
Clinical Evaluation of Collaborative Artificial Intelligence Systems:
Lessons from the Case of Robot-Assisted Surgery
Alexandre Coste
1a
, Frédéric Barbot
2b
and Thierry Chevalier
3,4,5 c
1
EuroMov Digital Health in Motion, Univ. Montpellier, IMT Mines Ales, Montpellier, France
2
INSERM CIC 1429, Raymond Poincaré Hospital APHP, France
3
CHU Nîmes, Department of Biostatistics, Epidemiology, Public Health and Innovation in Methodology,
30029 Nîmes, France
4
Univ. Montpellier, INSERM, UMR 1302, Institute Desbrest of Epidemiology and Public Health, Montpellier, France
5
Tech4Health-FCRIN, France
Keywords: Clinical Evaluation, Robotic Surgery, Human-Machine Collaboration, Artificial Intelligence, Medical
Devices.
Abstract: Collaborative AI systems, which combine both forms of intelligence (i.e., human and machine), are attracting
increasing interest from the scientific and medical communities, with various applications in radiology
(clinical decision support systems) and surgery (robot-assisted surgery). However, despite their promise, these
systems face significant challenges in integrating into clinical practice due to a lack of transparency, trust, and
clinical validation. Drawing on the case of robotic surgery, the aim of this work was to analyse the scientific
evidence for ten surgical robots currently on the market (i.e., CE-marked or FDA-cleared/approved) that meet
the definition of a collaborative AI system. We found a low number of peer-reviewed publications and a lack
of transparency from authors and manufacturers, particularly regarding the functioning of their devices, which
are often considered as ‘black boxes’. Furthermore, the term ‘artificial intelligence’ is under-utilised in
scientific publications, regulatory submissions, and commercial materials. Based on these findings, we
propose three recommendations to promote the integration of these medical devices: 1) promote the
transparency, explainability, and comprehensibility of AI devices by encouraging manufacturers to provide
more detailed information about their systems and their functioning, including the interrelationship with the
user; 2) promote randomised controlled multicentre trials to provide stronger evidence on the performance
and safety of these devices; 3) encourage the publication of scientific results in peer-reviewed journals to
expose them to scientific scrutiny and improve transparency. These recommendations have been carefully
formulated to cover a wide range of AI/ML-enabled medical devices, beyond the case of surgical robots
reviewed here.
1 INTRODUCTION
Artificial intelligence (AI) is expanding rapidly,
particularly in the healthcare sector. Technological
advances, particularly in computer science, have led
to increasingly powerful AI systems, but
paradoxically only a limited number of these systems
have been integrated into clinical practice, a
phenomenon known as the ‘AI chasm’ (e.g.,
Aristidou et al. 2022, Reyna et al. 2022). Key limiting
a
https://orcid.org/0000-0002-4497-6473
b
https://orcid.org/0000-0002-4648-9134
c
https://orcid.org/0000-0002-5110-6273
factors include a lack of transparency, trust,
interpretability, adaptability and scientific evidence.
In particular, many concerns have been raised in
recent years about the fact that certain AI systems
have been tested and validated using retrospective, in
silico data, which does not reflect real-world clinical
practice. Moreover, few studies have taken into
account the specificities of so-called ‘collaborative’
AI systems. These systems, which are based on the
close collaboration between two forms of
intelligence, human and artificial, (Vasey et al. 2022),
852
Coste, A., Barbot, F. and Chevalier, T.
Clinical Evaluation of Collaborative Artificial Intelligence Systems: Lessons from the Case of Robot-Assisted Surgery.
DOI: 10.5220/0012598500003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 852-857
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.

present significant methodological challenges due to
their inherent complexity. This complexity arises
primarily from the ongoing interplay between human-
related factors, such as the learning curve, the level of
expertise or the physical and mental fitness of the
operators, and AI-model factors, including
algorithmic specificities, the evolutionary nature for
continuous learning models, and the quality of the
learning data that shapes the model and its
performance.
