
Unleashing the Potential of Reinforcement Learning for Personalizing
Behavioral Transformations with Digital Therapeutics: A Systematic
Literature Review
Thure Georg Weimann
a
and Carola Gißke
Research Group Digital Health, TUD Dresden University of Technology, Dresden, Germany
Keywords:
Digital Therapeutics, Reinforcement Learning, Personalized Medicine, mHealth, Health Behavior Change.
Abstract:
Digital Therapeutics (DTx) are typically considered as patient-facing software applications delivering behavior
change interventions to treat non-communicable diseases (e.g., cardiovascular diseases, obesity, diabetes). In
recent years, they have successfully developed into a new pillar of care. A central promise of DTx is the idea
of personalizing medical interventions to the needs and characteristics of the patient. The present literature
review sheds light on using reinforcement learning, a subarea of machine learning, for personalizing DTx-
delivered care pathways via self-learning software agents. Based on the analysis of 36 studies, the paper
reviews the state of the art regarding the used algorithms, the objects of personalization, evaluation methods,
and metrics. In sum, the results highlight the potential and could already demonstrate the medical efficacy.
Implications for practice and future research are derived and discussed in order to bring self-learning DTx
applications one step closer to everyday care.
1 INTRODUCTION
Digital Therapeutics (DTx) promise the delivery of
personalized therapies independent of place or time
directly to the patient (F
¨
urstenau et al., 2023; Nahum-
Shani et al., 2023). Internally, most DTx applica-
tions provide behavior change interventions via ded-
icated techniques (e.g., self-monitoring, feedback,
goal-setting) like a virtual coach (Lee et al., 2023;
Weimann et al., 2022). Therefore, DTx are, especially
for behavior-modifiable diseases such as obesity, dia-
betes, or substance use disorders, an emerging therapy
complement or alternative (Hong et al., 2021).
However, one of the grand challenges is to tech-
nically realize the idea of ”personalized medicine”
within DTx. Following the definition proposed by the
European Union (2015), personalized medicine gen-
erally refers to “tailoring the right therapeutic strat-
egy for the right person at the right time,. . . ”. Prior
research on digital health interventions revealed that
personalization is not only vital for the intervention
outcome but also system use adherence (Kankanhalli
et al., 2021; Wei et al., 2020). For example, when the
patient becomes disengaged and stops using the sys-
tem because it does not address personal needs, the
a
https://orcid.org/0000-0002-2762-6121
system cannot have any further impact at all.
When implementing personalized digital health
interventions, developers broadly face two options:
handcrafted rules and data-driven machine learn-
ing/artificial intelligence (AI) (Nahum-Shani et al.,
2018). Rule-based approaches are particularly suit-
able when there is enough explicit knowledge avail-
able on how to adapt the system for the user based
on the values of contextual variables. However, static
rules are limited by the given expert knowledge on
the individual, and the system is not able to adapt the
rules by itself (i.e., self-learning). For instance, these
rule-based systems do not take into account that the
preferences of the user might even change unforesee-
ably over time. In contrast, algorithms from the area
of reinforcement learning (RL) do not necessarily rely
on knowledge about the user beforehand and are able
to capture dynamic changes over the course of the in-
tervention.
Generally speaking, RL algorithms learn to make
decisions from a continuous interaction loop with the
environment, to which the patient also belongs (Sut-
ton and Barto, 2018). Consequently, the system gets
to know the user better and better over the course of
interaction. This enables the system to learn which
interventions led to the intended effects (i.e., were
230
Weimann, T. and Gißke, C.
Unleashing the Potential of Reinforcement Learning for Personalizing Behavioral Transformations with Digital Therapeutics: A Systematic Literature Review.
DOI: 10.5220/0012474700003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 2, pages 230-245
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.

beneficial for the user) and repeat them while leaving
room for changes over the therapy course. Compared
to the other machine learning paradigms (supervised
and unsupervised learning), RL algorithms learn sim-
ilarly to humans by “trial and error” and, therefore, do
not necessarily rely on existing training data (Janiesch
et al., 2021). Thus, RL goes beyond and provides a
powerful framework for building intelligent software
agents delivering personalized health behavior change
interventions.
Previous literature reviews focused on the use of
RL for personalization in general (across domains)
(den Hengst et al., 2020), machine learning in per-
sonalized health systems (also for behavior change)
(Oyebode et al., 2023) or the use of RL in the en-
tire health domain (e.g., also medical imaging, diag-
nostics) (Coronato et al., 2020). However, the most
recent published review by Oyebode et al. (2023)
identified a mere of three papers which applied RL.
This highlights the need for a more targeted literature
search and analysis to capture the full spectrum of RL
in the context of health behavior change applications.
To the best of our knowledge, no paper provided a de-
tailed “zoom-in” view on the state of the art here yet.
The present paper aims to address this gap by answer-
ing the following research questions:
• RQ1: Which RL algorithms have been applied?
• RQ2: What do the algorithms exactly personalize
regarding the specific health scenario?
• RQ3: How was the efficacy, efficiency, and clini-
cal safety of the algorithms evaluated?
The remainder of this paper is structured as fol-
lows. Section 2 presents the theoretical background of
the RL problem and available classes of learning algo-
rithms. Afterward, the research method is described.
The results of the review are presented in section 4,
and trends, implications for practice, and research are
discussed in section 5.
2 BACKGROUND
The reinforcement learning problem is characterized
by an intelligent agent (i.e., the software application)
that interacts with the environment (i.e., the patient)
in a continuous feedback loop (Sutton and Barto,
2018). The intelligent agent chooses an action drawn
from a set of actions (e.g., health recommendations)
that might change the state of the environment ob-
served by the agent (e.g., therapy-relevant parame-
ters change). Afterward, the agent receives a reward
produced from the environment that numerically ex-
presses how successful the action has been regarding
a particular goal (e.g., increasing the daily activity of
the patient). The mathematics behind RL is grounded
in Markov decision theory consisting of states, ac-
tions, a reinforcement signal (reward), and transition
function (Kaelbling et al., 1996). Thus, the task of the
agent is to learn an optimal policy to choose an action
(based on the current state) that maximizes the reward
received from the environment. However, in order to
maximize the reward, the agent needs to explore dif-
ferent actions and their effects to be able to exploit
“the best” (optimal) discovered action. To address
this exploration-exploitation tradeoff, several strate-
gies have been proposed (Lattimore and Szepesv
´
ari,
2020).
For example, the so-called epsilon-greedy strat-
egy chooses the best action sometimes (with a prob-
ability of ε) and explores randomly otherwise (prob-
ability of 1-ε). In contrast, more advanced strategies
are Upper Confidence Bound (UCB) or Thompson-
Sampling (White, 2013). UCB decides on the action
with the highest potential for maximizing the reward
by calculating the sum of the current action value and
a measure of uncertainty. The uncertainty measure
decreases as the agent gains more knowledge about
the user. Consequently, the balance of exploration
and exploitation automatically changes towards ex-
ploitation over time instead of a constant probabil-
ity (e.g., epsilon-greedy). In comparison, Thomp-
son Sampling chooses the action with the highest ex-
pected reward by estimating the reward distribution
based on Bayesian inference (Agrawal and Goyal,
2012). These strategies are fundamentally rooted in
the concept of the bandit problem (Sutton and Barto,
2018).
Multi-armed bandit (MAB) algorithms assume a
simplified RL setting. The basic idea of MABs can be
explained in analogy to slot machines in casinos (also
called “one-armed bandits”). Assuming that there are
multiple bandits in a row with different reward distri-
butions, the general goal is to explore and then exploit
the bandit that maximizes payoff. Problems where
these reward distributions can change over time (e.g.,
patient preferences change), are referred to as non-
stationary (Mintz et al., 2020). MABs can be fur-
ther classified into algorithms that do not consider
the context at all (i.e., stateless, just learn an action-
reward relationship) and contextual MABs that do.
Contextual MABs (CMABs) rely on building a pre-
dictive model (e.g., logistic regression) for estimating
the arm’s value based on the context (Li et al., 2010).
Overall, MABs and CMABs assume a one-step deci-
sion horizon and thus only maximize the immediate
reward. In contrast, multi-step RL (“full RL”) as-
sumes that a decision for an action can also impact
Unleashing the Potential of Reinforcement Learning for Personalizing Behavioral Transformations with Digital Therapeutics: A Systematic
Literature Review
231
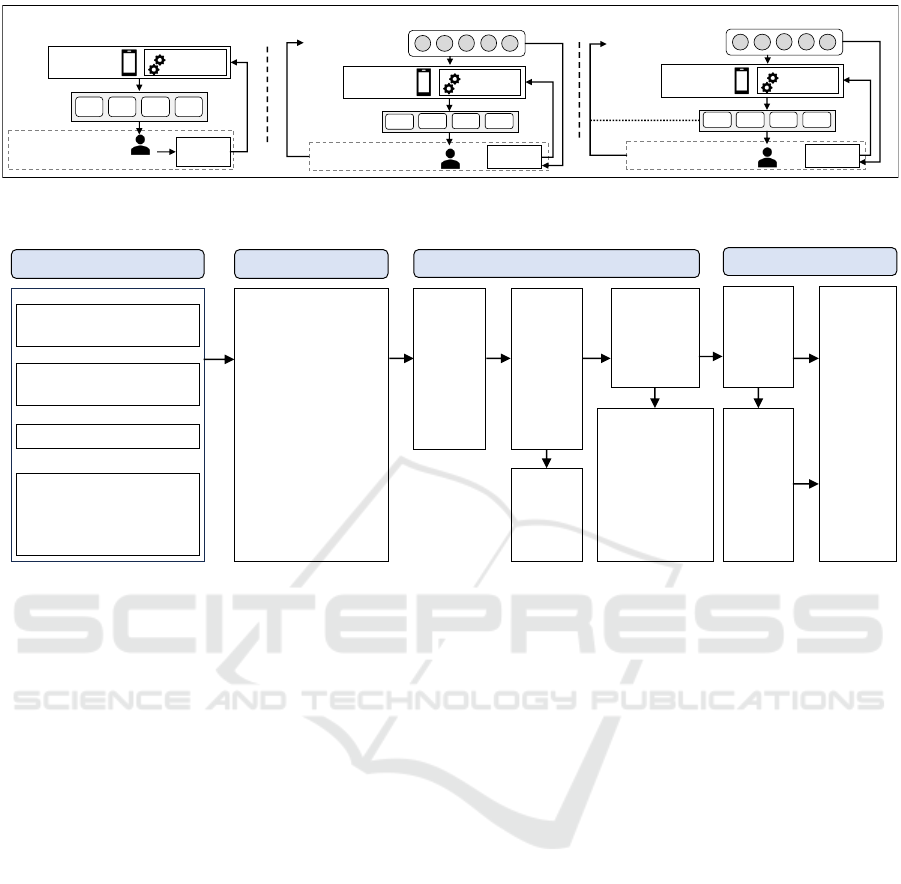
Model
Agent
(DTx App)
Multi-Armed Bandit Contextual Multi-Armed Bandit
Reinforcement Learning
A
1
A
2
A
3
A
n
Environment
Reward
Patient
Actions
(Recomm.)
