
Evaluation of the Performance of Wearables’ Inertial Sensors for the
Diagnosis of Resting Tremor in Parkinson’s Disease
Carlos Polvorinos-Fernández
1a
, Luis Sigcha
2b
, Laura Pereira de Pablo
1
, Luigi Borzí
4,5 c
,
Paulo Cardoso
3d
, Nelson Costa
3e
, Susana Costa
3f
, Juan Manuel López
6g
,
Guillermo de Arcas
1h
and Ignacio Pavón
1i
1
Instrumentation and Applied Acoustics Research Group (I2A2), Mechanical Engineering Department, ETSI Industriales,
Universidad Politécnica de Madrid, Spain
2
Department of Physical Education and Sports Science, Health Research Institute, & Data-Driven Computer Engineering
(D2 iCE) Group, University of Limerick, Limerick, V94 T9PX, Ireland
3
ALGORITMI Research Center, School of Engineering, University of Minho, Guimarães, Portugal
4
PolitoBIOMed Lab–Biomedical Engineering Lab, Politecnico di Torino, 10129 Turin, Italy
5
ANTHEA Lab–Data Analytics and Technologies for Health Lab, Department of Control and Computer Engineering,
Politecnico di Torino, 10129 Turin, Italy
6
Instrumentation and Applied Acoustics Research Group (I2A2), Physical Electronics,
Electrical Engineering and Applied Physics Department, ETS de Ingeniería y Sistemas de Telecomunicación,
Universidad Politécnica de Madrid, Spain
Keywords: Motor Symptoms, Wearables, Accelerometer, Gyroscope, Machine Learning.
Abstract: Currently, objective monitoring of resting tremor in Parkinson’s disease (PD) involves wearable devices and
machine learning. Smartwatches may present an affordable method for remote and unintrusive tremor
monitoring. However, the development of optimized systems is necessary to perform accurate monitoring in
free-living settings. In this study, the potential of inertial sensors to detect resting tremors is evaluated. A
smartwatch was placed on the wrist of six subjects with PD during the execution of MDS-UPDRS motor tasks.
Data were collected over eight weeks from triaxial accelerometer and gyroscope simultaneously and used to
implement machine learning algorithms to detect resting tremor. The best performance (accuracy 97.0% in
tremor detection) was achieved using accelerometer data analysed with a Random Forest classifier, while the
gyroscope showed lower performance (93.0%). The results indicates that the use of the accelerometer in
commercial smartwatches can offer effective results for detecting resting tremors, while reducing
computational workload. These results show opportunities for the development of robust free-living tremor
monitoring systems using commodity devices and algorithms using a single sensor.
1 INTRODUCTION
Parkinson's disease is a neurodegenerative disease
affecting the central nervous system, leading to motor
and non-motor manifestations. PD occurs when
a
https://orcid.org/0000-0002-4594-9477
b
https://orcid.org/0000-0002-9968-5024
c
https://orcid.org/0000-0003-0875-6913
d
https://orcid.org/0000-0002-7924-0060
e
https://orcid.org/0000-0002-9348-8038
f
https://orcid.org/0000-0001-7440-8787
g
https://orcid.org/0000-0001-7847-8707
h
https://orcid.org/0000-0003-1699-7389
i
https://orcid.org/0000-0003-0970-0452
neurons do not produce enough of the chemical
"dopamine" (Wirdefeldt, Adami, Cole, Trichopoulos,
& Mandel, 2011).
Globally, 7–10 million individuals are currently
affected by PD, with an upward trend in recent years.
PD is rare before the age of 50 and exhibits a greater
820
Polvorinos-Fernández, C., Sigcha, L., Pereira de Pablo, L., Borzí, L., Cardoso, P., Costa, N., Costa, S., López, J., de Arcas, G. and Pavón, I.
Evaluation of the Performance of Wearables’ Inertial Sensors for the Diagnosis of Resting Tremor in Parkinson’s Disease.
DOI: 10.5220/0012571600003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 2, pages 820-827
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.

prevalence among men compared to women. The
incidence of PD increases with age, affecting around
1% of the population aged 60 or older (Rocca, 2018).
The development of the disease over time is
dependent on the person who suffers from it. During
the disease, patients progress through different stages,
associated with the severity of the symptoms and
physical disability caused. Currently, the diagnosis
and monitoring of the disease is conducted by a
medical specialist who assesses a series of exercises
performed by the patients using standardized
guidelines (Bhidayasiri & Martinez-Martin, 2017).
Among the motor symptoms in PD, the most
common and diagnostically distinct motor symptoms
is tremor (Halli-Tierney, Luker, & Carroll, 2020).
Tremor can be defined as an involuntary oscillatory
movement of parts of the human body, such as in the
hands or feet. There are different types of tremors
associated with PD, classified as resting tremor, that
presents when patients are relaxed; and action tremor,
which occurs when holding a position against gravity
or during any voluntary movement (Gironell, 2018).