The surgical field is undoubtedly one of the most
representative areas for collaborative AI systems
(Mayor et al. 2022), where the integration of human
expertise with AI capabilities shows remarkable
potential for advancing surgical practices. In
particular, the proposed benefits include: i) enhancing
the surgeon's perceptual abilities through three-
dimensional imaging; ii) improving the precision of
surgical gestures, particularly in minimally invasive
procedures, by filtering out tremors and reducing
differences associated with laterality preferences.
While autonomous surgery was the main
motivation for the pioneers (e.g., the PROBOT for
prostate resection), it is the robots that assist the
surgeon (i.e., teleoperated or co-manipulated), not
intended to replace him, that have become
widespread over the last twenty years. There are now
hundreds of surgical robots (on the market or under
development), covering various medical indications,
from general surgery, to gynaecology, orthopaedics
and even cardiac surgery. The Da Vinci surgical
system, developed by Intuitive Surgical, currently
dominates the market with more than 6,000 units sold
worldwide and more than 7 million procedures
carried out with the robot (figures given by the
manufacturer on its website
https://www.intuitive.com/). However, little is known
about the clinical evaluation of these medical devices
required for both European (Medical Device
Regulation, MDR) and American (FDA) compliance.
In this context, the objective of the present research is
to provide an overview of commercially available
collaborative AI systems in robotic surgery and to
review the associated scientific evidence.
2 METHODS
Following a similar methodology to Wu et al. (2021),
Benjamens et al. (2020), and van Leeuwen et al.
(2021), we identified ten robotic surgical systems
currently available in the market (i.e., compliant with
European regulations or FDA approved/cleared, see
Figure 1).
2.1 Search Strategy and Selection
Criteria
The surgical robots selection process was carried out
in two phases. First, we used the following
resources/databases:
i) FDA's database:"AI/ML-Enabled Medical
Devices," listing FDA approved AI/ML-
based medical devices.
ii) The recent review by Muehlematter,
Daniore & Vokinger (2021) listing 462 AI-
based devices approved in Europe and the
U.S. from 2015 to 2020.
iii) The new European Medical Devices
Database (Eudamed).
iv) The list of communications for Class IIa, IIb,
and III medical devices and implantable
medical devices from the ANSM (French
National Agency for Medicines and Health
Products Safety), covering devices in the
market from 2010 to 01/12/2021 (n =
83129).
v) PubMed
®
and Google Scholar
®
databases.
To ensure relevant results, precise keywords were
identified using the bilingual version of the INSERM
(French National Institute of Health and Medical
Research) MeSH (Medical Subject Headings)
lexicon. Keywords included terms related to robotic
surgery, artificial intelligence and machine learning.
A search of these keywords against those in the
above databases identified approximately one
hundred potential devices. A detailed analysis
involving cross-referencing with various sources,
including manufacturers' websites and commercial
documentation, led to the selection of devices that
met the following criteria:
vi) Surgical robots commercially available in
the European or American markets (i.e., EU-
MDR or FDA compliant).
vii) Collaborative surgical robots involving
human-machine interaction (i.e., co-
manipulated or teleoperated).
viii) Surgical robots incorporating AI, machine
learning, or deep learning processes.
