
Improved PID Control Based on Temperature Compensation for the
Incubation Plate of Chemiluminescent Immunoassay Analyzer
Zhaoyang Wang
1a
, Jing Wang
2
, Bo Liang
1
, Xuesong Ye
1
and Congcong Zhou
2b
1
Biosensor National Special Laboratory, College of Biomedical Engineering and Instrument Science,
Zhejiang University, Hangzhou, Zhejiang, 310027, China
2
Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University,
National Engineering Research Center for Innovation and Application of Minimally Invasive Devices,
East Qingchun Road, Hangzhou, Zhejiang, 310016, China
Keywords: Temperature Compensation, Improved PID Control, Incubation Plate, Constant Temperature Incubation.
Abstract: This paper proposes an improved PID control based on temperature compensation strategy, which can
dynamically adjust the target temperature value of the PID controller according to the ambient temperature
and the preset temperature compensation curve, thus basically eliminating the influence of ambient
temperature on the reaction liquid temperature, and ultimately achieving stable reaction liquid at the preset
temperature under different ambient temperature conditions. Through experiments, it was found that after
adding temperature compensation strategy to the PID control, the maximum steady-state temperature
difference of the reaction liquid decreased from 0.31℃ to 0.11℃, and the coefficient of variation (CV) of
Relative Light Units (RLU) decreased from 4.22% to 1.43%.
1 INTRODUCTION
Chemiluminescence immunoassay (CLIA) is an
analytical method based on the principle of immune
reaction. It utilizes the specific binding between
antigen and antibody to detect the presence and
quantity of specific substances in samples, including
proteins, hormones, tumor markers, etc(Boolani et al.,
2019; Khan et al., 2023; Xiao & Xu, 2020). Constant
temperature incubation is an important step in CLIA,
aiming to enhance the efficiency of antigen-antibody
binding and reduce non-specific reactions, thus
improving the sensitivity and accuracy of detection
results(Suan Ng, Ling Lee, Bothi Raja, & Doong,
2022). In chemiluminescent immunoassay analyzers,
constant temperature incubation is usually performed
in an incubation plate, with the incubation
temperature typically set at 37℃, similar to human
body temperature(Yalcin, oezkan, & Shah, 2022).
Studies have shown that different incubation
temperatures can affect the rate of antigen-antibody
binding and the accuracy and reproducibility of the
luminescence signal(Yufeng, Shizhou, Bo, &
a
https://orcid.org/0009-0004-8996-8743
b
https://orcid.org/0000-0001-8397-1491
Jianwen, 2010). Therefore, it is of great significance
to control the temperature of the reaction solution in
the incubation plate at a stable 37℃ for the accuracy
and reliability of detection results.
The current incubation strategy of the instrument
is to control the temperature of the incubation plate to
remain stable at the preset temperature, and then place
the reaction cups containing the reaction liquid into
the incubation plate for incubation(Feng-Mei, Da-
Qing, Jian-Ning, & Pan-Fei, 2019). Due to the open
top of the reaction cup, the reaction liquid is in direct
contact with the air, and in fact, the temperature of the
reaction liquid is affected by both the temperature of
the incubation plate and the ambient temperature.
In temperature sensing technology, studies have
shown that monitoring environmental temperature
has a significant impact on sensor design and
measurement results(Ren, Zhang, Ye, & Zhou, 2023).
However, most existing instruments set the
incubation temperature on a fixed value using the PID
algorithm, without considering the influence of
environmental temperature. This leads to differences
in the incubation temperature of the reaction liquid at
Wang, Z., Wang, J., Liang, B., Ye, X. and Zhou, C.
Improved PID Control Based on Temperature Compensation for the Incubation Plate of Chemiluminescent Immunoassay Analyzer.
DOI: 10.5220/0012573300003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 811-816
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
811
different ambient temperatures, resulting in variations
in the detection results of the same sample.
This paper proposes an improved PID control
based on temperature compensation, which can
dynamically adjust the target temperature value of the
PID controller based on different environmental
temperatures. The ultimate goal is to ensure that the
reaction liquid remains stable at the preset
temperature under different ambient temperatures.
The application of this improved PID control based
on temperature compensation for the incubation plate
will help improve the accuracy and reliability of
chemiluminescent immunoassay detection results,
especially in cases where laboratory environmental
temperatures fluctuate significantly. Furthermore,
this control strategy can also be applied to other
experimental scenarios requiring precise temperature
control, demonstrating a certain degree of
universality and practicality.