The frequency range in which this type of tremor
manifest is in the range of 3.5-7 Hz (Salarian, et al.,
2003), while common human movements are usually
found in the 0-20 Hz band (Mannini, Intille,
Rosenberger, Sabatini, & Haskell, 2013).
Currently, levodopa is the principal drug used to
treat PD actively (LeWitt, 2008). It acts by converting
to dopamine in the brain and works vigorously on
controlling tremors in patients.
The subjective nature of motor assessment based
in observation techniques and the sporadic follow-up
commonly performed in clinical settings hinders the
implementation of precise therapies. For this reason,
the need of tools to improve the diagnosis and
continuous monitoring is still required.
The use of smart technologies for diseases such as
PD is currently on the rise. Wearable technologies,
stand out for their low cost, battery life, non-
invasiveness, can bring an excellent technological
support to implement monitoring systems for PD.
The use of inertial sensors such as accelerometers
and gyroscopes included in wearable devices with an
appropriate processing of these data and a subsequent
implementation of artificial intelligence algorithms
can be a promising alternative for the monitoring of
motor symptoms in PD in free-living conditions.
In this work, it has been evaluated which of the
inertial sensors integrated in a smartwatch,
accelerometer or gyroscope, could provide better
performance in terms of accuracy for the
classification of tremor in PD patients using machine
learning models. The dataset (Sigcha, et al., 2023)
used contains weekly records from several
Parkinson's disease patients during various planned
activities, while they were wearing a smartwatch.
2 BACKGROUND
Currently, the most used method for the assessment
of PD is the Movement Disorders Society's review of
the Unified Parkinson's Disease Rating Scale (MDS-
UPDRS) (Goetz, et al., 2008). Motor symptoms are
evaluated in the part III of this guide on a scale of 0
to 4, with 0 assigned to the non-existence of
symptoms and 4 the label for the most severe value.
Despite this scale is widely used, the evaluation
can be subjective by the physician and depends on his
or her perception at the time, which may vary from
one neurologist to another. This, together with the
fact that patients make very occasional visits to the
clinic, has led many authors to study the possibility of
remote and objective symptom monitoring.
Therefore, in recent years, numerous studies have
evaluated the possibility of using wearable devices
for healthcare applications. Some studies have
focused on the development of specific devices, while
others have used commercial devices for the
evaluation of PD pathologies (Sigcha, et al., 2023).
Regardless of how the monitoring has been
approached, MEMS (Micro Electronic Mechanical
Systems) type sensors have been used due to their
small size and low cost. The most common sensors
used in motor symptom monitoring are the
accelerometer and the gyroscope. A study conducted
by (San-Segundo, et al., 2020) used accelerometers to
compare tremor detection in free-living conditions
and in the laboratory environment, achieving a 10%
and 5% error. (López-Blanco, et al., 2019) conducted
one year of monitoring using a smartwatch that
collected data from a gyroscope yielded a Spearman
coefficient between the mean of the resting tremor
scores and smartwatch measurements was 0.81.
The combination of accelerometer and gyroscope
data for tremor detection was evaluated in (Sun, et al.,
2021), where a watch integrating both sensors was
developed, achieving an accuracy of over 94%.
Despite the progress in tremor monitoring,
previous studies have not focused on evaluating
which inertial sensor (accelerometer or gyroscope)
can provide more information to assess this symptom.
This paper will to study the potential of inertial
sensors in a commercial smartwatch to detect restring
tremor and evaluate which dataset, the one collected
from the accelerometer or the one obtained from the
gyroscope, could provide more useful information.
Evaluation of the Performance of Wearables’ Inertial Sensors for the Diagnosis of Resting Tremor in Parkinson’s Disease
821

3 MATERIALS AND METHODS
3.1 Data Collection
The data used in this study were collected during the
TECAPARK project (TECAPARK, n.d.), using a
proprietary m-health application named Monipar
(Sigcha, et al., 2023). A consumer-grade smartwatch
and a smartphone were used to monitor motor
symptoms in PD patients. The Monipar dataset
contains weekly records from Parkinson's disease
patients during planned activities, including
standardized exercises and resting periods for their
upper limbs, while they were wearing a smartwatch.
Data was collected from 6 PD patients (3 males/3
females, 64.2 ± 8.2 years). These subjects were in
early stages of the disease according to the Hoehn and
Yahr scale (Hoehn & Yahr, 1998) (H&Y = 1).
Three participants did not present tremors while
the other three presented tremors. A trained specialist
evaluated tremor according to MDS-UPDRS 3.17,
assigning score from 0 (no tremor) to 2 (mild tremor).
The data collection was conducted over 8 weeks,
and, during the study, all patients maintained their
usual medication regimen.
To perform tasks such as signal labeling,
preprocessing and feature extraction, MATLAB
software (R2017a) was employed. For the evaluation
and the training of the models, Python (3.6), and the
libraries Pandas, and Scikit learn were chosen.