2.2 Analysis of Scientific Evidence and
Clinical Evaluation Methodology
The level of scientific evidence and clinical
evaluation methodology for the ten selected devices
were examined using two methods. Firstly, a
systematic search of PubMed, Google Scholar and
Clinical Evaluation of Collaborative Artificial Intelligence Systems: Lessons from the Case of Robot-Assisted Surgery
853
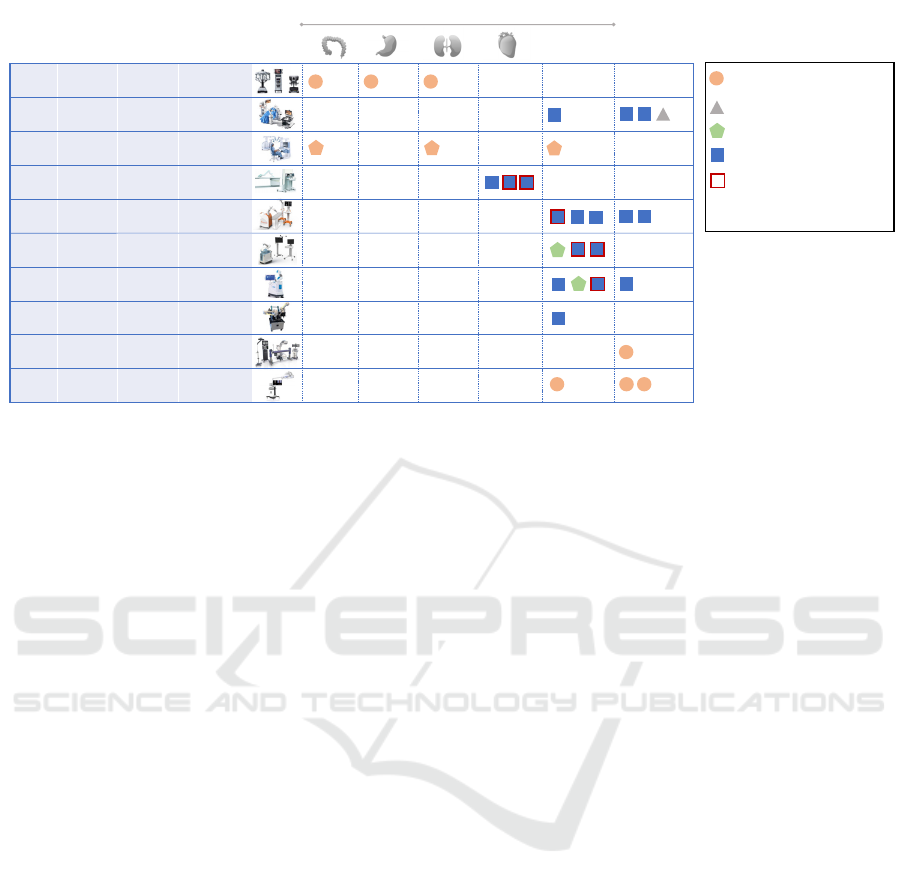
Figure 1: Overview of the ten collaborative surgical robots integrating AI/ML processes marketed in the U.S. and/or Europe.
IEEE Xplore was performed using the trade name
and/or the manufacturer name of each robot. This
allowed us to extract peer-reviewed articles from
1988 to April 2023. The ClinicalTrials.gov registry
and the medRxiv biomedical research preprint
platform were also consulted to avoid potential
publication bias and to obtain a comprehensive view
of ongoing research.
Secondly, the FDA and European Commission
(Eudamed) databases were consulted to access
detailed information on devices, including preclinical
and clinical data submitted by manufacturers to
regulatory authorities for the conformity assessment.
3 RESULTS
Figure 1 presents the ten collaborative surgical robots
selected for the analysis, categorized according to
their trade name, their manufacturer, their type
(teleoperated or co-manipulated), the associated
scientific publications, and the type of validation
studies.