2 IMPROVED PID CONTROL
BASED ON TEMPERATURE
COMPENSATION
2.1 Principle of the Improved PID
Control
Conventional PID control is a closed-loop control
method based on three components: proportional,
integral, and derivative(Borase, Maghade, Sondkar,
& Pawar, 2021). It is used to regulate the output of a
system to stabilize target variables such as
temperature at a set value. The proportional, integral,
and derivative components work together to adjust
the controller's output to influence the actuator,
thereby achieving precise control of the target
variable of the controlled object(Kaul, Tiwari, Yadav,
& Kumar, 2021; Phu Nguyen, Hung Nguyen,
Ahmadian, & Senu, 2020). The schematic diagram of
conventional PID control is shown in the Figure 1,
where r(t) represents the set value, y(t) represents the
measurement value, e(t) is the error value, and u(t) is
the output value of the PID controller.
Figure 1: Conventional PID control.
In conventional PID control, the set value r(t) is a
fixed value(Joseph, Dada, Abidemi, Oyewola, &
Khammas, 2022; Kaul et al., 2021). However, in a
constant-temperature incubation module, the
temperature of the reaction solution may be affected
by the ambient temperature. Therefore, we propose an
improved PID control based on temperature
compensation, where the set value of the PID
controller can be dynamically adjusted according to
the ambient temperature. Specifically, at high
ambient temperatures, the set value r(t) needs to be
appropriately reduced to avoid overheating of the
reaction solution, while at low ambient temperatures,
the set value r(t) needs to be correspondingly
increased to maintain stable temperature of the
reaction solution. The schematic diagram of the
improved PID control is shown in the Figure 2.
Figure 2: Improved PID control.
The dynamic adjustment of the set value r(t) is
mainly achieved through the ambient temperature and
a preset temperature compensation curve. The
adjustment block diagram of r(t) is shown in the
Figure 3. The temperature sensing sensor collects the
ambient temperature in the first step. In the second
step, the controller calculates the temperature
compensation value for the incubation plate based on
the acquired ambient temperature and the preset
temperature compensation curve. Finally, the target
temperature value for the incubation plate r(t) is
determined in the third step.
Figure 3: Adjustment block diagram of the set value r(t).
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
812
2.2 Acquisition of the Temperature
Compensation Curve
The core of improved PID control lies in obtaining
the temperature compensation curve. The steps to
obtain the temperature compensation curve are as
follows:
Step 1: Place the incubation plate in an
environment with adjustable temperature and use the
ambient temperature as the independent variable 𝑥
、
𝑥
、
⋯
、
𝑥
. Adjust the incubation plate
temperature to stabilize the reaction liquid
temperature at a preset temperature of 37℃. Record
the corresponding incubation plate temperature
compensation values 𝑦
、
𝑦
、
⋯
、
𝑦
at different
ambient temperatures. Obtain a set of data points
𝑝
𝑥
,𝑦
,𝑖1,2,⋯,𝑛.
Step 2: Obtain the temperature compensation
curve through polynomial fitting. Select the fitting
curve based on the principle of minimizing the sum
of squared deviations, ensuring that the fitted curve
deviates minimally from the actual temperature
compensation curve 𝑦𝑓𝑥.
The process of polynomial least squares fitting is
as follows:
1. Assume the 𝑘
-order polynomial for the fitting is
given by equation (1):
𝑦𝑎
𝑎
𝑥⋯𝑎
𝑥
(1)
2. The sum of squared deviations, which represents
the distance between each temperature
compensation value point and the fitted curve, is
denoted as the sum of squared errors in equation
(2).
𝑅
𝑦
𝑎
𝑎
𝑥
⋯𝑎
𝑥
(2)
3. In order to minimize the sum of squared errors, 𝑎
、𝑎
、 ⋯、𝑎
should satisfy the condition that
the partial derivatives of equation (2) with respect
to 𝑎
are equal to zero.
2
𝑦
𝑎
𝑎
𝑥
⋯𝑎
𝑥
0
2
𝑦
𝑎
𝑎
𝑥
⋯𝑎
𝑥
𝑥
0
⋯
2
𝑦
𝑎
𝑎
𝑥
⋯𝑎
𝑥
𝑥
0
(3)
4. By simplifying each equation in equation (3) and
representing them in matrix form, we obtain the
matrix shown in equation (4).