Contextual State
(Features)
Environment
Reward
Model
Agent
A
1
A
2
A
3
A
n
Contextual State
(Features)
Environment
Reward
Model
Agent
A
1
A
2
A
3
A
n
Figure 1: Multi-Armed Bandits (MABs), Contextual-Multi-Armed Bandits (CMABs) and the Full-Reinforcement Learning
(RL) problem (based on McInerney et al. (2018) and Kaelbling et al. (1996)).
Personalization
Identification Screening
Search Terms
(Title, Abstract, (Keywords); AND combined)
Health
Reinforcement Learning
Information & Communication
Technologies
Records identified from
Databases (n = 1242)
• PubMed (n = 174)
• Scopus (n = 702)
• Web of Science (n = 366)
Filter: Journal Articles and
Conference Papers,
Language: English, 2010 –
2023
Other sources:
• RL papers included in the
literature r eview by
Oyebode et al. (n = 3)
Records
after
duplicates
removed
(n = 764)
Included
Records
excluded
(n = 679)
Records
screened
(n = 764)
Full-text articles
assessed for
eligibility
(n = 85)
Full text-articles
excluded, with
reasons (n = 59)
• E1: (n = 5)
• E2: (n = 6)
• E5: (n = 7)
• E6: (n = 7)
• E7: (n = 15)
• E8: (n = 6)
• E9: (n = 13)
n = 42
papers
included in
the review
(n = 36
independent
studies)
Additional
records
identified
through
citation
chaining
(n = 16)
"personali*" OR "tailor*" OR "adapt*" OR
"custom*" OR "individual* " OR "cont extual*"
OR "context aware*" OR "context sens*"
OR "proactive*" OR "just in time"
"health*" OR "therap*" OR "medical" OR
"clinical" OR "rehabilitation" OR "behav ior
change" OR "behaviour change" OR
"fitness" OR "wellbeing" OR "wellness"
"reinforcement learning" OR "bandit*"
"digital" OR "techn*" OR "mobile" OR "app"
OR "application" OR "smartphone*" OR
"phone*" OR "electronic " OR "web" OR
"int ernet" OR "information system*" OR
"int elligent syst em*" OR "recommender
system*” OR "support system*" OR "tutoring
system*" OR "virtual coach*" OR "e-coach*"
OR "virtual assistan*"
n = 26
Figure 2: Flow diagram of the literature search and selection process (based on PRISMA (Moher, 2009)).
the choice of subsequent decisions and influence the
state. For example, when the intelligent agent has
prompted the patient to go out for a walk in the morn-
ing, the same recommendation for a second activity
in the afternoon might not maximize the long-term
cumulative reward as the patient could become bored
(Philipp et al., 2019). Figure 1 illustrates the differ-
ences between MABs, CMABs, and the full RL prob-
lem.
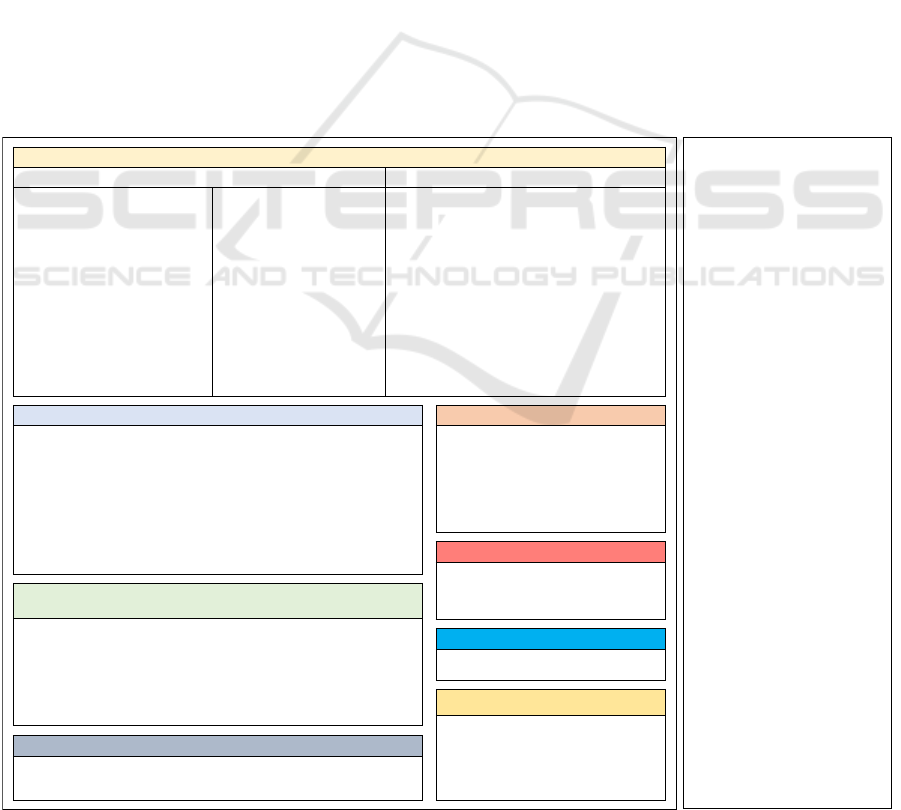
3 METHOD
For addressing the formulated research questions, a
systematic literature review based on the methodol-
ogy by Webster and Watson (2002) and the PRISMA
guidelines (Moher, 2009) was conducted. For the lit-
erature search, the databases Scopus, Web of Science,
and PubMed were selected. The search query (see
Figure 2) was composed of terms describing the con-
cept of personalization, health, reinforcement learn-
ing, as well as digital technologies and delivery chan-
nels (concatenated with an “AND” operator). Two au-
thors were involved in the overall literature search and
analysis process. The literature search was initially
conducted in February and was updated at the begin-
ning of November 2023. The scope of the literature
search was limited to the fields of title, abstract, and
keywords (only Web of Science and Scopus). Only
peer-reviewed journal articles and conference papers
written in English and published between 2010 and
2023 were included in the review. Overall, the liter-
ature search in databases yielded 1242 results. Ad-
ditionally, three studies were included that employed
RL algorithms and were identified in the related work
by Oyebode et al. (2023). After duplicate removal,
the 764 remaining papers were screened for relevance
to the research goals based on title and abstract. Af-
ter screening for relevance, the full text was screened.
Due to the still low maturity of this research topic,
we decided also to include study protocols. How-
ever, we excluded review articles (E1), articles that
did not focus on long-term health behavior change
(E2), not report what was exactly personalized using
RL (E3), not describe the algorithmic approach (E4),
not focus (or plan to focus) on an application for the
patient as end-user (E5), not conducted or described
an evaluation of the RL approach (e.g., mere system
description) (E6), and studies that only used a syn-
thetic data set to investigate the RL algorithm (i.e.,
no real-world data including (states), actions and re-
wards) (E7). When related papers described the same
application and had the same research goals, we in-
cluded only the paper(s) with the most comprehensive
evaluation in terms of real-world evidence and ex-
cluded the others (E8). This criterion aims to ensure
HEALTHINF 2024 - 17th International Conference on Health Informatics
232
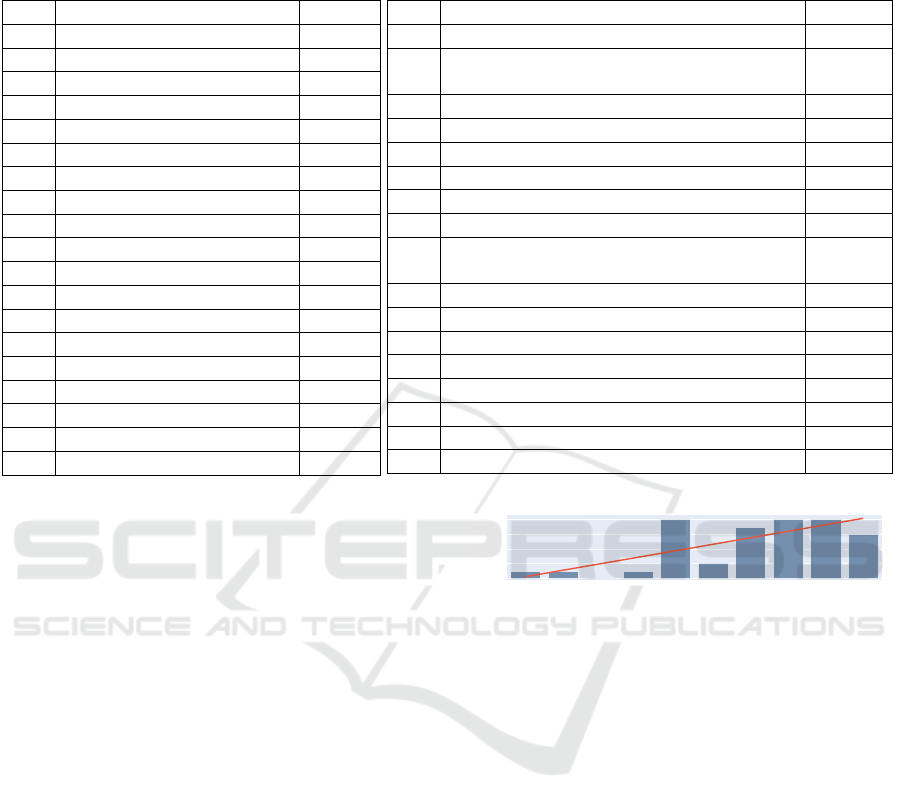
Table 1: Included studies (ST = Stress, PA = Physical Activity, D = Diet, HL = Health Literacy, UE = User engagement, WL
= Weight loss, EM = Emotion/Mood, S = Sleep, MA = Medication adherence, QR = Questionnaire response rate, E = Effort,
SAR = Substance abuse risk).
ID Ref. Opt.