3.1 Collaborative Surgical Robots: A
Highly Heterogeneous Landscape
Among the ten collaborative surgical robots, four are
teleoperated (Da Vinci Xi
®
, Versius
®
, Senhance
®
, R-
One+
TM
), and six are co-manipulated (Epione
®
,
Mako
®
, Rosa Knee System
®
, Maestro
®
, Pulse
System
TM
, 7D Surgical System
®
). All teleoperated
robots belong to the same risk class, i.e., Class IIb for
EU-MDR compliance (4/4) and Class II for FDA
compliance when obtained (2/4: Da Vinci Xi
®
and
Senhance
®
). In contrast, the risk class for co-
manipulated devices is more heterogeneous, ranging
from Class I to Class IIb for EU-MDR compliance
and from Class I to II for FDA compliance. This
diversity is partly explained by the variety of
technologies used and the range of covered
indications, including orthopaedic, cardiac, spinal,
and general laparoscopic surgery. Notably, the
Maestro
®
robot stands out by being classified in the
lowest risk class (Class I), contrary to the general
trend where active devices are typically classified at
least in Class IIa according to the MDR. Also, it is
important to note that all the analysed surgical robots
have obtained U.S. compliance through the 510(k)
procedure, a simplified procedure highly coveted by
Da
Vinci
Xi
®
Intuitive
Surgical
Teleoperated
> 22000 publications
including
[1]
and
[2]
using AI processes
Ver sius
®
CMR
Surgical
Teleoperated
[3],
[4],
[5],
[6]
Senha nce
®
Asensus Surgical
Teleoperated
[7], [8], [9]
R-One+™
Robocath
Teleoperated
Ø
3
studies
referenced
on
ClinicalTrials.gov
Epione
®
Quantum Surgical
Co-manipulated
[10],
[11],
[12]
+
2
studies
referenced on
ClinicalTrials.gov
Mak o
®
Stryker
Co-manipulated
[13],
[14],
[15]
Rosa
®
Knee
Sys tem
Zimmer Biomet
Co-manipulated
[16], [17], [18]
Mae s tr o
®
Moon Surgical
Co-manipulated
Ø
1
clinical
investigation
referenced on the
Eudamed
portal
Pulse
TM
Sys tem
NuVasive
Co-manipulated
[19]
7D
Sur gical
Sys tem
7D
Surgical
Inc.
Co-manipulated
17
publications
including
[20] and [21]
1
1
1
Otherorgans/
Multiplesites
1
Insilico/
cadaveric
model
Invivo
0 0
0
31
4
1
1
1
0
1
0
2a
4
1
3
2b
3
01
4
1
0
0 0
2b
Prospectivestudytovalidateasystem
basedonAIprocesses
Multicentricstudy
Othertypeofstudy
0-4
ValidationstageaccordingtotheIDEAL
recommandations
Usabilitystudy
Retrospectivestudy
[1] Cheng, Q., & Dong, Y. (2022). Da Vinci Robot-Assisted Video Image Processing under Artificial Intelligence Vision Processing Technology. Computational and Mathematical Methods in Medicine.
[2] Azad, R. I., Mukhopadhyay, S., & Asadnia, M. (2021). Using explainable deep learning in da Vinci Xi robot for tumor detection. International Journal on Smart
Sensing and Intelligent Systems, 14(1), 1-16.
[3] Kelkar, D. S., Kurlekar, U., Stevens, L., Wagholikar, G. D., & Slack, M. (2023). An early prospective clinical study to evaluate the safety and performance of the versius surgical system in robot-assisted cholecystectomy. Annals of Surgery, 277(1), 9.
[4] Kayser, M., Krebs, T. F., Alkatout, I.,
Kayser, T., Reischig, K., Baastrup, J., ... & Bergholz, R. (2022). Evaluation of the Versius robotic surgical system for procedures in small cavities. Children, 9(2), 199.
[5] Haig, F., Medeiros, A. C. B., Chitty, K., & Slack, M. (2020). Usability assessment of Versius, a new robot-ass isted surgical device for use in minimal access
surgery. BMJ Surgery, Interventions, & Health Technologies, 2(1).
[6] Morton, J., Hardwick, R. H., Tilney, H. S., Gudgeon, A. M., Jah, A., Stevens, L., ... & Slack, M. (2021). Preclinical evaluation of the versius surgical system, a new robot-assisted surgical device for use in minimal access general and colorectal procedures. Surgical
endoscopy, 35,
2169-2177.
[7] Sasaki, T., Tomohisa, F., Nishimura, M., Arifuku, H., Ono, T., Noda, A., & Otsubo, T. (2023). Initial 30 cholecystectomy procedures performed with the Senhance digital laparoscopy system. Asian Journal of Endoscopic Surgery, 16(2), 225-232.