⎣
⎢
⎢
⎢
⎢
⎢
⎢
⎢
⎢
⎡
𝑛𝑥
⋯𝑥
𝑥
𝑥
⋯𝑥
⋮⋮⋱⋮
𝑥
𝑥
⋯𝑥
⎦
⎥
⎥
⎥
⎥
⎥
⎥
⎥
⎥
⎤
𝑎
𝑎
⋮
𝑎
⎣
⎢
⎢
⎢
⎢
⎢
⎢
⎢
⎢
⎡
𝑦
𝑥
𝑦
⋮
𝑥
𝑦
⎦
⎥
⎥
⎥
⎥
⎥
⎥
⎥
⎥
⎤
(4)
5. Let matrix X, A, and Y be defined as follows:
𝑿
11⋯1
𝑥
𝑥
⋯𝑥
⋮⋮⋱⋮
𝑥
𝑥
⋯𝑥
,𝑨
𝑎
𝑎
⋮
𝑎
,𝒀
𝑦
𝑦
⋮
𝑦
(5)
According to equation (4), we have 𝑿𝑿
𝑻
𝑨𝑿𝒀.
Therefore, the coefficient matrix of the fitting
polynomial can be obtained 𝑨
𝑿𝑿
𝑻
𝟏
𝑿𝒀. This
provides us with the coefficients of the fitting
polynomial and thus the temperature compensation
curve.
With the help of Matlab, the results of third-order
polynomial fitting can be visualized. By inputting
different ambient temperatures and their
corresponding temperature compensation values, the
polyfit function can be used to achieve polynomial
curve fitting and obtain the temperature
compensation curve. The third-order polynomial
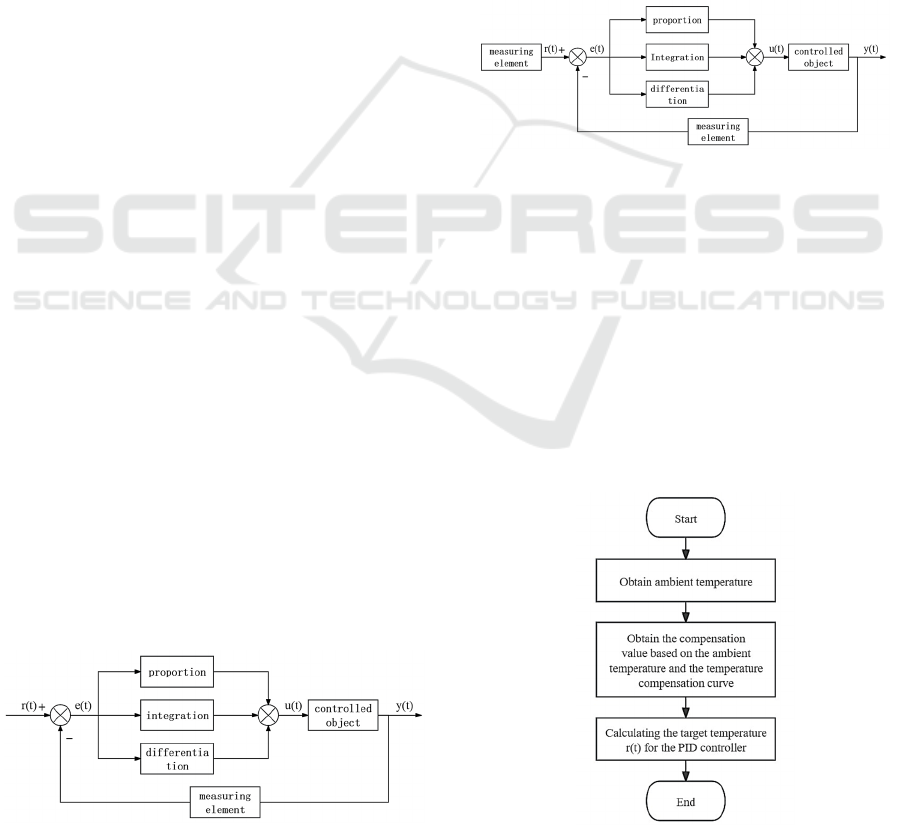
fitting result is shown in Figure 4.
Figure 4: The results of third-order polynomial fitting.