3.2 Acquisition Device (Smartwatch)
A consumer-grade smartwatch was used as the data
acquisition device during the measurement sessions
and was placed on the wrist of the most affected side.
The wearable device was used to collect vibration
signals in the time domain using the bult-in inertial
sensors (accelerometer and gyroscope) in three axes.
In the case of the accelerometer, in m/s
2
, and, for the
gyroscope, in rad/s.
In this study, a smartwatch with dimensions of
46.6×51.8×12.9 mm and a weight of 32.5g was used,
with WearOs® as the operating system. This
smartwatch is equipped with an LSM6DS3 type
package, which includes a 3-axis digital gyroscope
and a 3-axis digital accelerometer. The triaxial
accelerometer has a maximum measurement
amplitude of ±2 g, while the triaxial gyroscope has a
measurement range of ±2000 dps.
The smartwatch was set to record data at a
sampling rate of 50 Hz. This frequency has been
established as it is appropriate for the analysis of
human movement, as common human movements are
usually found in the 0-20 Hz band, while it also
allows recording the typical PD tremors in the range
of 3.5-7 Hz (Salarian, et al., 2003).
3.3 Experimental Protocol
In each measurement session, each patient performed
8 exercises designed to assess the motor status,
including resting periods between exercises. These
exercises were conducted using Monipar application,
which guides the user through exercises by displaying
the tasks to be performed on the mobile screen.
In specific, each exercise belongs to the MDS-
UPDRS part III. The exercises proposed are related to
the amplitude of resting and postural tremor of the
hands, movement of the hands towards the chest,
finger tapping, hand movements, pronation-
supination of the hands, getting up and gait.
Each exercise has a different duration
(explanation plus execution); some take 15 seconds,
while others may require 50 seconds. Furthermore,
there is a 30-second break between exercises, making
7 minutes the approximate duration of each single
measurement session. Furthermore, each patient's
sessions were video recorded for subsequent labeling.
For the completion of this work, only the data
related to resting tremor amplitude, assessed through
section 3.17 of the MDS-UPDRS were used. In this
task, the patient should sit quietly in a chair with
hands resting on the armrest (not on the lap) and feet
resting comfortably on the floor, for 10 seconds,
without any other indication. Figure 1 shows the
interface of the resting tremor exercise.
Figure 1: Exercise explanation in mobile application.
3.4 Data Labelling
For data labelling, Monipar automatic generated
labels were used for each of the exercises. The
HEALTHINF 2024 - 17th International Conference on Health Informatics
822
sequence of exercises is numbered from 1 to 8
according to the order in which they are performed.
The resting tremor exercise corresponds to label 1.
For tremor labeling, data were automatically
labeled using thresholds according to magnitude
analysis in the tremor band (3.5-7.5 Hz). Then, these
labels were verified and corrected using video
recordings for each test. Data was labeled according
to the MDS-UPDRS section 3.17 guidelines. In
specific, the following were assigned to the data: 0
(Normal) if no tremor is observed, 1 (Slight) if the
maximum amplitude of the movement is less than 1
cm, 2 (Mild) if the maximum amplitude is between 1
and 3 cm, 3 (Moderate) if it is between 3, and 10 cm
and as 4 (Severe) if it is greater than 10 cm.
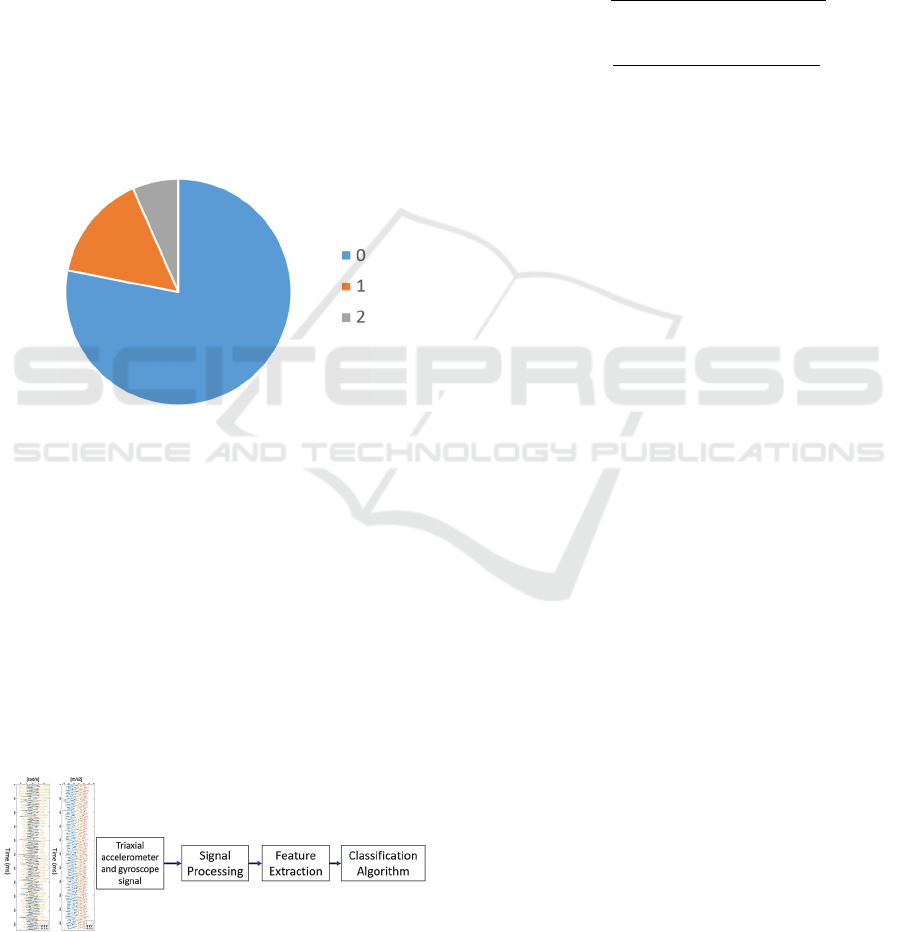
Figure 2 shows the distribution of tremor labels.