[8] Sasaki, M., Hirano, Y., Yonezawa, H., Shimamura, S., Kataoka, A., Fujii, T., ...
& Koyama, I. (2022). Short-term results of robot-assisted colorectal cancer surgery using Senhance Digital Laparoscopy System. Asian Journal of Endoscopic Surgery, 15(3), 613-618.
[9] Holzer, J., Beyer, P., Schilcher, F., Poth, C., Stephan, D., von Schnakenburg, C., ... & Staib, L. (2022). First Pediatri c Pyeloplasty Using the Senhance® Robotic System—A Case Report. Children,
9(3), 302.
[10] de Baère, T., Roux, C., Noel, G., Delpla, A., Deschamps, F., Varin, E., & Tselikas, L. (2022). Robotic assistance for percutaneous needle insertion in the kidney: preclinical proof on a swine animal model. European Radiology Experimental, 6(1), 13.
[11] de Baère, T., Roux, C., Deschamps, F., Tselikas, L.,
& Guiu, B. (2022). Evaluation of a New CT-Guided Robotic System for Percutaneous Needle Insertion for Thermal Ablation of Liver Tumors: A Prospective Pilot Study. Cardiovascular and Interventional Radiology, 45(11),
1701-1709.
[12] Gunderman, A. L., Musa, M., Gunderman, B. O., Banovac, F., Cleary, K., Yang, X., & Chen, Y. (2023). Autonomous
Respiratory Motion Compensated Robot for CT-Guided Abdominal Radiofrequency Ablations. IEEE Transactions on Medical Robotics and Bionics.
[13] Sires, J. D., Craik, J. D., & Wilson, C. J. (2021). Accuracy of bone resection in MAKO total knee robotic-assisted surgery. The journal of knee surgery, 34(07), 745-748.
[14] Young, S. W., Zeng, N.,
Tay, M. L., Fulker, D., Esposito, C., Carter, M., ... & Walker, M. (2022). A prospective randomised controlled trial of mechanical axis with soft tissue release balancing vs functional alignment with bony resection balancing in total knee replacement—a
study using Stryker Mako robotic arm-assi sted technology. Trials, 23(1), 1-10.
[15] Ando, W., Takao, M.,
Hamada, H., Uemura, K., & Sugano, N. (2021). Comparison of the accuracy of the cup position and orientation in total hip arthroplasty for osteoarthritis secondary to developmental dysplasia of the hip between the Mako robotic arm-assisted system and
computed tomography-based navigation. International orthopaedics, 45, 1719-1725.
[16] Vanlommel, L., Neven, E., Anderson, M. B.,
Bruckers, L., & Truijen, J. (2021). The initial learning curve for the ROSA® Knee System can be achieved in 6-11 cases for operative time and has similar 90-day complication rates with improved implant alignment compared to
manual instrumentation in total knee arthroplasty. Journal of Experimental Orthopaedics, 8, 1-12.
[17] Parratte, S., Price, A.
J., Jeys, L. M., Jackson, W. F., & Clarke, H. D. (2019). Accuracy of a new robotically assisted technique for total knee arthroplasty: a cadaveric study. The Journal of arthroplasty, 34(11), 2799-2803.
[18] Anderson, M. B. ROSA
®
Knee System 2022 Clinical Evidence Summary.
[19] Beisemann, N., Gierse, J., Mandelka, E., Hassel, F., Grützner, P. A., Franke, J., & Vetter, S. Y. (2022). Comparison of three imaging and navigation systems regarding accuracy of pedicle screw placement in a sawbone model. Scientific Reports, 12(1), 12344.
[20] Guha, D., Jakubovic, R., Gupta, S.,
Fehlings, M. G., Mainprize, T. G., Yee, A., & Yang, V. X. (2019). Intraoperative error propagation in 3-dimensional spinal navigation from nonsegmental registration: a prospective cadaveric and clinical study. Global Spine Journal, 9(5), 512-520.