S1 Paredes et al. (2014) ST
S2 Rabbi et al. (2015) PA, D
S3 Yom-Tov et al. (2017) PA
S4 Zhu et al. (2018a) PA
S5 Zhu et al. (2018b) PA
S6 Zhu et al. (2018c) PA
S7 Rabbi et al. (2018) PA
S8 Zhou et al. (2018a,b) PA
S9 Delmas et al. (2018) HL
S10 Gonul et al. (2018) UE
S11 Pelle et al. (2019, 2020) UE
S12 Forman et al. (2019) WL
S13 Mintz et al. (2020) PA
S14 Liao et al. (2020) PA
S15 Daskalova et al. (2020) S
S16 Gasparetti et al. (2020) WL
S17 Etminani et al. (2021) MA
S18 Lauffenburger et al. (2021) MA
S19 Figueroa et al. (2021) EM
ID Ref. Opt.
S20 Zhu et al. (2021) PA
S21 Wang et al. (2021a,b),
Sporrel et al. (2022)
PA
S22 Tomkins et al. (2021) PA
S23 Hu et al. (2021) QR
S24 Di et al. (2022) HS
S25 El Hassouni et al. (2022) UE
S26 Piette et al. (2022) HS
S27 Ameko et al. (2020), Beltzer et al. (2022) EM
S28 Aguilera et al. (2020),
Figueroa et al. (2022)
PA
S29 Bertsimas et al. (2022) PA
S30 Albers et al. (2022) E
S31 Zhou et al. (2023) UE
S32 Piette et al. (2023) SAR
S33 Tragos et al. (2023) UE
S34 Tong et al. (2023) ST
S35 Gray et al. (2023) PA
S36 Kinsey et al. (2023) HS
that our review is based on the most robust and exten-
sive research available. However, related papers pro-
viding complementary insights for our research ques-
tions were included. Finally, papers that did not fit
within the scope of our review were excluded (E9).
In total, n = 26 papers were identified from the
database search and satisfied the selection criteria.
To identify additional studies, a forward and back-
ward search was conducted based on these papers, re-
sulting in the identification of 16 additional papers.
Papers were categorized as part of the same overar-
ching study (denoted as S) when both the medical
use case and the algorithm employed remained un-
changed. Overall, the final analysis set comprised 42
papers referring to 36 studies (see Table 1). Figure 2
illustrates the overall literature search process.
4 RESULTS
4.1 General Characteristics
When conducting a descriptive analysis regarding the
time of publication of the analyzed papers, it becomes
evident that there has been a growing interest regard-
ing RL for personalizing health behavior change in-
terventions (see Figure 3).
When taking a look at the study participants based
on the origin of the training data, n = 18 studies inves-
2014 2015 2016 2017 2018 2019 2020 2021 2022 2023
0
2
4
6
8
Year
Frequency
Figure 3: Number of publications by year.
tigated or plan to investigate the algorithm in the gen-
eral population (see Table 2). Although DTx gener-
ally go beyond primary prevention, the findings from
these studies remain significant for their advancement
due to the limited number of papers available and the
emerging nature of the field. However, several studies
also investigated RL algorithms with data from peo-
ple with hypertension (n = 4), diabetes type 2 (n = 4),
anxiety and/or depression (n = 3), overweight/obesity
(n = 3), chronic pain (n = 2), asthma, osteoarthritis,
opioid-abuse risk or tobacco-smoking behavior (n =
1).
Regarding the delivery platform of the RL-based
intervention (see Table 3), a significant amount of
studies (n = 15) delivered the intervention via a mo-
bile app. However, several studies (n = 13) also used
data collected in prior mHealth studies from elec-
tronic medical records (EMRs) or an online weight
loss platform and used it to train the RL algorithm
in a simulation testbed. Further delivery platforms
were web applications (n = 5), SMS (n = 2), phone
calls (automatic interactive voice response calls and
Unleashing the Potential of Reinforcement Learning for Personalizing Behavioral Transformations with Digital Therapeutics: A Systematic
Literature Review
233
by health professionals) (n = 2) or mobile messages
and phone calls (n = 1).
4.2 Optimization Goal (Reward)
By taking the nature of the reward function variables
into account, the optimization goals could be broadly
classified as health- and system-usage-related. Both
health- and usage-related goals were operationalized
subjectively (e.g., perceived easiness (S7), motivation
(S20), subjective stress assessment (S1)) or through
more objectively gathered measures (e.g., step count
(S4, S5, S6, S22), minutes of activity performance
(S3, S7), questionnaire completion (S23)). Interest-
ingly, one study (S12) considered economic aspects
in the reward function by taking into account the time
a therapist spends on an intervention as an alternative
to solely computer-generated messages.
For the present literature analysis, the research
was analyzed based on the nature of the immediately
underlying (i.e., proximal) constructs that were oper-
ationalized in the reward function. For example, even
though an app designed to promote physical activity
often also leads to weight loss, it was assigned to the
”physical activity” category in this study unless body
weight was also operationalized.
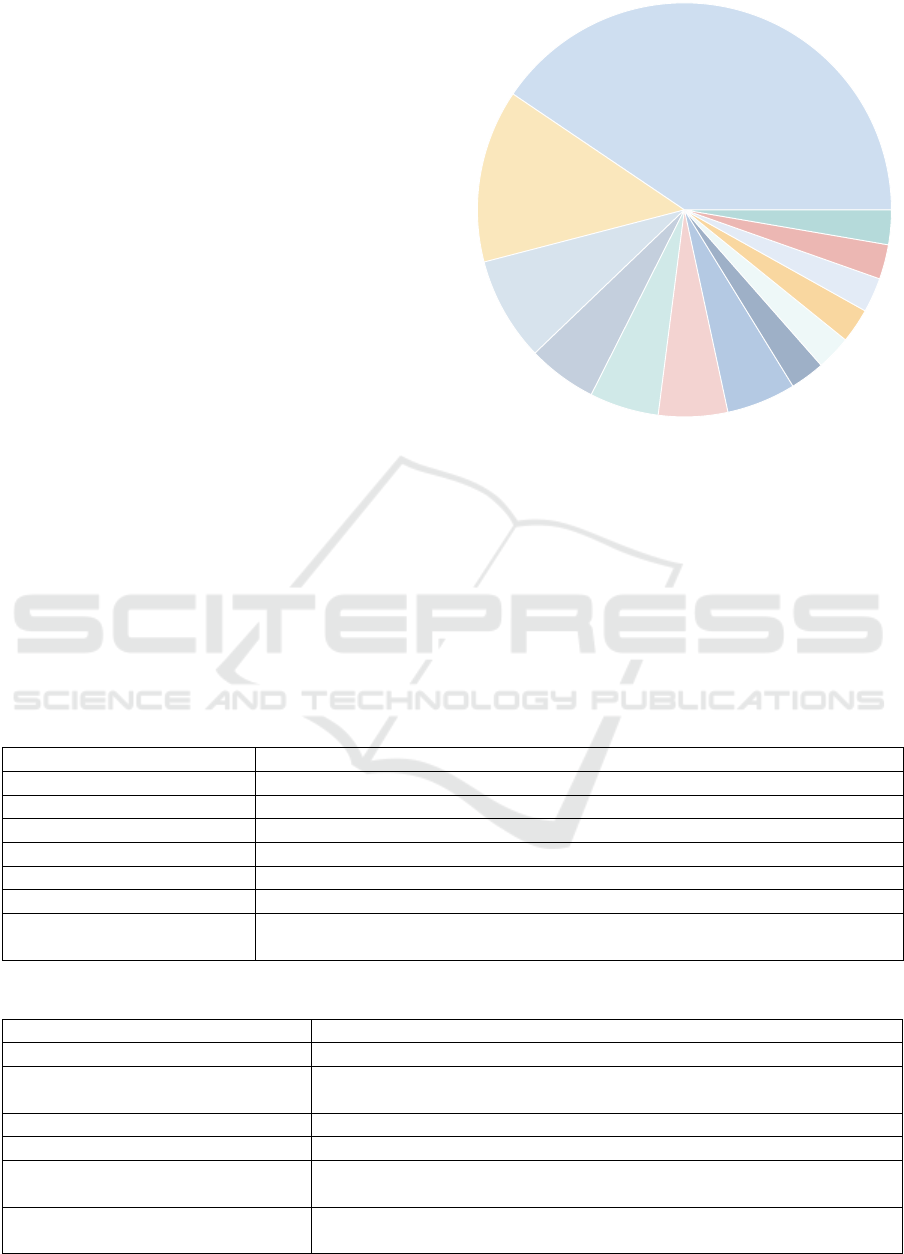
As depicted in Figure 4, a significant proportion of
the analyzed studies (n = 15) focused on optimizing
the physical activity of the patient using correspond-
ing reward measures. It should be noted that the re-
Physical Activity (n = 15)
User Engagement (n = 5)
Health status (n = 3)
Emotion/Mood (n = 2)
Medication Adherence (n = 2)
Stress (n = 2)
Weight loss (n = 2)
Diet (n = 1)
Effort (n = 1)
Health Literacy (n = 1)
Questionnaire response rate (n = 1)
Sleep (n = 1)
Substance abuse risk (n = 1)
Figure 4: Studies by optimization goal.
ward of the agent can be also composed of multiple
variables (e.g., in an additive or multiplicative man-
ner). For example, in S7, a reward score was calcu-
lated by multiplying the perceived easiness (daily sur-
vey) and minutes of activity. Likewise, n = 3 studies
aimed to optimize the patient’s more holistic health
status and therefore combined multiple variables ex-
pressing different dimensions (e.g., physical activity,
health literacy, pain, HbA1c reduction, quality of life)
(S24, S26). Other studies aimed to optimize user
Table 2: Overview of the user data populations.
Population of user data Studies
General population (n = 18) S1, S2, S4, S5, S6, S8, S10, S15, S16, S19, S20, S21, S25, S28, S33-S36
Patients with hypertension S14, S17, S22, S29
Patients with type 2 diabetes S3, S18, S24, S28
Anxiety and/or depression S23, S27, S28
Overweight/obese people S12, S13, S31
Patients with chronic pain S7, S26
Other Children with asthma (S9), Patients with osteoarthritis (S11), Tobacco smoking
people (S30), Patients with opoid-abuse risk (S32)
Table 3: Overview of the employed RL-based intervention delivery platforms.