Only labels 0,1,2 are available, with 0 being the most
common label, present in 78% of the data.
Figure 2: Observations distributed by tremor label.
In this paper, the tremor label was used in two
ways. On the one hand, to classify among MDS-
UPDRS scores. And on the other hand, to
differentiate between the presence or not of tremor,
so labels 1 and 2 will be grouped into a single label.
3.5 Algorithmic Approach
This paper presents machine learning models that
predict the level of tremor amplitude using
accelerometer and gyroscope data to identify which
of them is more useful for prediction. This work was
developed following the schema shown in Figure 3.
Figure 3: Algorithm development diagram.
To train and evaluate the proposed models, the
signal obtained from the smartwatch has been
processed. First, the signal obtained from each of the
three axes of each sensor was combined into one by
means of Euclidean Norm according to equations (1)
and (2). This is since the inertial sensors embedded in
the wearable device can have a random orientation,
so this combination has been performed to avoid
errors. In addition, the computational load during
training and prediction can be reduced.
𝐴
𝑐𝑐𝑒𝑙
𝑎𝑐𝑐𝑒𝑙
𝑎𝑐𝑐𝑒𝑙
𝑎𝑐𝑐𝑒𝑙
(1)
𝐺𝑦𝑟𝑜
𝑔𝑦𝑟𝑜
𝑔𝑦𝑟𝑜
𝑔𝑦𝑟𝑜
(2)
After calculating the Eucliden norm the signal
was filtered using a Butterworth band-pass filter of
order 3 to select the signal between 0.5 and 10 Hz.
This frequency range is suitable for human activity
recognition and relevant for the tremor present in PD
(Khan, Hammerla, Mellor, & Plötz, 2016).
Following, signal segmentation was performed
using 128-sample windows (2.56 seconds) using 50%
overlap. A total of 5158 windows have been defined.
This combination of segmentation and overlapping is
recommended for PD tremor analysis (Patel, et al.,
2009) using inertial sensors.
To establish the tremor amplitude label in each of
the windows, if the label is repeated for more than
half of the observations in each window, the assigned
value will be that label. If this does not occur, such
windows will be excluded.
Finally, the signal was transformed to the
frequency domain using the Fast Fourier Transform
(FFT), since it has been shown (Ahlrichs & Samà,
2014) that the signal in the frequency domain could
be representative to evaluate the tremor.
Both time and frequency domain features will be
obtained. The time variables are the easiest to obtain
since do not entail a very high computational cost;
however, they do not lead to very robust conclusions
due to their difficult interpretation in this domain in
view of the tremors associated with human
movement. Nevertheless, the frequency variables
allow an improvement in the detection of the tremors
despite being more computationally complex to
acquire due to the need to calculate the FFT.
For each domain, the same type of features was
obtained. The Table 1 shows the extracted features
with a brief explanation of each of them. The database
used will be composed of 18 characteristics, 9 from
the time domain and 9 from the frequency domain.