[21] Peh, S., Chatterjea, A., Pfarr, J., Schäfer, J. P., Weuster, M., Klüter, T., ... &
Lippross, S. (2020). Accuracy of augmented reality surgical navigation for minimally invasive pedicle screw insertion in the thoracic and lumbar spine with a new tracking device. The Spine Journal, 20(4),
629-637.
Device Trade
Name
Manufacturer Type of robot
Associated
scientific
publications
Illustration
ClinMed 2024 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
854

manufacturers. Indeed, the manufacturers must only
demonstrate that their device is as safe and effective,
i.e., substantially equivalent, to a legally marketed
device.
3.2 A Significant Lack of Transparency
Surprisingly, 70% of robot manufacturers do not
explicitly mention the use of artificial intelligence or
machine learning processes. Some manufacturers,
such as Asensus Surgical
®
, use instead terms like
‘augmented intelligence’ without explicit mention of
AI in regulatory documents. Nuvasive
®
is one of the
few manufacturers explicitly using the term ‘artificial
intelligence’ on its website, but the term does not
appear in any compliance submission documents. AI
or not AI: it seems that talking about artificial
intelligence can be beneficial in certain cases, less so
in others, particularly with regulatory authorities.
3.3 Lack of Scientific Evidence
A detailed examination of publications associated
with the devices reveals varied levels of scientific
evidence. While some devices have limited or no
peer-reviewed articles, others, like the Da Vinci
®
,
have extensive literature due to their longer market
presence. Importantly, the number of studies
specifically dedicated to evaluating AI algorithm
performance and safety is extremely limited, even for
the well-established robot like Da Vinci
®
. Moreover,
most of these studies focus on preclinical stages or
involve a very small number of patients. Only 20% of
the analysed studies (6 out of 30) are multicentric,
emphasizing the need for more comprehensive
research.
4 DISCUSSION
The aim of this work was to delineate the contours
and inherent challenges in the clinical validation of
collaborative AI systems, particularly those involving
close collaboration between human and artificial
intelligence, with a particular focus on robotic
surgery. As mentioned above, these systems present
new methodological challenges in clinical evaluation
due to the inherent variability of individual factors
and those related to the AI system itself. The
introduction of an AI component adds a new
dimension and complexity to the existing challenges
of validating technological innovation in surgery.
Previously, it was known that different levels of
expertise could lead to different levels of
performance, creating a performance bias in favour of
established technologies (Rudicel & Esdaile, 1985).
Now, the performance of collaborative AI systems
can vary between different user profiles.
Furthermore, the capabilities of continuously learning
systems can evolve over time, either positively or
negatively.
4.1 Current Regulatory Framework
and Issues
The current regulatory framework, both in Europe
with the MDR and in the U.S., does not adequately
address the specificities of AI systems, especially
collaborative AI systems. Formulated at a time when
devices had limited interactivity and infrequent
updates, the existing framework struggles to
accommodate the evolving and interactive nature of
new technologies, particularly those incorporating AI
(Gilbert et al., 2023). However, this is changing, with
the imminent arrival of the first regulation on
artificial intelligence (i.e., ‘EU AI Act’) and the
FDA's draft guidance ‘Marketing Submission
Recommendations for a Predetermined Change
Control Plan for Artificial Intelligence/Machine
Learning (AI/ML) - Enabled Device Software
Functions / Draft Guidance for Industry and Food and
Drug Administration Staff’. The latter specifically
aims to address the evolving nature of these new
devices, which are capable of real-time or near real-
time learning. These regulatory advances are
welcome, particularly in light of the observations
made in this work.
4.2 Promoting Transparency,
Explainability, and Intelligibility of
Devices
A key finding of this study is that leading surgical
robots on the market currently lack sufficient detailed
information about the technologies used, despite
recommendations from the World Health
Organization in its core ethical principle number 3
(i.e., ensure transparency, explainability, and
intelligibility) and the ISO/IEC TR 24028:2020.