Intervention delivery platform Studies
Mobile App (n = 15) S1, S2, S7, S8, S10, S11, S15, S17, S18, S21, S22, S25, S28, S33, S36
Simulation testbed with data from
mHealth study (n = 11)
S4, S5, S6, S13, S14, S16, S21, S22, S23, S27, S29
Web application S9, S20, S30, S34, S35
SMS S3, S19
Phone calls (automated and by hu-
man)
S26, S32
Other Simulation testbed with data from EMR (S24), data from online weight
loss platform (S31); Mobile messages and phone calls (S12)
HEALTHINF 2024 - 17th International Conference on Health Informatics
234
Multi-Armed-Bandits (MABs)
n = 8
Contextual-Multi-Armed-Bandits (CMABs)
n = 17
Full-Reinforcement Learning (Full-RL)
n = 11
• Thompson Sampling (S15)
• UCB (S12)
• EXP3 (S2)
• Knapsack using 𝝐-Greedy (S7)
• ZPDES (S9)
• ROGUE UCB, 𝝐-ROGUE (S13)
• Shapley Bandits (S35)
• Unspecified (S20)
• Linear Thompson Sampling (S14, S22,
S28, S31)
• LinUCB (S11, S26, S32)
• Random Forest with UCB (S1, S34)
• Binary classificator/logistic regression
(off policy learning) (S17, S27)
• Linear regression (S3, S19)
• GLMM-based (S23)
• Microsoft Personalizer (S18)
• Optimal Policy Trees + (OPT+) (S29)
• Robust Actor-Critic (S4)
• Q-Learning (S16, S24, S30)
• LSTDQ (S5, S25)
• REINFORCE (S21)
• Online-Actor Critic LSTDQ (S6)
• Proximal Policy Optimization
Algorithm (PPO) (S33)
• SARSA (with adaptations) (S10)
• Behavioral Analytics Algorithm
(BAA) (S8)
• Deep Q-Networks (DQN) (S36)
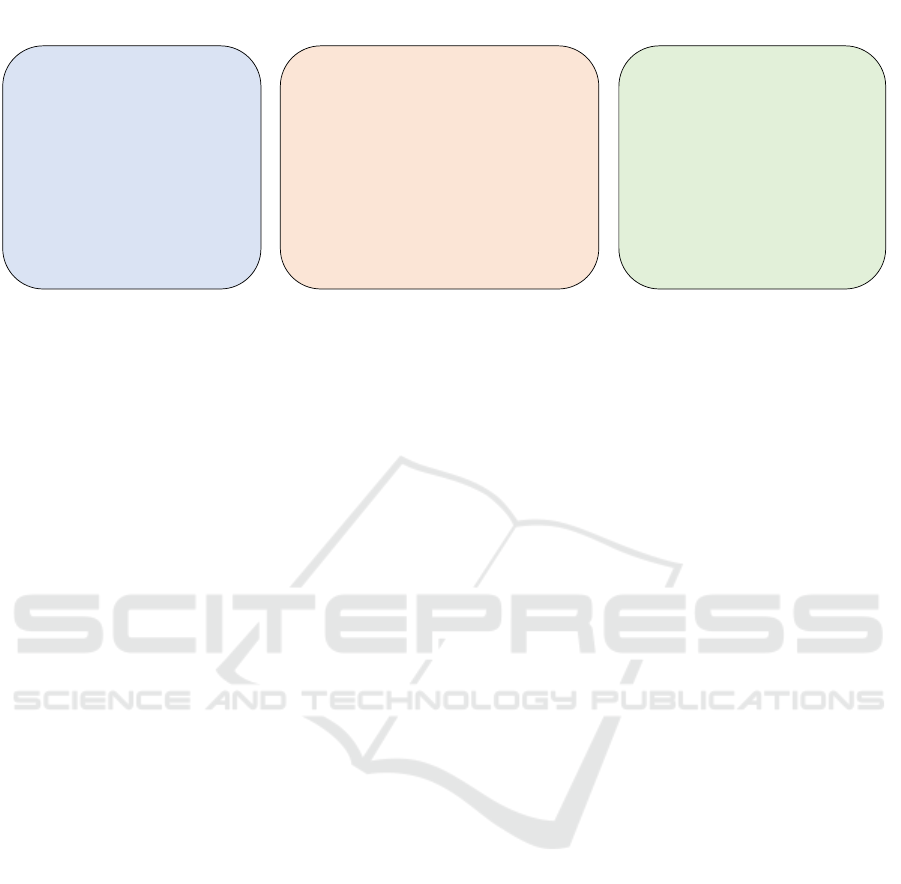
Figure 5: Overview of the reinforcement learning algorithms used.
engagement (n = 5), emotion/mood (n = 2), weight
loss (n = 2), medication adherence (n = 2), stress (n
= 2), dietary change, health literacy, substance abuse
risk, questionnaire response rate, the user’s effort, and
sleep (n = 1).
4.3 Used Reinforcement Learning
Algorithms
The majority of the studies (n = 25) employed ban-
dit algorithms (esp. CMABs or MABs), while the
remaining studies (n = 11) employed algorithms de-
signed to address the complete RL problem (see Fig-
ure 5). Overall, the studies varied in their level of
detail when describing the algorithm, which made it
challenging to classify and directly compare them.
MABs: The arguably most straightforward and
simplified approach to address the RL problem are
MABs, which have been investigated in n = 8 studies.
The study by Rabbi et al. (S2) was one of the pio-
neering studies in this field and used the EXP3 bandit
algorithm. A promising approach regarding the use
of RL in chronic disease scenarios was described in
a later study by the same authors (S7) and is referred
to as “bandits with knapsacks”. The general idea of
this approach is to set an upper activity bound (e.g.,
60 minutes) and select a set of recommendations that
meet this constraint in total. According to the study’s
authors, the reason behind this approach is that the
therapeutic goal is not necessarily focused on pro-
moting maximum activity but rather on consistently
adhering to a predetermined goal.
Mintz et al. (S13) proposed another sophisticated
technique called ROGUE-bandits, utilizing UCB and
ε-greedy strategies, specifically designed for non-
stationary scenarios. In these settings, it is assumed
that rewards can vary over time due to habituation
phenomena, such as the patient’s changing prefer-
ences during the intervention. If a particular inter-
vention option is delivered excessively, the patient’s
receptiveness to this action may decrease. However,
over time, the intervention option can “recover”, and
the patient becomes receptive again.
In the study S9, a bandit algorithm was applied
that originated from the field of pedagogical agents
called “Zone of Proximal Development and Empiri-
cal Success” (ZPDES). The approach explores an ac-
tivity graph using a hierarchical structure comprising
multiple layers for determining the health educational
theme (e.g., “my body”, “my tips”), the level of diffi-
culty, and the type of delivery (e.g., game, quiz).
A promising approach from an ethical point of
view was recently described in S35 and is called
”Shapley Bandits”. The algorithm implements fair-
ness constraints to optimize the group performance in
an exergame that presents social comparison rankings
to the user. The remaining studies used Thompson
Sampling or UCB. It is noteworthy that the reported
exploration rates (ε) vary, ranging from 1% (due to an
upfront forced exploration, see S35), 10% (S2) up to
20% (S7).
CMABs: Almost half of the analyzed studies (n
= 17) used contextual bandits. Among these pa-
pers, Linear Thompson sampling was commonly em-
ployed (n = 4). In addition, several papers utilized lin-
ear regression with Boltzmann-Sampling (S3, S19),
a random-forest regression algorithm with UCB (S1,
S34), while other studies adapted generalized lin-
ear mixed models (S23) or trained a binary classi-
fier/logistic regression in an offline-fashion with his-
toric data and employed the doubly robust estimator
(S17, S27). One noteworthy approach described in
S18 aimed to leverage “Microsoft Personalizer”, a no-
code configurable CMAB, for their study. Another
distinctive approach was proposed in S4, where an
Actor-Critic bandit was investigated, which consists
of two components (actor and critic). While the ac-
tor learns the actual policy and thus determines the
actions based on the context, the critic component as-
Unleashing the Potential of Reinforcement Learning for Personalizing Behavioral Transformations with Digital Therapeutics: A Systematic
Literature Review
235

sesses the policy’s value to drive its update.
For constructing a CMAB, one may train one
model for each arm (i.e., action) as proposed in the
commonly cited LinUCB algorithm (Li et al., 2010).
In contrast, Yom-Tov et al. (S3) considered a con-
catenated feature vector (Kesler construction) encom-
passing the context and the actions for predicting
the change in activity for each action with only one
model.
Full-RL: A comparatively small amount of stud-
ies (n = 11) used algorithms designed to address full
RL problems. Among these studies, several employed
Q-learning, which is widely recognized as one of
the most well-known and traditional RL approaches
(Watkins and Dayan, 1992). The study S16 used
the simplest form with Q-tables, while S24 employed
Gradient Boosted Regression Trees to estimate the Q-
function. In the study S25, the LSTDQ algorithm was
used, which can be considered a special type of Q-
learning. Building on LSTDQ, the study S6 proposed
an online Actor-Critic algorithm .
The studies by Wang and colleagues (S21) used
the “REINFORCE” algorithm that directly estimates
the policy (i.e., policy-based). Q-learning algorithms,
in contrast, estimate the value of a state-action pair
and thus estimate the policy indirectly (i.e., value-
based) (Watkins and Dayan, 1992). Notably, the pro-
posed approach by Wang et al. incorporated a con-
straint in the algorithm on the maximum reminders
per week to avoid intervention fatigue on the patient’s
side by sending too many reminders.
Another distinctive approach was described in
S8, where the so-called “Behavioral Analytics Algo-
rithm” was proposed combining “inverse RL” and tra-
ditional RL. Inverse RL is used to derive parameters
of the patient which are the user’s baseline level of
steps, the user’s responsiveness to the step goal, and
the user’s self-efficacy. Afterward, the estimated pa-
rameters are used to conduct a direct policy search by
solving a mixed integer linear program to get an opti-
mal step goal (RL step).
Remarkably, one study used deep neural networks
with RL (”deep RL”) and extended the DQN algo-
rithm (S36). Other algorithms reported in the litera-
ture were proximal policy optimization (PPO) (S33)
and SARSA (S10).
Personalization Approaches: The described al-
gorithms have been used with data collected at differ-
ent levels to enable personalization. Broadly speak-
ing, three approaches are discussed in the literature
that are a “one model for all” or “pooled” data ap-
proach (e.g., S1, S3, S18, S19, S24, S26), a “group-
driven” or “clustered” approach (e.g., S5, S6, S25)
and a “separate” or “N-of 1” approach (e.g., S2,
S7, S17). However, not all papers have explicitly
described on which level they used the data, leav-
ing room for interpretation and making categorization
and quantitative evaluation difficult. In general, all
three approaches have benefits and drawbacks. While
training one model for all patients by making use of
their entire pooled data accelerates the learning pro-
cess, the personalization may be too broad and not
precisely enough for the individual. The underlying
assumption of this approach is that the “best action”
is unknown, but users tend to be homogeneous in their
responses. On the other hand, building a separate
model for each user addresses their heterogeneity but
significantly slows down the learning process due to
the sparsity and noisiness of the data. Nonetheless,
the ability to build a model for the user’s local device
(i.e., decentralized) can have advantages in terms of
privacy (Rabbi et al., 2015).