Evaluation of the Performance of Wearables’ Inertial Sensors for the Diagnosis of Resting Tremor in Parkinson’s Disease
823
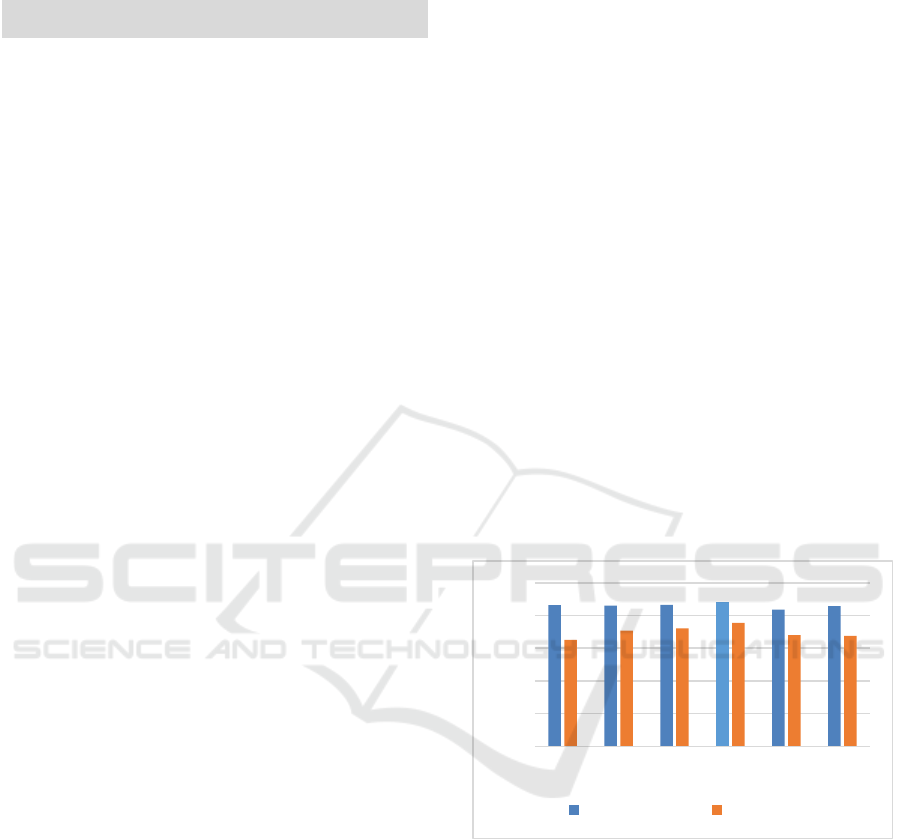
Table 1: Features extracted from the filtered accelerometer
and gyroscope signals in each domain.
Feature Description
Standard
Deviation
Returns the standard deviation of
the si
g
nals in each domain.
Mean
Calculates the median value of all
the measurements.
Median
Finds the median value of the
filtered si
g
nals in each domain.
Percentile 25
The 25th percentile of the input
data for each domain si
g
nal slice.
Percentile 75
The 75th percentile of the input
data for each domain si
g
nal slice.
Skewness
Asymmetry of the filtered signals
in each domain.
Max
Finds the maximum of the values
for each window in each domain.
Min
Finds the minimum of the values
for each window in each domain.
Entropy
Returns the entropy of the filtered
si
g
nals in each domain.
To these 18 features, those obtained from the FFT
of the signal must be added. In this case, 65 additional
features were obtained. So, a total of 83 features were
calculated for each of the 5158 defined windows.
For the development of machine learning models,
the database was divided using Hold Out Validation.
In this case, 80% (4126 windows) of the data for
algorithm training and 20% (1032 windows) for
algorithm validation. Although all measurements
were collected during the same task, since human
movement, especially PD, is different from one to
another, the train-test distribution has been
randomized among the entire dataset.
As the target variable is categorical, the models
proposed are classification models. In this study, the
following models are proposed: Gradient Boosting
(XGB), AdaBoost (ADAB), KNeighbours (KNN),
Random Forest (RF), Logistic Regression (LR) and
Decision Tree (TREE). Evaluation of the models was
performed using accuracy, sensitivity, specificity,
precision and F1-score metrics.
4 EXPERIMENTS AND RESULTS
This section presents the results obtained in the
present study. Multiple experiments were conducted
to evaluate and determine which sensors provide the
best performance. Section 4.1 presents the results
related to the evaluation using a binary classification
between tremor and non-tremor, obtaining the best
sensor with the results of the best model obtained.
Section 4.2 presents the results using the
classification of the tremor level thresholds according
to the MDS-UPDRS scale and establishing the best
sensor and model obtained.
4.1 Results of the Training of Binary
Models
The proposed classification models shown in Section
3.5 were implemented and trained using the set of
features extracted from the time and frequency
domains for each triaxial signal. In specific, two
different sets of features were extracted to each
inertial sensor (accelerometer and gyroscope).
In this case, as an unbalanced database is used,
metrics such as accuracy or precision, which measure
the proportion of correct predictions out of the total
number of predictions, can be misleading as they
provide a very high percentage of correct predictions,
but they could be only correct predictions of no
tremor. Thus, the study has focused on analyzing the
F1-score, because this metric combines precision and
recall using their harmonic mean, so a maximum F1-
score implies maximizing both precision and recall
simultaneously. Figure 4 show the training results
obtained, as reflected in the F1-score for each of the
models for the accelerometer and the gyroscope.
Figure 4: F1-score comparison for each algorithm using
accelerometer and gyroscope data.