2.3 The Construction of a Constant
Temperature Incubation Platform
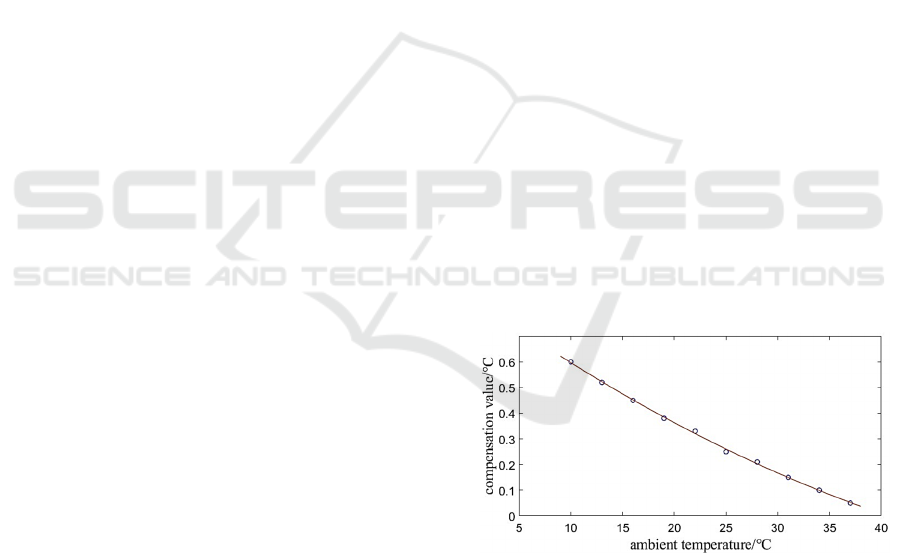
We constructed a constant temperature incubation
platform according to the experimental requirements,
as shown in the
Figure 5
. The constant temperature
incubation platform includes an aluminum incubation
plate, heating film, temperature sensor, and controller.
The aluminum incubation plate is used to hold the
Improved PID Control Based on Temperature Compensation for the Incubation Plate of Chemiluminescent Immunoassay Analyzer
813
reaction vessels, providing a stable incubation
environment for the reaction solution. The heating
film is pasted around the incubation plate to heat it.
The constant temperature incubation platform is
equipped with two temperature sensors, one of which
is embedded inside the incubation plate to collect the
temperature of the incubation plate, while the other
temperature sensor is placed in the air to collect the
ambient temperature. The core of the controller is an
STM32 microcontroller, which changes the duty
cycle of the Pulse-Width Modulation (PWM) signal
output pin through the PID temperature control
algorithm to achieve precise control of the
temperature of the incubation plate.
Figure 5: Constant temperature incubation platform.
3 RESULTS AND DISCUSSION
3.1 Effects of Improved PID Control
To compare the effects of temperature compensation
strategies versus no temperature compensation
strategy on the incubation temperature of the reaction
solution, we placed the incubation plate in a
temperature-controlled incubator and varied the
temperature of the incubator to simulate changes in
ambient temperature. We added 300 μL of reaction
solution with an initial temperature of 7°C to the
reaction vessels and measured the temperature
changes of the reaction solution during incubation at
different ambient temperatures.
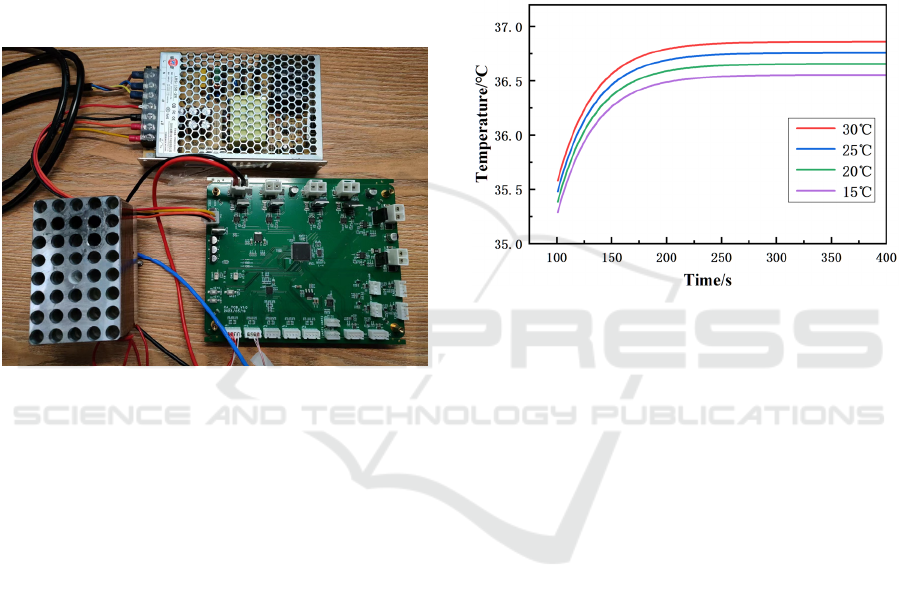
In the first set of experiments, the PID
temperature control algorithm did not consider
temperature compensation strategies. The incubation
plate temperature was fixed at 37°C, and the
temperature changes of the reaction solution were
observed at ambient temperatures of 15°C, 20°C,
25°C, and 30°C. To clearly observe the steady-state
temperature differences of the reaction solution, only
the temperature change curves from 100-400 s after
placing the reaction solution in the incubation plate
are shown in the
Figure 6
. From the test results, it can
be observed that the ambient temperature has a
significant impact on the steady-state temperature of
the reaction solution. At an ambient temperature of
30°C, the steady-state temperature of the reaction
solution is 36.86°C. However, at an ambient
temperature of 15°C, the steady-state temperature of
the reaction solution is 36.55°C, resulting in a
difference of 0.31°C.