Balancing Between Pooling and Separation:
Recent literature proposed sophisticated approaches
to handle these two extremes. Therefore, RL is
frequently combined with unsupervised learning ap-
proaches. A comparatively straightforward approach
is described in the studies S5 and S25 where the k-
means clustering algorithm was used to build groups
of similar users and then a model was learned for each
group. For dealing with the longitudinal character
of the data, the study S25 used the Dynamic Time
Warping approach for calculating the similarities (dis-
tances) between the users. Another approach is dis-
cussed in S6, where a network graph of users was
built, and then the k-nearest neighbors algorithm was
applied. In contrast to the mentioned approaches, the
so-called “IntelligentPooling” approach by Tomkins
et al. (S22) adaptively pools more data if homogene-
ity is observed in the data, while it adapts to a rather
separate approach with reduced pooling when the data
is heterogeneous.
Additional Algorithms: Beyond balancing be-
tween the “personalization extremes”, the analyzed
studies also used additional algorithms for other pur-
poses. For example, the recent study S31 used deep
learning for generating representations (embeddings)
combining static patient attributes along with sequen-
tial patterns (health behavior pathways), which ex-
tract useful information. The user embedding vector
then serves in conjunction with an item embedding
as a recommendation context for a CMAB. In con-
trast to using further algorithms for pre-processing,
additional algorithms may be also placed behind the
RL algorithm. The study by Rabbi et al. (S2) com-
bined human customization with RL-based sugges-
tions. Users are allowed to remove unsuitable sugges-
tions by themselves from a list and re-order sugges-
HEALTHINF 2024 - 17th International Conference on Health Informatics
236

tions according to their individual preferences. Af-
ter the MAB has determined a set of suggestions, a
Pareto-frontier algorithm is triggered. This algorithm
serves to balance the activity recommendations be-
tween the user’s preferences and efforts to perform
the activities to maximize further the likelihood that
the user follows the suggestion.
4.4 Contextual Data Inputs
Overall, those studies that used contextualized algo-
rithms (i.e., CMAB and Full-RL) integrated a variety
of variables which have been mainly measured via ac-
tive and passive sensing approaches.
Actively sensed contextual data comprised self-
reported data based on questionnaires such as the pa-
tient’s mood (e.g., S1, S25), pain intensity (e.g., S26),
the motivation to change or location (e.g., S27).
In contrast, passively sensed input data referred
to the physical activity behavior using the pedome-
ter or acceleration sensor (e.g., S1, S8, S14, S22,
S27), the GPS sensed location (S1), system use ad-
herence variables (esp., time since last smartphone
lock event (S1), number of notifications received/read
(S25), number of app screens (S14), number of times
the app has been opened (S23)), health adherence
variables (S18), the intervention history (S3) or vari-
ables that were directly derived from the underlying
operating system (S1). Beyond actively or passively
sensed data on the user’s side, one study used data
from the EMR of the patient (e.g., hospital admis-
sions, emergency room visits,. . . ), i.e., data generated
and collected by health professionals (S24).
Several studies distinguished between baseline
variables (e.g., age, gender, user traits and prefer-
ences) that do not change during the course of in-
tervention and dynamic variables (e.g., S1, S3, S11,
S17, S18). Dynamic variables broadly referred to
the health-behavior, health-status and system usage-
related metrics, the intervention history but also envi-
ronment variables such as time of day, current week-
day or weather (e.g., S1, S14, S25, S27). In particular,
the intervention history could be expressed in terms of
which intervention was delivered and when it was de-
livered (e.g., S3, S14, S17, S18, S19, S21, S22, S23,
S31). For example, Yom-Tov et al. (S3) included the
recommendation history as a contextual variable in
terms of the number of days since each message cate-
gory was sent. The justification for this strategy is to
repetitively use the same messages if deemed advan-
tageous by the learning algorithm or introduce more
diversity. Similarly, other studies (e.g., S17, S18,
S19, S31) incorporated the intervention history into
the model (e.g., time when message was sent, days
since message was sent, or interventions performed
in the past). For incorporating the users’s interven-
tion receptivity, the studies S1 and S21 accessed the
user’s calendar to obtain data such as the number of
free calendar records or the time until next meeting.
4.5 Objects of Personalization
For categorizing the studies regarding the objects of
personalization, we followed the ”Behavioral Inter-
vention Technology Model” by Mohr et al. (2014).
Based on this model, we derived the categories of per-
sonalizing the type of intervention (what?), the timing
(when?), the intervention goals (why?), and the deliv-
ery channel or representation format (how?). Figure 6
depicts an overview of the objects of personalization
found in the literature.
Type of Intervention: Regarding the objects of
personalization, almost half of the analyzed studies (n
= 17) used the RL algorithm to select an intervention
type from a set of alternative actions. A common ap-
proach is to categorize the intervention options such
as groups of activities or message types (e.g., S1, S17,
S19). The RL algorithm then typically determines the
intervention category from which a pre-formulated
content instance (e.g., message) is randomly drawn.
These categorizations of messages are often guided
by theoretical and empirical findings from the field of
health behavior change (e.g., S1, S3, S18, S19, S28).
Beyond traditional media content (i.e., text, images,
audio, or video), intervention options could be also
games, questionnaires, or quizzes. However, a dif-
ferentiated approach can be found in the study S20,
where RL was used to optimize social comparison
rankings (upward, downward, mixward) to stimulate
physical activity. It should be noted that studies that
used the terms “goals” or “challenges” were also as-
signed to this category if it was apparent that certain
behavioral activities to achieve the goal were directly
associated (e.g., S11, S31).
Timing: By integrating a “no intervention” or
“provide nothing” action into the action space, the
problems of finding the “best” intervention and time
to deliver the intervention can be implicitly framed in
one model. Overall, n = 6 studies have been identi-
fied which include a ”no intervention” action of the
agent among several others. If only one type of in-
tervention exists, this “hybrid” approach is framed as
a binary problem (intervention vs. no intervention)
(n = 7). Another option to address the timing prob-
lem is to include possible time windows into the ac-
tion space (e.g., 3 categories: 9 a.m. - 12 p.m.; 12
- 3 p.m.; 3 - 6 p.m.) (see S19, S23). It should be
noted that although RL can be used to determine suit-
Unleashing the Potential of Reinforcement Learning for Personalizing Behavioral Transformations with Digital Therapeutics: A Systematic
Literature Review
237
• Text/Image/Audio/Video content (S1,
S2, S7, S11, S13, S15, S17, S19, S24,
S27, S31, S33, S34, S36)
• Social comparison ranking (S20, S35)
• Questionnaires/Quizzes (S9, S36)
• Games (S9)
• Categorized time windows
(S19, S23)
• Intervention vs. no-
intervention (S4, S5, S6, S10,
S14, S21, S29)
• Numeric step goal (S8)
• Categorized step goals
(S16)
• Automated text message vs. Text-
exchange vs. Phone-call by therapist (S12)
• Interactive voice response call vs. phone
call by therapist (different durations) (S26,
S32)
• Complexity/level of difficulty (S9)
Type of Intervention (n = 17)
?
Timing of Intervention (n = 9)
Goal values (n = 2)
Delivery Channel/
Representation (n = 4)
• Multiple intervention options + no intervention
(S3, S18, S22, S25, S28, S30)
(n = 6)
Figure 6: Overview of the objects of personalization.
• RL vs. Random (S1, S2, S7, S19, S20, S21, S28, S35)
• RL vs. Standard care (S11, S12, S17, S18, S26, S32)
• Observational study (S2, S11, S17, S21, S25)
• RL vs. Static (S3, S8, S10, S36)
• Micro-randomized trial (S14, S22, S28)
• RL vs. Random vs. None (S34)
• RL vs. Other RL Algorithms (S30)
• RL vs. Other Non-RL Algorithms (S33)
• Pre-Post Study (S9)
• Comparison of different intervention lengths (S15)
• Comparison of different personalization levels (S5, S6, S22, S23)
• RL vs. Other RL Algorithms (S13, S14, S29)
• RL vs. Random vs. Other Algorithms (S16, S21)
• RL vs. Other RL and Non-RL Algorithms (S31)
• RL vs. Standard care (S24)
• RL vs. Static (S4)
• Comparison of metrics to prior reference values (S23)
• Comparison of different constraint integration approaches (S21)
• Comparison of different grouping of actions (S27)
• Comparison of different estimators for offline policy learning (S27)
Real-World Studies (n = 25) Simulation testbed evaluations (n = 13)
Figure 7: Overview of the employed evaluation approaches.
able timings, the algorithm itself still needs to be in-
voked at certain times. These “decision points” are
often pre-defined times of a day/week or repeating
intervals (Nahum-Shani et al., 2018). For example,
the algorithm described in S3 is triggered each morn-
ing while other studies invoke the algorithm multiple
times a day (e.g., S14) or in specific intervals (e.g.,
S21). The time horizon between the triggers could be
also larger than at the daily level (e.g., weekly (S26)
or monthly (S24)). Beyond system-initiated timings,
the algorithm could be also triggered by the user (e.g.,
S20).
Goals: A comparatively small amount of studies
(n = 2) investigated the use of RL to optimize goal pa-
rameters in terms of specific goal values (daily steps).
Therefore, the approach described in S8 uses inverse
RL to predict a challenging but attainable number of
daily steps that satisfy restrictions related to the user’s
responsiveness and self-efficacy. In contrast, the ap-
proach proposed in S16 recommends a range of steps
drawn from 5 categories.
Delivery Channel/Representation: Some stud-
ies used the RL algorithm to determine the interven-
tion delivery channel or representation of the content
(how?). For example, the studies S12, S26, and S32
balanced between automated text messages or calls
and human interventions by therapists. Noteworthy,
the study S26 integrated the intervention duration into
the action space, leading to a “hybrid” model address-
ing also the time aspect of the intervention (duration).
In contrast, the study S9 used RL to adapt the com-
plexity (difficulty) of the presented content.
4.6 Evaluation Approach
The evaluation approaches described in the analyzed
studies were broadly categorized as real-world stud-
ies (i.e., conducted with human subjects) and testbed
evaluations in a computer-simulated environment (see
Figure 7).
Real-World-Studies: Overall, n = 25 studies
were conducted or planned in real-world settings, in-
cluding an experimental comparison of RL vs. Ran-
dom policy (typically uniform sampling), RL vs.