From the observation of figures 4, it is evident that
results obtained from the data provided by the
accelerometer suppose a better performance of the
prediction model over those achieved through the
gyroscope. The average F1-score value of the models
trained with the accelerometer data was 0.86 while
that obtained with the gyroscope dataset is 0.70.
However, to determine which is the best
classification model obtained, all calculated metrics
were considered. Table 2 and Table 3 show the
training results for each of the models.
0.00
0.20
0.40
0.60
0.80
1.00
XGB ADAB KNN RF LR TREE
Accelerometer Gyroscope
HEALTHINF 2024 - 17th International Conference on Health Informatics
824

Among all the machine learning algorithms
implemented, it is observed that the Random Forest
algorithm offers the best metrics, and in particular the
balance of sensitivity and specificity is highlighted.
Table 2: Metrics obtained for each machine learning
algorithm from accelerometer data.
Accuracy
Sensitivity
Specificity
Precision
F1-score
XGB
0,94 0,86 0,96 0,87 0,87
ADAB
0,94 0,87 0,96 0,86 0,86
KNN
0,94 0,86 0,97 0,88 0,87
RF
0,95 0,87 0,97 0,90 0,88
LR
0,93 0,81 0,97 0,87 0,84
TREE
0,94 0,86 0,96 0,86 0,86
Table 3: Metrics obtained for each machine learning
algorithm from gyroscope data.
Accuracy
Sensitivity
Specificity
Precision
F1-score
XGB
0,88 0,51 0,99 0,92 0,65
ADAB
0,89 0,61 0,97 0,84 0,71
KNN
0,90 0,61 0,98 0,89 0,72
RF
0,90 0,68 0,97 0,86 0,76
LR
0,88 0,57 0,97 0,85 0,68
TREE
0,86 0,68 0,90 0,67 0,68
The test data, 20% of the total dataset, has been
evaluated with the best model, Random Forest, to
identify which of the two dataset yields better results.
The metrics are shown in Table 4 while the related
normalized confusion matrices are shown in Figure 5.
Table 4: Metrics associated with Random Forest algorithm
for accelerometer and gyroscope for test set.
[%]
Accelerometer Gyroscope
Accuracy 96.98 92.99
Sensitivity 91.06 75.07
Specificity 98.56 97.70
Precision 94.38 89.54
F1-score 92.69 81.67
It can be appreciated that both inertial sensors
have above 75% in all the metrics that were
considered. However, there are notable differences
based on the selected inertial sensor. For all these
metrics, the accelerometer database shows better
performance than the gyroscope. While both sensors
obtain quite similar specificity values, significant
differences are shown in the other metrics, with the
most significant difference in sensitivity, where the
accelerometer provides a value of 91% while the
gyroscope obtains a value of 75%.
It is noteworthy that the gyroscope data produces
an error of 25% predicting no tremor when it is
tremor. 91.06% sensitivity and 98.56% specificity for
the data provided by the accelerometer indicate a very
high accuracy rate in the prediction of resting tremors.
Acceleromete
r
G
y
roscope
Figure 5: Confusion matrices for accelerometer and
gyroscope for test set.
4.2 Results of the Training of
Multiclass Models
This experiment will approach the lines of resting
tremor prediction in a more qualitative way, leaving
behind binary classification. It has sought to evaluate
the performance of the algorithms by faithfully
predicting the tremor label assigned by the MDS-
UPDRS scale, using multiple classification.
In this case, the model that has been proposed is
the one that has achieved the best results in the binary
classification model. The Table 5 shows the metrics
obtained from the model trained using the data from
the different data sources.
Table 5: Comparison of the accuracy of each class for
Random Forest (multiclass classification).
Accelerometer Gyroscope
[%]
0 1 2 0 1 2
Accuracy
96.8 89.3
Sensitivity
98.6 88.5 93.6 97.9 54.7 60.2
Specificity
98.6 88.5 93.6 97.9 54.7 60.2
Precision
97.9 88.9 100 93.7 64.6 75.5
F1-score
98.2 88.7 96.7 95.8 59.2 67.0
Evaluation of the Performance of Wearables’ Inertial Sensors for the Diagnosis of Resting Tremor in Parkinson’s Disease
825
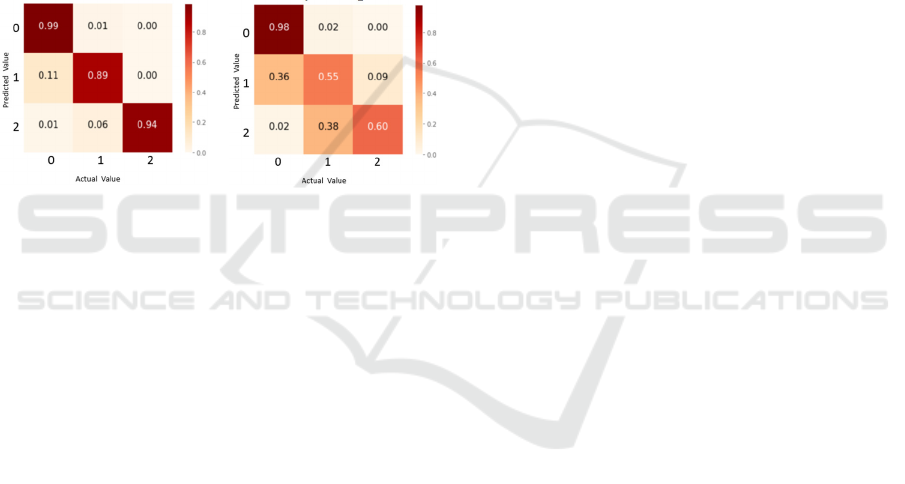
It can be noticed that the prediction of true resting
tremors of amplitude according to MDS-UPDRS
(scores 1 and 2) has a much higher hit rate with the
accelerometer data than by the gyroscope. For the
detection of no tremor (label 0), the values obtained
by the accelerometer and the gyroscope are quite
similar, however, it is again the dataset obtained from
the accelerometer that provides the best results.
