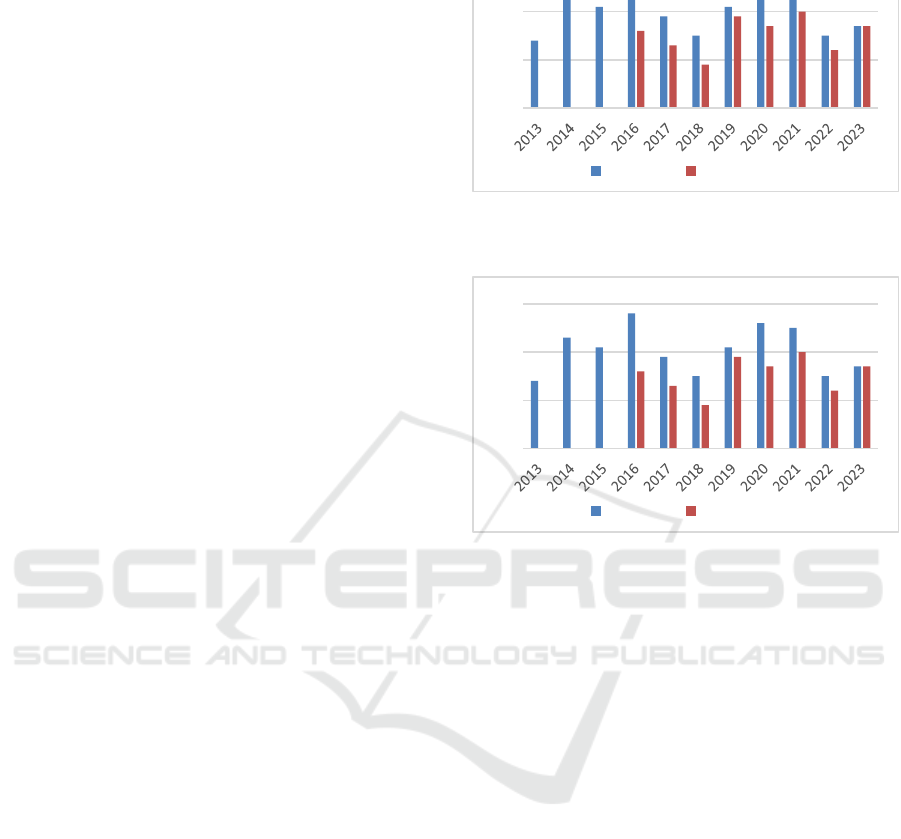
their teachers. Since 2013, over 200 learners were
graduated (
Figure) and their majority are now active in the RA
departments of the MD industry in France.
Figure 1: Demography of learners in Master ATRDM.
5 CONCLUSIONS
The elaboration of the academic curriculum of Master
ATRDM at Polytech Lyon, is following an Agile
development process with annual iterations (e.g
MDDMDR). This is compulsory because the MD-
RA evolve frequently and also because the missions
of MD-RA managers are constantly changing.
It was based on an important Partnership with
professionals from the industry of MD and with
SNITEM, the main union of the French MD industry.
Networking between students is strongly
encouraged through projects and with their individual
adherence to our private group on Linkedin (Alumni,
2016).
Each student is closely monitored by 2 tutors,
including a company tutor and an academic tutor
from the university. They correspond with each other
via an electronic website and during compulsory
visits in situ.
We participated in 2013 to launching the French
national network of Masters in Regulatory Affairs,
Quality and Clinical evaluations of MD (ARClimed
project) under the auspices of the AMI-CMA, a
French national initiative to foster the development of
0
10
20
Total Apprentices
0
10
20
30
Total Apprentices
ClinMed 2024 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
862

new training programs for new occupations in the
digital industry. We can now envision a possible
European deployment of our Master in MD-RA.
ACKNOWLEDGEMENTS
The authors would like to thank the education team
for their dedicated involvement in the development of
the ATRDM Master's programme, in particular Ing.
Alexia GARIN, Manager RA-DM, Ing. Benjamin
ROCHETTE, VP RA-DM and Professor Xavier
ARMOIRY.
REFERENCES
Bayrak T., Yilmaz S.E., What Will Be the Economic
Impact of the New Medical Device Regulation? An
Interrupted Time-Series Analysis of Foreign Trade
Data, Value Health Reg Issues. 2022; 29:1–7
MDD: https://www.legislation.gov.uk/eudr/1993/42/
MDR: Regulation (EU) 2017/745 of the European
Parliament and of the Council of 5 April 2017 on
medical devices, amending Directive 2001/83/EC,
Regulation (EC) No 178/2002 and Regulation (EC) No
1223/2009 and repealing Council Directives
90/385/EEC and 93/42/EEC
Perrin E. et al., "Biomedical engineering degrees at Lyon 1
University," 2007, 29th Annual International
Conference of the IEEE Engineering in Medicine and
Biology Society, Lyon, France, 2007, pp. 5932-5935,
doi: 10.1109/IEMBS.2007.4353698
Noury N., “Developing an educational program for future
Regulatory Affairs professionals in the Medical
Devices industry”., European Medtech 2022, March
2022, Brussels.
Makulova, AT, Theory and Practice of Competency-Based
Approach in Education, International Education
Studies, 2015, vol 8(8) p183-192
Snitem:, https://www.snitem.fr/
Alumni: https://www.linkedin.com/groups/8979939/.
A Skill based Educational Program for Future Regulatory Affairs Professionals in the Medical Devices Industry: A Top down Approach at
Polytech Lyon University, France
863