While recognising the highly competitive nature of
the robotic surgery market, characterised by a
constant drive for innovation and the protection of
intellectual property, it remains crucial to ensure a
minimum level of transparency, particularly in the
context of AI. Transparency goes beyond regulatory
compliance and is a key factor in building trust among
both practitioners and patients. The development of
Eudamed represents a real opportunity for greater
Clinical Evaluation of Collaborative Artificial Intelligence Systems: Lessons from the Case of Robot-Assisted Surgery
855

transparency on the part of manufacturers, as
envisaged by the European Commission in the
creation of this unique database, which will provide
public access to certain information on marketed
medical devices. in Europe (device identification,
reported incidents, ongoing clinical investiga-
tions, ...). However, it is regrettable that the Eudamed
database is not yet fully operational and is not as
comprehensive as the FDA databases. It is also
regrettable that the Summary of Safety and Clinical
Performance (SSCP), required by the Art. 32 of the
MDR, is limited to implantable devices and class III
devices. As we have observed, most surgical robots
fall into the IIa and IIb categories and are therefore
not directly subject to this obligation.
4.3 Promoting Randomized Controlled
Multicentre Studies and Scientific
Publications
Another important point is the lack of robust evidence
from rigorous clinical trials. Indeed, most of the
reported trials were monocentric and observational,
which can lead to significant methodological biases.
In particular, monocentric studies may produce
results that are not generalisable to geographically
diverse patient populations with different economic,
educational, social, behavioural, ethnic and cultural
characteristics (Kaushal et al, 2020). In addition,
randomised controlled trials (RCTs) are considered
the gold standard in clinical trials as they provide the
highest level of scientific evidence. In this regard,
authors/manufacturers can use various published
guidelines such as SPIRIT-AI (Rivera et al., 2020),
DECIDE-AI (Vasey et al., 2022), STARD-AI
(Sounderajah et al., 2021), TRIPOD-AI and
PROBAST-AI (Collins et al., 2021) to better develop
their research protocols and write their scientific
papers.
4.4 Limitations and Future
Perspectives
Naturally, this research has some limitations. Firstly,
it focuses exclusively on surgical robots, which limits
its representativeness in terms of the diversity of AI
solutions available on the market. However, these
surgical robots illustrate well the concept of
collaborative AI systems, and the recommendations
formulated herein are intended to be transversal and
applicable to a wider range of medical devices,
including autonomous or non-surgical devices.
Secondly, it is important to note that our analysis
exclusively concentrated on robots that are already on
the market (i.e., having obtained EU or US
conformity), specifically in the context of their
clinical validation This approach excludes the pre-
approval phases, including the development of the
idea into a product. Consequently, there might exist
additional barriers not identified within this study.
For a more comprehensive insight, future research
could expand its purview by examining a wider range
of medical devices, including aspects associated with
the development of medical devices. Furthermore, it
would be interesting to consider an extension of the
IDEAL protocol (i.e., IDEAL-AI, see McCulloch et
al. 2009) to include specificities related to the
validation of surgical technology innovations based
on AI/ML processes, such as the collaborative
surgical robots studied here.
5 CONCLUSIONS
In this work, we have identified several barriers to the
implementation of collaborative AI systems in
clinical practice, in particular the lack of transparency
and scientific publications. We have therefore formu-
lated a set of recommendations aimed at promoting the
integration of AI systems into clinical practice,
namely: i) promoting transparency, explainability and
intelligibility of AI devices, ii) promoting the conduct
of randomised controlled multicentre trials, and iii)
encouraging the publication of study results in peer-
reviewed journals. These recommendations have been
formulated to be as transversal as possible and
applicable to a wide range of AI/ML-enabled medical
devices, not just surgical robots.
ACKNOWLEDGEMENTS
AC thanks the Tech4Health network
(https://www.reseau-tech4health.fr) for its financial
support.