Figure 6: Temperature of reaction liquid under different
ambient temperatures (without temperature compensation).
In the second set of experiments, the PID
temperature control algorithm considered
temperature compensation strategies, and the set
temperature of the incubation plate was dynamically
adjusted according to the ambient temperature and the
preset temperature compensation curve. The
temperature changes of the reaction solution during
incubation at ambient temperatures of 15°C, 20°C,
25°C, and 30°C were observed. Similarly, the
temperature change curves from 100-400 s after
placing the reaction solution in the incubation plate
are shown in the
Figure 7
. From the test results, it can
be observed that with temperature compensation at
different ambient temperatures, the steady-state
temperature difference of the reaction solution is very
small. The steady-state temperature of the reaction
solution is maintained at 37 ± 0.1°C after temperature
compensation, with a maximum temperature
difference of 0.11°C.
Comparing the results of the
two experimental groups, it can be observed that
improved PID control can significantly reduce the
steady-state temperature difference during incubation
of the reaction solution at different environmental
temperatures.
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
814

Figure 7: Temperature of reaction liquid under different
ambient temperatures (with temperature compensation).
3.2 Impact of Improved PID Control
on the RLU
Taking the Interleukin-6 (IL-6) chemiluminescence
system as an example, we explored the influence of
improved PID control on the results of
chemiluminescence immunoassay. In the first set of
experiments, the PID temperature control algorithm
did not consider temperature compensation strategies.
The same samples were incubated at ambient
temperatures of 15°C, 20°C, 25°C, and 30°C for 30
minutes. After magnetic separation and washing, the
reaction cups were placed in a photomultiplier tube
for reading. In the second set of experiments, the PID
temperature control algorithm considered
temperature compensation strategies, and the
subsequent experimental procedures were the same as
those of the first set. By analyzing the differences of
RLU between the two sets of experiments, the final
results are shown in the Table 1.
Table 1: The impact of improved PID control.
Ambient
temperature/℃
RLU without
temperature
com
p
ensation
RLU with
temperature
com
p
ensation
15 1921244 2163580
20 2063019 2188961
25 2125171 2143365
30 2143024 2226606
CV 4.22% 1.43%
From preliminary experimental results, it can be
observed that when temperature compensation
strategy is not considered, there is a significant
difference in the steady-state temperature of the
reaction solution at different ambient temperatures,
resulting in a high CV in the RLU, reaching 4.22%.
However, when temperature compensation strategy is
taken into account, the steady-state temperature
difference of the reaction solution at different ambient
temperatures is reduced, leading to a lower CV in the
final RLU, which is only 1.43%.
The above experiments demonstrate that by
incorporating temperature compensation strategy into
the PID algorithm, the influence of environmental
temperature on the steady-state temperature of the
reaction solution can be effectively eliminated. This
ensures that the reaction solution remains stable at the
set temperature under different environmental
conditions. This finding is of great significance in
reducing the CV in the detection results and
improving the accuracy of the detection results.
4 CONCLUSIONS
In this work, we propose an improved PID control
algorithm based on temperature compensation. By
sensing the environmental temperature and obtaining
the temperature compensation value through the
preset temperature compensation curve, the influence
of environmental temperature on the reaction solution
temperature can be effectively eliminated. The
temperature compensation curve is fitted using a
third-order polynomial.
By comparing the steady-
state temperatures of the reaction solution before and
after considering the temperature compensation
strategy, it can be observed that the improved PID
control can reduce the maximum steady-state
temperature difference of the reaction solution from
0.31°C to 0.11°C.