From the observation of Figure 6, for the
gyroscope data, it is a difficult task to discern between
tremor and no tremor. It makes an error of 36%
predicting tremor 1 when it is really rest, and 38%
predicting tremor 2 when it is tremor 1. This is not the
case for the accelerometer, with very low percentages
of error between predictions for no tremor and
different level of tremor (11% and 6%, respectively).
Acceleromete
r
G
y
roscope
Figure 6: Confusion matrix for Random Forest model.
5 CONCLUSIONS
Monitoring PD individuals is crucial for precisely
tracking their progression and treatments. This study
seeks to optimize this process and aims to implement
efficient algorithms to facilitate the monitoring.
With the rise of technology in recent decades, this
study indicates that a commercial smartwatch can
provide useful data to monitor resting tremor in PD in
subjects. Several models for the prediction of resting
tremor were implemented using data provided by
inertial sensors embedded in a smartwatch during the
performance of eight standardized exercises.
The results suggest that the use of the
accelerometer as only inertial sensor can provide
optimal results for prediction of resting tremor. The
use of a single inertial sensor in wearables could help
improve the battery performance and power
consumption of the device, as well as reduce the
computational load needed for data.
However, it should be noted that this study has
certain limitations that need to be considered in future
projects. It has been worked with 6 PD patients for 8
weeks, which may be a small sample. In addition, the
database is unbalanced, with more than 75% of the
samples from the same label. So, a larger number of
measurement sessions would be necessary to increase
the reliability of the study.
The binary prediction of PD resting tremor
achieves its best hit rate using a Random Forest model
with the accelerometer data, obtaining 91.06 % in the
sensitivity metric and 98.56 % in the specificity
metric. These results are remarkably satisfactory for
the automatic detection of resting tremor, and are
similar to those proposed in (Sun, et al., 2021) and
(San-Segundo, et al., 2020), but with the advantage
that it has been achieved using a single sensor.
To find a prediction that fits better the true level
of resting tremor, an experiment has been conducted
in which the Random Forest algorithm was trained for
a multiple prediction that differentiates the resting
tremor collected according to the MDS-UPDRS
labelling guide. Based on the results, it is observed
that, using the data provided by the accelerometer, it
is possible to predict very reliably, with a sensitivity
and specificity rate of 98.6% in no presence of resting
tremor, 88.5 % for tremor with MDS-UPDRS score
1, and 93.6 % for resting tremor score 2, with the
biggest challenge for the algorithm being the
differentiation between no tremor and tremor score 1.
A multiclass classification gives a more specific
idea of the severity of the disease, and in a future real-
world application, would contribute to the clinician's
understanding and follow-up of the patient's data.
ACKNOWLEDGEMENTS
This research has been possible thanks to the
financing of the project BIOCLITE PID2021-
123708OB-I00, funded by MCIN/AEI/10.13039/
501100011033/ FEDER, EU; and by “Ayudas para
contratos predoctorales para la realización del
doctorado con mención internacional en sus escuelas,
facultad, centros e institutos de I+D+i”, funded by
Programa Propio I+D+i 2022 from Universidad
Politécnica de Madrid. The authors acknowledge to
the Physical Education and Sports Science (PESS)
department, the Health Research Institute (HRI), and
the Data-Driven Computer Engineering (D2iCE)
Group at University of Limerick, and the
Instrumentation and Applied Acoustics Research
Group (I2A2) at Universidad Politécnica de Madrid.
REFERENCES
Ahlrichs, C., & Samà, A. (2014). Is “frequency
distribution” enough to detect tremor in PD patients
HEALTHINF 2024 - 17th International Conference on Health Informatics
826

using a wrist worn accelerometer? Proceedings of the
8th International Conference on Pervasive Computing
Technologies for Healthcare, (pp. 65-71). Oldenburg,
Germany.