Static policy (fixed schedule of intervention delivery
or fixed type of message), RL vs. Standard care,
different advanced algorithms (RL and non-RL), the
investigation of different intervention lengths (using
RL), and a pre-post-study. Three studies described the
use of the RL algorithm in a micro-randomized trial,
which is a study design originating from the field of
JITAIs (Nahum-Shani et al., 2018) for getting insights
into the optimal timing, context, and effectiveness of
intervention options. Furthermore, several studies re-
ported results from observational studies.
Testbed Evaluations: A significant number of
papers (n = 13) investigated the RL algorithm in a
simulated test environment using real-world datasets
collected in previous studies. The comparisons in-
clude the comparison of different levels of personal-
ization (e.g., pooled-RL vs. separated-RL), the com-
parison of the proposed algorithm to other RL and/or
non-RL algorithm(s), comparison of different catego-
rizations of the action space, comparison of different
constraint integration approaches, comparison of esti-
mators for offline policy learning, comparison to prior
reference values, and RL vs. standard care.
HEALTHINF 2024 - 17th International Conference on Health Informatics
238
A commonly cited data set used for testbed evalu-
ations originates from the so-called “HeartSteps” (v1)
mHealth study (S4, S5, S6, S14, S22, S29). The
HeartSteps v1 study was a 42-day micro-randomized
trial including 44 healthy adults aimed to evalu-
ate context-aware recommendations to reduce seden-
tary behavior. According to Klasnja et al. (2019),
the cleaned data set includes 6061 decision points
linking recommendations to corresponding outcomes
(cause–effect relationship). Likewise, the study S13
also used an interventional data set from a mHealth
trial. Several papers also used data originating from
observational studies. These observational data sets
strongly differed in their sample size (from 114 to
>10.000 participants) and length (5 weeks up to 4
years) (S27, S21). However, as described, e.g., in
S16, inferring causal relationships from mere obser-
vational data comes with limitations, and the deriva-
tion of states-action pairs with their linkage to rewards
is challenging.
4.7 Evaluation Metrics
The evaluation metrics were categorized based on the
conceptualization of engagement in the context of
digital behavior change interventions by Perski et al.
(2017), the taxonomy of health behaviors by Nudel-
man and Shiloh (2015), and the taxonomy of clinical
outcomes proposed by Dodd et al. (2018).
While these categorizations refer to the patient-
centric perspective, an additional category was induc-
tively introduced for metrics directly related to the
RL algorithms (i.e., algorithm-centric). Algorithm-
centric measures were used in n = 17 studies, includ-
ing the average reward, regret, precision, and other
metrics. Patient-centric metrics in terms of deliv-
ery of care and engagement with systems or services
were frequently assessed (n = 23) and involved both
subjective measures (such as patient-reported adher-
ence) and objective measures (such as system-logged
interaction frequency). Exercise, nutrition, quality
of life, health-related knowledge, psychiatric out-
comes, general health measures (like body weight and
Delivery of care & Engagement with the system/services (n = 23)
Objective measures (n = 12)Subjectively collected measures (n = 20)
• Number of interactions with the interventions (e.g.,
clicks, videos, taps on reminders, messages read,
number of chosen goals, etc.) (S11, S15, S17, S21,
S25)
•
Self-reporting behavior (frequency of reports,
response rates) (S19, S23, S25)
•
Number of times the app was opened (S7, S21,
S28)
•
Number of times pillbox was opened (S18)
•
Time spent reading the messages (S28)
•
Logins per week (S11)
•
Reaction time in seconds (S10, S34)
•
Ratio of engaged interventions to total
interventions (S10)
•
Intervention completion time (S34)
•
Perceived easiness (S7)
and
capability following the
interv. (S21)
•
Care experiences (S28)
•
Impression of change (S26)
•
Perceived effort put in
activities (S30)
•
Curiosity (S9)
•
Receptivity (S21)
•
Recommendability of the
app to others (S7)
•
Social support (S28)
•
Social comparison
preference (S20)
•
User experience & usability (S2,
S8, S11, S17, S19, S20, S21, S28,
S34)
•
Followed/adhered to interv. (S2,
S7, S9, S15, S17, S18)
•
Motivation, Enjoyment (S9, S20,
S21, S30, S33, S35)
•
Needs, wishes, opinions (S7,
S15, S17, S21, S28)
•
Perceived
effectiveness/helpfulness (S1,
S3, S7, S12), relatedness (S2)
•
Ratings of the recommendation
(S1, S2, S17)
•
Intention to follow the interv.
(S2,
S7)
Patient-centric (n = 28)
Algorithm-centric (n = 17)
•
(Average) reward (S4, S5, S6, S13,
S14, S21, S25, S27, S32)
•
Regret (S13, S22)
•
Precision (S16, S31)
•
Mean daily number of messages
(per user) proposed by algorithm
(S10, S29)
•
Recall (S31)
•
Accuracy (S16)
•
Adj. R
2
(linear regression) (S3)
•
Agreement between model and
health professional (S24)
•
Similarity of recommendations
(S31)
•
Normalized cumulative gain (S31)
•
Mean average precision (S31)
•
Improvements regarding known
user preferences (S31)
•
Mean squared error of policy
parameters (S23)
•
Value rati o for estimated policy
(S23)
To deeper unders tand t he m odel and t he
learning process, studies also analyzed:
•
Importance of contextual variables
(feature importance) (S3, S27)
•
Treatment selecti on probabil i ties,
action distribution (S22, S25, S32)
•
Changes in parameters over time
(S3, S22)
•
Reward distribution (S25)
• Randomization probability over time
(S14)
•
Posterior mean estimates of
treatment effect over time (S14)
Exercise (n = 14)
•
Total dai l y step count (S8, S12, S20, S28, S35),
•
Self-reported physical activity (S11, S28, S36)
•
Walking/day (min) (S2, S3, S7), Rate of walking (hz/day) (S3)
•
Step goal attainment (S8)
•
Non-walking exercises/day (min) (S7)
•
Avg. 30 min step count after a decision time (S14)
•
Exercise/day (calories) (S2)
•
Physical activity rate (weekly) (S17)
•
Number of activities triggered by a reminder (performance of activity) (S21)
•
Physical activity (accelerometer data) (S21)
•
Sedentary time (S29)
Metabolism and Nutrition (n = 6)
•
Glycemic control (HbA1c) (S3, S18, S24)
•
Calories (each meal, daily) (S2, S12)
•
Self-reported dietary behavior (S36)
Quality of Life (QoL) (n = 4)
•
Overall QoL (S11, S17, S24, S26)
•
Disease-related QoL (S24)
Health-related knowledge (learn and apply),
attitudes and beliefs (n = 7)
•
Health literacy (disease-related or general) (S9, S17, S36)
•
Illness perception (S9, S11)
•
Coping skills (S1)
•
Self-management behavior in terms of patient activation (S11)
•
Application of disease-related self-care activities (S24)
•
“Did you know questions” and gamification tests (S17)
•
Self-efficacy for managing chronic diseases (S28)
•
Treatment bel iefs (S11)
Psychiatric outcomes (n = 6)
•
Depression symptoms (S1, S19, S26,
S28, S34)
•
Emotional state/Mood (S7, S19, S28)
•
Anxiety symptoms (S19, S28, S34)
•
Stress (S1, S34)
•
Sleep disturbance (S34)
•
Loneliness (S28)
•
Behavioral activation (S28)
Resource use (n = 3)
•
Human coaching hours (S12, S26)
•
Number of self-
reported consultations in
healthcare (S11)
•
Direct medical costs (S11)
Other health-related measures / General outcomes (n = 7)
•
Weight loss (S12, S32)
•
Patient’s overall functioning in daily life (S11, S26)
•
Pain (S7, S26), Blood pressure (S17), Sleep behavior (S15)
Figure 8: Overview of evaluation metrics reported in the analyzed studies.
Unleashing the Potential of Reinforcement Learning for Personalizing Behavioral Transformations with Digital Therapeutics: A Systematic
Literature Review
239
Table 4: Overview of interventional real-world studies comparing RL to another (non-RL) condition.
ID Comparison Length, n Key result
S1
RL vs. Ran-
dom
4 weeks,
n = 95
RL-based intervention facilitated greater stress reduction over four weeks
compared to random recommendations
S2
RL vs. Ran-
dom
14 weeks,
n = 16
Significant improvements in participant’s physical activity, consumed
calories, perceived relatedness of suggestions and the number of sugges-
tions followed or wanted tofollow
S7
RL vs. Ran-
dom
5 weeks,
n = 10
RL-based suggestions were easier to follow; differences regarding the
physical activity and reported pain were non-significant; decreasing app
use over time
S20
RL vs. Ran-
dom
3 weeks,
n = 53
No significant increase in physical activity, though motivation levels were
reported higher in the RL group (small to moderate effect size)
S21
RL vs. Ran-
dom
4 weeks,
n = 23
No significant differences in physical activity behavior and perceptions,
opinions, and user experience between the RL and random prompts group
S28
RL vs. Ran-
dom
6 weeks,
n = 93
RL group membership did not significantly influenced the daily steps
S34
RL, Rand.,
Self, None
4 weeks,
n = 69
RL-based and self-proposed interventions had significant higher stress re-
duction than random interventions
S35
RL vs. Ran-
dom
3 weeks,
n = 46
Significant improvement of the user’s motivation in the ShapleyBandit
group compared to random intervention but not to the greedy strategy
S3
RL vs.
Static
26 weeks,
n = 27
RL-based messaging significantly improved physical activity (walking
behavior); significant decrease of HbA1c levels in diabetes patients
S8
RL vs.
Static
10 weeks,
n = 64
RL-based group had a lower decrease of daily steps over time compared
to the static group (10.000 steps/day)
S10
RL vs.
Static
2 weeks,
n = 17
Improvements of adapted SARSA over static policy in terms of number
of engaged interventions and reaction times
S36
RL vs.
Static
6 months,
n = 1049
Improvements of the user’s diabetes-related health literacy, self-reported
physical activity and diet
S33
RL vs.
PSO
15 weeks,
n = 69
Significant effect of RL over particle swarm optimization (PSO) algorithm
on physical activity enjoyment scale
S11
RL vs. Stan-
dard care
6 months,
n = 427
RL-based app did not alter the frequency of consultations, no clinically
relevant improvements of pain or symptoms
S12
RL vs. Stan-
dard care
12 weeks,
n = 52
The RL-based conditions (individual- and group optimized) achieved
equivalent weight loss results with reduced human coaching hours com-
pared to the solely human delivered intervention
S26
RL vs. Stan-
dard care
10 weeks,
n = 278
RL-based selection of delivery mode led to improvements in pain scores
with less therapist time
S32
RL vs. Stan-
dard care
12 weeks,
n = 228
Improvements of the opioid-abuse risk score, particularly for patients with
the highest risk at the start of the study
blood pressure), and economic resource use were also
evaluated, highlighting a comprehensive and multi-
faceted approach to assessing RL-based health behav-
ior change interventions. Figure 8 summarizes the
identified evaluation metrics.