The improved PID control also
reduces the CV of the detection results for the same
sample at different environmental temperatures. By
considering the temperature compensation strategy,
the CV of the RLU decreases from 4.22% to 1.43%.
This is of significant importance in improving
instrument performance and enhancing the accuracy
of the detection results.
In future work, we plan to explore alternative fitting
methods to further reduce the steady-state temperature
difference of the reaction solution under different
environmental temperatures. Additionally, we will also
explore other chemiluminescence systems to further
investigate the impact of improved PID control on the
results of chemiluminescence detection.
ACKNOWLEDGEMENTS
This work was supported in part by Zhejiang
Provincial Natural Science Foundation of China
Improved PID Control Based on Temperature Compensation for the Incubation Plate of Chemiluminescent Immunoassay Analyzer
815

under Grant No. LY22H180006 and the National Key
R&D Program of China under Grant No.
2017YFF0210803.
REFERENCES
Boolani, A., Channaveerappa, D., Dupree, E. J.,
Jayathirtha, M., Aslebagh, R., Grobe, S., . . . Darie, C.
C. (2019). Trends in Analysis of Cortisol and Its
Derivatives. In A. G. Woods & C. C. Darie (Eds.),
Advancements of Mass Spectrometry in Biomedical
Research, 2nd Edition (Vol. 1140, pp. 649-664).
Borase, R. P., Maghade, D. K., Sondkar, S. Y., & Pawar, S.
N. (2021). A review of PID control, tuning methods and
applications. International Journal of Dynamics and
Control, 9(2), 818-827. doi:10.1007/s40435-020-
00665-4
Feng-Mei, F., Da-Qing, L., Jian-Ning, Z., & Pan-Fei, H.
(2019). Performance Verification of CA125 in Roche
Cobas6000 Automatic Electrochemiluminescence
Immunoassay Analyzer. World Latest Medicine
Information.
Joseph, S. B., Dada, E. G., Abidemi, A., Oyewola, D. O., &
Khammas, B. M. (2022). Metaheuristic algorithms for
PID controller parameters tuning: review, approaches
and open problems. Heliyon, 8(5).
doi:10.1016/j.heliyon.2022.e09399
Kaul, S., Tiwari, N., Yadav, S., & Kumar, A. (2021).
Comparative Analysis and Controller Design for BLDC
Motor Using PID and Adaptive PID Controller. Recent
Advances in Electrical & Electronic Engineering,
14(6), 671-682. doi:10.2174/235209651466621082315
2446
Khan, M., Shah, S. H., Salman, M., Abdullah, M., Hayat,
F., & Akbar, S. (2023). Enzyme-Linked
Immunosorbent Assay versus Chemiluminescent
Immunoassay: A General Overview. Global Journal of
Medical Pharmaceutical and Biomedical Update,
18(1). doi:10.25259/gjmpbu_77_2022
Phu Nguyen, D., Hung Nguyen, N., Ahmadian, A., & Senu,
N. (2020). A New Fuzzy PID Control System Based on
Fuzzy PID Controller and Fuzzy Control Process.
International Journal of Fuzzy Systems, 22(7), 2163-
2187. doi:10.1007/s40815-020-00904-y
Ren, X., Zhang, Y., Ye, X., & Zhou, C. (2023). Study of
perfusion based theoretical model and experimental
evaluation for wearable CBT measurement.
Measurement, 206. doi:10.1016/j.measurement.2022.1
12338
Suan Ng, S., Ling Lee, H., Bothi Raja, P., & Doong, R.-a.
(2022). Recent Advances in Nanomaterial-based
Optical Biosensors as Potential Point-of-Care Testing
(PoCT) Probes in Carcinoembryonic Antigen
Detection. Chemistry-an Asian Journal, 17(14).
doi:10.1002/asia.202200287
Xiao, Q., & Xu, C. (2020). Research progress on
chemiluminescence immunoassay combined with
novel technologies. Trac-Trends in Analytical
Chemistry, 124. doi:10.1016/j.trac.2019.115780
Yalcin, S., oezkan, S., & Shah, T. (2022). Incubation
Temperature and Lighting: Effect on Embryonic
Development, Post-Hatch Growth, and Adaptive
Response. Frontiers in Physiology, 13. doi:10.3389/
fphys.2022.899977
Yufeng, Y., Shizhou, L., Bo, H., & Jianwen, Z. (2010).
Automated Chemiluminescence Immunoassay
Analyzer. Paper presented at the 2010 International
Conference on Intelligent Computation Technology
and Automation.
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
816