Bhidayasiri, R., & Martinez-Martin, P. (2017). Clinical
Assessments in Parkinson's Disease: Scales and
Monitoring. International Review of Neurobiology,
132: 129-182.
Gironell, A. P.-S.-L. (2018). Tremor types in Parkinson
disease: a descriptive study using a new classification.
Parkinson's Disease, 2018: 4327597.
Goetz, C., Tilley, B., Shaftman, S., Stebbins, G., Fahn, S.,
Martinez-Martin, P., Dodel, R. (2008). Movement
Disorder Society-sponsored revision of the Unified
Parkinson's Disease Rating Scale (MDS-UPDRS):
Scale presentation and clinimetric testing results.
Movement Disorders, 23(15): 2129-2170.
Halli-Tierney, A. D., Luker, J., & Carroll, D. G. (2020).
Parkinson disease. American family physician,
102(11):679-691.
Hoehn, M., & Yahr, M. (1998). Parkinsonism: Onset,
progression, and mortality. Neurology, 17(5):427-42.
Khan, A., Hammerla, N., Mellor, S., & Plötz, T. (2016).
Optimising sampling rates for accelerometer-based
human activity recognition. Pattern Recognit, 73: 33-
40.
LeWitt, P. A. (2008). Levodopa for the treatment of
Parkinson's disease. New Englad Journal of Medicine,
359(23):2468-76.
López-Blanco, R., Velasco, M., Méndez-Guerrero, A.,
Romero, J., Del Castillo, M. D., Serrano, I. R., &
Benito-León, J. (2019). Smartwatch for the analysis of
rest tremor in patients with Parkinson's disease. Journal
of the Neurological Sciences, 401:37-42.
Mannini, A., Intille, S., Rosenberger, M., Sabatini, A., &
Haskell, W. (2013). Activity Recognition Using a
Single Accelerometer Placed at the Wrist or Ankle.
Medicine & Science in Sports & Exercise, 45(11):2193-
203.
Patel, S., Lorincz, K., Hughes, R., Huggins, N., Growdon,
J., Standaert, D., Bonato, P. (2009). Monitoring Motor
Fluctuations in Patients With Parkinson's Disease
Using Wearable Sensors. IEEE Transactions on
Information Technology in Biomedicine, 13(6):864-73.
Rocca, W. (2018). The burden of Parkinson's disease: a
worldwide perspective. The Lancet Neurology, 17(11);
928-929.
Salarian, A., Russmann, H., Vingerhoets, F., Burkhard, P.,
Blanc, Y., Dehollain, C., & Aminian, K. (2003). An
ambulatory system to quantify bradykinesia and tremor
in Parkinson’s disease. Proceedings of the 4th
International IEEE EMBS Special Topic Conference on
Information Technology Applications in Biomedicine,
(pp. 35–38). Birmingham.
San-Segundo, R., Zhang, A., Cebulla, A., Panev, S., Tabor,
G., Stebbins, K., Hodgins, J. (2020). Parkinson’s
Disease Tremor Detection in the Wild Using Wearable
Accelerometers. Sensors, 20(20):5817.
Sigcha, L., Borzì, L., Amato, F., Rechichi, I., Ramos
Romero, C., Cárdenas, A., Olmo, G. (2023). Deep
learning and wearable sensors for the diagnosis and
monitoring of Parkinson’s disease: A systematic
review. Expert Systems with Applications, 229A,
1205412.
Sigcha, L., Pavón, I., De Arcas, G., Costa, N., Costa, S.,
Arezes, P., & Polvorinos-Fernández, C. (2023).
Monipar Database: smartwatch movement data to
monitor motor competency in subjects with Parkinson's
disease (1.0) [Data set]. Retrieved from Zenodo:
https://doi.org/10.5281/zenodo.8104853
Sigcha, L., Polvorinos-Fernández, C., Costa, N., Costa, S.,
Arezes, P., Gago, M., Pavón, I. (2023). Monipar:
Movement data collection tool to monitor motor
symptoms in Parkinson's disease using smartwatches
and smartphones. Frontiers in Neurology, 14.
Sun, M., Watson, A., Blackwell, G., Jung, W., Wang, S.,
Koltermann, K., Pretzer-Aboff, I. (2021).
TremorSense: Tremor Detection for Parkinson's
Disease Using Convolutional Neural Network.
IEEE/ACM Conference on Connected Health:
Applications, Systems and Engineering Technologies
(CHASE), (pp. 1-10). Washington, DC, USA.
TECAPARK. (n.d.). Retrieved from https://www.i2a2.upm.
es/tecapark/
Wirdefeldt, K., Adami, H., Cole, P., Trichopoulos, D., &
Mandel, J. (2011). Epidemiology and etiology of
Parkinson’s disease: a review of the evidence.
European Journal of Epidemiology, 26 Suppl 1:S1-58.
Evaluation of the Performance of Wearables’ Inertial Sensors for the Diagnosis of Resting Tremor in Parkinson’s Disease
827