4.8 Evidence
Table 4 provides a summary of interventional real-
world studies comparing RL to a non-RL condition
in order to obtain a general view of the overall evi-
dence (n = 17). It is noteworthy that bandit algorithms
were used in n = 12 of the 17 real-world studies pre-
sented. Overall, the RL algorithms have been com-
pared to random policies, static policies (e.g., always
sending the same message or sending a message at
the same time), other algorithms, or to the standard
care involving human therapists. Despite mixed re-
sults, several studies could demonstrate the potential
to improve existing traditional or digital interventions.
5 DISCUSSION
5.1 Principal Findings
The high-level findings of this literature review are
summarized in a framework for integrating RL within
HEALTHINF 2024 - 17th International Conference on Health Informatics
240
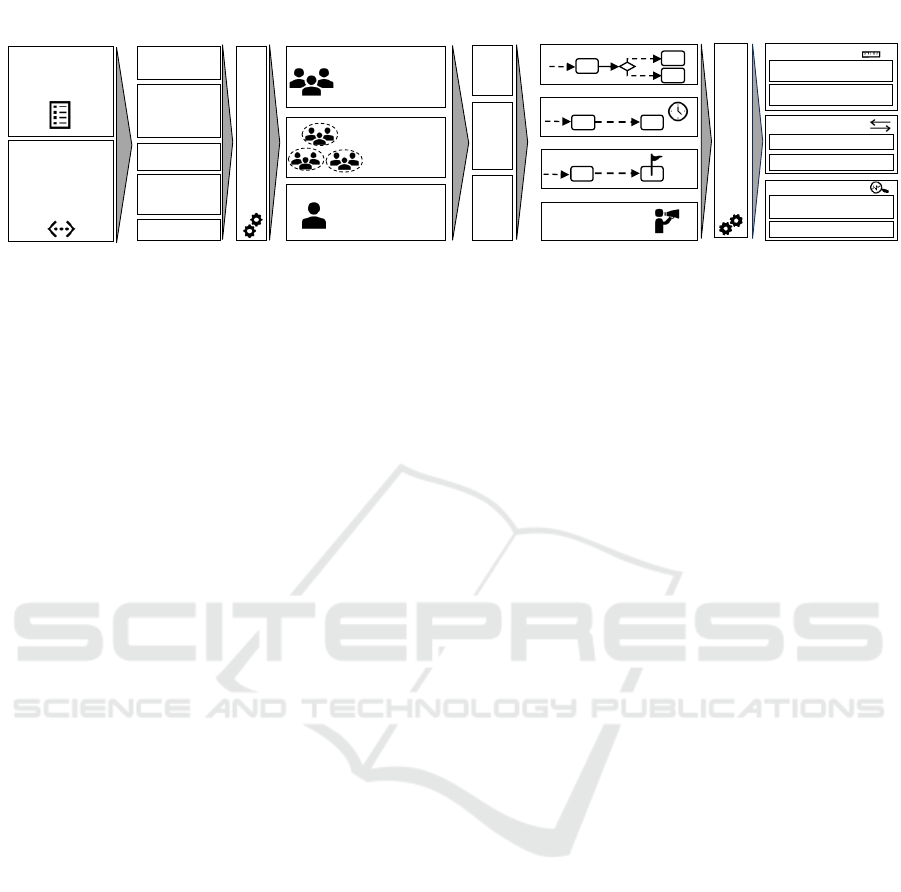
Pooled Data
Individual
Data
Preprocessing Algorithms
Data
(Context & Reward)
Level of
Personalization
Full RL
MAB
CMAB
Agent
Object of
personalization
Passively
Sensed &
System derived
data
Actively Sensed
(Self-reported)
Baseline
variables
Current and
past health
behavior
Time
Recommend.
History
…
Type of Intervention
Postprocessing Algorithms
?
Timing of Intervention
Outcomes/Goals
Delivery Channel/
Representation
Evaluation
Clustered
Data
Algorithm-centric
Patient-centric
Simulated Testbed
Real-World
Other Algorithm
(Random, Static,…)
Environment
Metrics
Comparison
Standard Care
Figure 9: Framework for integrating Reinforcement Learning within DTx-supported Care Pathways.
DTx-supported care pathways (see Figure 9) and are
discussed in detail below.
Data Considerations: Collectively, the findings
from real-world studies underscore the potential of
RL within DTx but also highlight the need for larger,
longer-term studies beyond 6 months to validate these
results. Despite this need, the present literature re-
view may still clear up the misbelief that RL needs
millions of data rows (data points) to obtain superior
results compared to random or static policies. Ob-
taining such amounts of data is difficult in the con-
text of behavior change interventions where rewards
are delayed as the effects of the recommendations can
be observed only several hours, days, or weeks later.
However, there is still a need for publicly available
data sets of health behavior change interventions. Of
note is the recent study by Albers et al. (2022), which
contributes in this regard by sharing their dataset con-
sisting of 2366 persuasive messages delivered to 671
people to promote smoking cessation.
Our literature review revealed that a variety of data
sources (actively and passively sensed data) may be
included to capture the contextual state of the user
and/or serve as a reward signal for the algorithm.
In particular, self-reported data collected via ques-
tionnaires is a typical ingredient of RL-based behav-
ior change interventions. However, when using self-
reported data for training the algorithm, ensuring high
data quality and mitigating biases (e.g., social desir-
ability) is crucial as the learning process can be di-
rectly manipulated. We argue that future research
should also throw an eye on advancing user interfaces
to make the questionnaire completion more engag-
ing and motivating for the user and thus improve data
quality (e.g., using chatbots or gamification).
Algorithmic Considerations: In addition to con-
siderations regarding the data, the literature analysis
revealed that good results can be achieved with com-
paratively ”simple” algorithms (MABs and CMABs).
However, given their application for one-step decision
problems, the personalization of the care pathway is
then rather limited to certain points than the entire
path.
To address the balance between personalization at
a pooled data level and on an individual level, the al-
gorithms can be enhanced, for instance, by incorpo-
rating pre-processing methods like clustering. This
approach seems promising to accelerate the learning
process and mitigate data quality issues. Closely re-
lated to this trade-off between pooling and individ-
ualization are considerations regarding the privacy-
preserving use of AI. Up to now, this issue has been
largely unaddressed in the analyzed literature. One
promising approach could be so-called ”federated”
reinforcement learning where the user data remains
on the local device, and only the trained models are
shared and then aggregated on a centralized server
(Khalid et al., 2023). Further algorithmic challenges
that are not yet completely solved are related to non-
stationarity (e.g., see Mintz et al. (2020), Tomkins
et al. (2021)). This line of research focuses on tak-
ing into account that the learned user’s preferences
can vary over time, including the phenomenon that
the learned model itself may change the user and con-
sequently lose accuracy. Zhu et al. (2021) call this the
”Personalization Paradox”.
In view of the fact that DTx applications fall un-
der the medical device legislation, patient safety must
given special consideration, especially when AI tech-
niques are used. For example, prompting a patient
suffering from a chronic disease who may be on the
brink of overexertion to increase exercise over and
over again can cause medical damage. However,
these concerns have been only addressed in a very
small amount of papers by setting boundaries to the
algorithm in terms of an upper time-bound of physi-
cal activity per day (e.g., 60 minutes) (S7) or by re-
stricting the number of notifications per day (S21).
We argue that future studies should take concerns re-
garding patient safety more deliberately into account
when designing the algorithmic approach (”safety by
design”). Directly associated with the need for patient
safety is the need for model interpretability when AI
is integrated into medical devices. Within the ana-
Unleashing the Potential of Reinforcement Learning for Personalizing Behavioral Transformations with Digital Therapeutics: A Systematic
Literature Review
241

lyzed literature, the potential of explainable AI (XAI)
has not yet been fully exploited, although most of the
studies used largely transparent approximation mod-
els (esp., linear regression or regression trees). Future
research could integrate explanations into the action
space (e.g., see McInerney et al. (2018)) and/or em-
ploy post-processing algorithms to enhance the per-
suasiveness of health recommendations.
Considerations Regarding the Optimization
Goal and Objects of Personalization: Overall, the
results of the literature review suggest that a signif-
icant amount of papers sought to optimize physical
activity. In contrast, application areas such as op-
timizing questionnaire response rates are compara-
tively understudied, although self-reporting is a typ-
ical ingredient of DTx (Lee et al., 2023). Therefore,
we encourage researchers and practitioners to study
the use of RL beyond physical activity promotion. For
instance, future studies could focus more intensively
on using RL to optimize the delivery channel, content
representation, or specific goals for the patient.
Methodically Support the Development of ”In-
telligent” DTx Interventions: To simplify the tech-
nical process of integrating RL within DTx, a promis-
ing approach are low-/no-code development plat-
forms. Within the literature review, one study could
be identified that used such a platform to set up the
RL algorithm (S18). Overall, we argue that there is
a large potential in this regard as the first domain-
specific platforms for DTx development are just start-
ing to emerge (Liu et al., 2022).
5.2 Strengths and Limitations
The current literature review offers a comprehensive,
multi-perspective insight into a comparatively under-
explored and unstructured field. An extensive litera-
ture search was undertaken, encompassing a detailed
search string and three databases, supplemented by
snowballing relevant articles. However, our work also
comes with several limitations. Firstly, applying RL
to behavior change interventions is still in its early
stage, which means that the field is rapidly evolving,
and despite employing a rather inclusive search strat-
egy, some pertinent studies might have been missed.
Secondly, given the heterogeneity of this emerging
field, and the exploratory nature of this review, the
methodological quality of the studies was not explic-
itly assessed. Thirdly, the interpretation of data and
results leaves room for subjective bias. However, the
involvement of two authors may have helped to re-
duce this potential bias.
6 CONCLUSIONS
The paper aimed to offer a detailed examination of
the application of RL algorithms for enhancing be-
havior change interventions delivered via DTx. Based
on our analysis of the state of the art, we derived im-
plications for practice and future research by elucidat-
ing the employed algorithms, the objects of personal-
ization, and outlining their evaluation approaches and
metrics. Overall, there is a need for larger studies go-
ing beyond the scope of primary prevention to gain
more evidence on the suitability and efficacy of using
RL within DTx. Nonetheless, several real-world stud-
ies could indicate the advantageousness compared to
conventional approaches and demonstrate their im-
pact on health outcomes. Future research perspectives
may also include self-reporting approaches that are
able to maintain high data quality and user engage-
ment over time, the integration of privacy-preserving
AI techniques, problems arising from the ”personal-
ization paradox”, mechanisms to ensure patient safety
and methods supporting the holistic development of
self-learning DTx. In conclusion, this study may of-
fer a foundational starting point for those exploring
the design, implementation, and evaluation of self-
learning DTx. It contributes to the growing body of
research in this field.