REFERENCES
Aristidou, A., Jena, R., & Topol, E. J. (2022). Bridging the
chasm between AI and clinical implementation. The
Lancet, 399(10325), 620.
Benjamens, S., Dhunnoo, P., & Meskó, B. (2020). The state
of artificial intelligence-based FDA-approved medical
devices and algorithms: an online database. NPJ digital
medicine, 3(1), 118.
Collins, G. S., Dhiman, P., Navarro, C. L. A., Ma, J., Hooft,
L., Reitsma, J. B., ... & Moons, K. G. (2021). Protocol
ClinMed 2024 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
856

for development of a reporting guideline (TRIPOD-AI)
and risk of bias tool (PROBAST-AI) for diagnostic and
prognostic prediction model studies based on artificial
intelligence. BMJ open, 11(7), e048008.
Gilbert, S., Fenech, M., Hirsch, M., Upadhyay, S.,
Biasiucci, A., & Starlinger, J. (2021). Algorithm change
protocols in the regulation of adaptive machine
learning–based medical devices. Journal of Medical
Internet Research, 23(10), e30545
Kaushal, A., Altman, R., & Langlotz, C. (2020).
Geographic distribution of US cohorts used to train
deep learning algorithms. Jama, 324(12), 1212-1213.
Mayor, N., Coppola, A. S., & Challacombe, B. (2022). Past,
present and future of surgical robotics. Trends in
Urology & Men's Health, 13(1), 7-10.
McCulloch, P., Altman, D. G., Campbell, W. B., Flum, D.
R., Glasziou, P., Marshall, J. C., & Nicholl, J. (2009).
No surgical innovation without evaluation: the IDEAL
recommendations. The Lancet,
374(9695), 1105-1112.Muehlematter, U. J., Daniore, P., &
Vokinger, K. N. (2021). Approval of artificial
intelligence and machine learning-based medical
devices in the USA and Europe (2015–20): a
comparative analysis. The Lancet Digital Health, 3(3),
e195-e203.
Reyna, M. A., Nsoesie, E. O., & Clifford, G. D. (2022).
Rethinking algorithm performance metrics for artificial
intelligence in diagnostic medicine. JAMA, 328(4),
329-330.
Rivera, S. C., Liu, X., Chan, A. W., Denniston, A. K.,
Calvert, M. J., Ashrafian, H., ... & Yau, C. (2020).
Guidelines for clinical trial protocols for interventions
involving artificial intelligence: the SPIRIT-AI
extension. The Lancet Digital Health, 2(10), e549-
e560.
Rudicel, S., & Esdaile, J. (1985). The randomized clinical
trial in orthopaedics: obligation or option? JBJS, 67(8),
1284-1293.
Sounderajah, V., Ashrafian, H., Golub, R. M., Shetty, S.,
De Fauw, J., Hooft, L., ... & Liu, X. (2021). Developing
a reporting guideline for artificial intelligence-centred
diagnostic test accuracy studies: the STARD-AI
protocol. BMJ open, 11(6), e047709.
Vasey, B., Nagendran, M., Campbell, B., Clifton, D. A.,
Collins, G. S., Denaxas, S., ... & McCulloch, P. (2022).
Reporting guideline for the early-stage clinical
evaluation of decision support systems driven by
artificial intelligence: DECIDE-AI. Nature medicine,
28(5), 924-933.
van Leeuwen, K. G., Schalekamp, S., Rutten, M. J., van
Ginneken, B., & de Rooij, M. (2021). Artificial
intelligence in radiology: 100 commercially available
products and their scientific evidence. European
radiology, 31, 3797-3804.
Wu, E., Wu, K., Daneshjou, R., Ouyang, D., Ho, D. E., &
Zou, J. (2021). How medical AI devices are evaluated:
limitations and recommendations from an analysis of
FDA approvals. Nature Medicine, 27(4), 582-584.
Clinical Evaluation of Collaborative Artificial Intelligence Systems: Lessons from the Case of Robot-Assisted Surgery
857