REFERENCES
Agrawal, S. and Goyal, N. (2012). Analysis of thomp-
son sampling for the multi-armed bandit problem. In
Mannor, S., Srebro, N., and Williamson, R. C., edi-
tors, Proceedings of the 25th Annual Conference on
Learning Theory, volume 23 of Proceedings of Ma-
chine Learning Research, pages 39.1–39.26, Edin-
burgh, Scotland. PMLR.
Aguilera, A., Figueroa, C. A., Hernandez-Ramos, R.,
Sarkar, U., Cemballi, A., Gomez-Pathak, L., Mira-
montes, J., Yom-Tov, E., Chakraborty, B., Yan, X.,
Xu, J., Modiri, A., Aggarwal, J., Jay Williams, J.,
and Lyles, C. R. (2020). mHealth app using machine
learning to increase physical activity in diabetes and
depression: clinical trial protocol for the DIAMANTE
Study. BMJ Open, 10(8):e034723.
Albers, N., Neerincx, M. A., and Brinkman, W.-P. (2022).
Addressing people’s current and future states in a re-
inforcement learning algorithm for persuading to quit
smoking and to be physically active. PLOS ONE,
17(12):e0277295.
Ameko, M. K., Beltzer, M. L., Cai, L., Boukhechba, M.,
Teachman, B. A., and Barnes, L. E. (2020). Offline
Contextual Multi-armed Bandits for Mobile Health
Interventions: A Case Study on Emotion Regulation.
In Fourteenth ACM Conference on Recommender Sys-
tems, pages 249–258, Virtual Event Brazil. ACM.
HEALTHINF 2024 - 17th International Conference on Health Informatics
242

Beltzer, M. L., Ameko, M. K., Daniel, K. E., Daros, A. R.,
Boukhechba, M., Barnes, L. E., and Teachman, B. A.
(2022). Building an emotion regulation recommender
algorithm for socially anxious individuals using con-
textual bandits. British Journal of Clinical Psychol-
ogy, 61(S1):51–72.
Bertsimas, D., Klasnja, P., Murphy, S., and Na, L. (2022).
Data-driven Interpretable Policy Construction for Per-
sonalized Mobile Health. In 2022 IEEE International
Conference on Digital Health (ICDH), pages 13–22,
Barcelona, Spain. IEEE.
Coronato, A., Naeem, M., De Pietro, G., and Paragliola, G.
(2020). Reinforcement learning for intelligent health-
care applications: A survey. Artificial Intelligence in
Medicine, 109:101964.
Daskalova, N., Yoon, J., Wang, Y., Araujo, C., Beltran,
G., Nugent, N., McGeary, J., Williams, J. J., and
Huang, J. (2020). SleepBandits: Guided Flexible Self-
Experiments for Sleep. In Proceedings of the 2020
CHI Conference on Human Factors in Computing Sys-
tems, pages 1–13, Honolulu HI USA. ACM.
Delmas, A., Clement, B., Oudeyer, P.-Y., and Sauz
´
eon, H.
(2018). Fostering Health Education With a Serious
Game in Children With Asthma: Pilot Studies for As-
sessing Learning Efficacy and Automatized Learning
Personalization. Frontiers in Education, 3:99.
den Hengst, F., Grua, E. M., el Hassouni, A., and Hoogen-
doorn, M. (2020). Reinforcement learning for person-
alization: A systematic literature review. Data Sci-
ence, 3(2):107–147. Number: 2.
Di, S., Petch, J., Gerstein, H. C., Zhu, R., and Sherifali, D.
(2022). Optimizing Health Coaching for Patients With
Type 2 Diabetes Using Machine Learning: Model De-
velopment and Validation Study. JMIR Formative Re-
search, 6(9):e37838.
Dodd, S., Clarke, M., Becker, L., Mavergames, C., Fish,
R., and Williamson, P. R. (2018). A taxonomy has
been developed for outcomes in medical research to
help improve knowledge discovery. Journal of Clini-
cal Epidemiology, 96:84–92.
El Hassouni, A., Hoogendoorn, M., Ciharova, M., Kleiboer,
A., Amarti, K., Muhonen, V., Riper, H., and Eiben,
A. E. (2022). pH-RL: A Personalization Architec-
ture to Bring Reinforcement Learning to Health Prac-
tice. In Nicosia, G., Ojha, V., La Malfa, E., La Malfa,
G., Jansen, G., Pardalos, P. M., Giuffrida, G., and
Umeton, R., editors, Machine Learning, Optimiza-
tion, and Data Science, volume 13163, pages 265–
280. Springer International Publishing, Cham. Series
Title: Lecture Notes in Computer Science.
Etminani, K., G
¨
oransson, C., Galozy, A., Norell Pejner,
M., and Nowaczyk, S. (2021). Improving Medica-
tion Adherence Through Adaptive Digital Interven-
tions (iMedA) in Patients With Hypertension: Proto-
col for an Interrupted Time Series Study. JMIR Re-
search Protocols, 10(5):e24494.
European Union (2015). Council conclusions on person-
alised medicine for patients. Official Journal of the
European Union, (C 421).
Figueroa, C. A., Deliu, N., Chakraborty, B., Modiri, A., Xu,
J., Aggarwal, J., Jay Williams, J., Lyles, C., and Aguil-
era, A. (2022). Daily Motivational Text Messages to
Promote Physical Activity in University Students: Re-
sults From a Microrandomized Trial. Annals of Be-
havioral Medicine, 56(2):212–218.
Figueroa, C. A., Hernandez-Ramos, R., Boone, C. E.,
G
´
omez-Pathak, L., Yip, V., Luo, T., Sierra, V., Xu,
J., Chakraborty, B., Darrow, S., and Aguilera, A.
(2021). A Text Messaging Intervention for Cop-
ing With Social Distancing During COVID-19 (Stay-
Well at Home): Protocol for a Randomized Controlled
Trial. JMIR Research Protocols, 10(1):e23592.
Forman, E. M., Kerrigan, S. G., Butryn, M. L., Juarascio,
A. S., Manasse, S. M., Onta
˜
n
´
on, S., Dallal, D. H.,
Crochiere, R. J., and Moskow, D. (2019). Can the ar-
tificial intelligence technique of reinforcement learn-
ing use continuously-monitored digital data to opti-
mize treatment for weight loss? Journal of Behavioral
Medicine, 42(2):276–290.
F
¨
urstenau, D., Gersch, M., and Schreiter, S. (2023). Digital
Therapeutics (DTx). Business & Information Systems
Engineering.
Gasparetti, F., Aiello, L. M., and Quercia, D. (2020).
Personalized weight loss strategies by mining activ-
ity tracker data. User Modeling and User-Adapted
Interaction, 30(3):447–476. Number: 3 Publisher:
Springer.
Gonul, S., Namli, T., Baskaya, M., Sinaci, A. A., Cosar,
A., and Toroslu, I. H. (2018). Optimization of Just-
in-Time Adaptive Interventions Using Reinforcement
Learning. In Mouhoub, M., Sadaoui, S., Ait Mo-
hamed, O., and Ali, M., editors, Recent Trends and
Future Technology in Applied Intelligence, volume
10868, pages 334–341. Springer International Pub-
lishing, Cham. Series Title: Lecture Notes in Com-
puter Science.
Gray, R. C., Villareale, J., Fox, T. B., Dallal, D. H., On-
tanon, S., Arigo, D., Jabbari, S., and Zhu, J. (2023).
Improving Fairness in Adaptive Social Exergames via
Shapley Bandits. In Proceedings of the 28th Inter-
national Conference on Intelligent User Interfaces,
pages 322–336, Sydney NSW Australia. ACM.
Hong, J. S., Wasden, C., and Han, D. H. (2021). Introduc-
tion of digital therapeutics. Computer Methods and
Programs in Biomedicine, 209:106319.
Hu, X., Qian, M., Cheng, B., and Cheung, Y. K. (2021). Per-
sonalized Policy Learning Using Longitudinal Mobile
Health Data. Journal of the American Statistical As-
sociation, 116(533):410–420.
Janiesch, C., Zschech, P., and Heinrich, K. (2021). Machine
learning and deep learning. Electronic Markets.
Kaelbling, L. P., Littman, M. L., and Moore, A. W. (1996).
Reinforcement Learning: A Survey. Journal of Artifi-
cial Intelligence Research, 4:237–285.
Kankanhalli, A., Xia, Q., Ai, P., and Zhao, X. (2021).
Understanding Personalization for Health Behavior
Change Applications: A Review and Future Direc-
tions. AIS Transactions on Human-Computer Inter-
action, pages 316–349.
Khalid, N., Qayyum, A., Bilal, M., Al-Fuqaha, A., and
Qadir, J. (2023). Privacy-preserving artificial intel-
ligence in healthcare: Techniques and applications.
Computers in Biology and Medicine, 158:106848.
Kinsey, S., Wolf, J., Saligram, N., Ramesan, V., Walavalkar,
M., Jaswal, N., Ramalingam, S., Sinha, A., and
Nguyen, T. (2023). Building a Personalized Mes-
Unleashing the Potential of Reinforcement Learning for Personalizing Behavioral Transformations with Digital Therapeutics: A Systematic
Literature Review
243

saging System for Health Intervention in Underpriv-
ileged Regions Using Reinforcement Learning. In
32nd International Joint Conference on Artificial In-
telligence, IJCAI 2023, pages 6022–6030. Interna-
tional Joint Conferences on Artificial Intelligence.
Klasnja, P., Smith, S., Seewald, N. J., Lee, A., Hall,
K., Luers, B., Hekler, E. B., and Murphy, S. A.
(2019). Efficacy of Contextually Tailored Sugges-
tions for Physical Activity: A Micro-randomized Op-
timization Trial of HeartSteps. Annals of Behavioral
Medicine, 53(6):573–582.
Lattimore, T. and Szepesv
´
ari, C. (2020). Bandit algorithms.
Cambridge University Press, Cambridge ; New York,
NY.
Lauffenburger, J. C., Yom-Tov, E., Keller, P. A., McDon-
nell, M. E., Bessette, L. G., Fontanet, C. P., Sears,
E. S., Kim, E., Hanken, K., Buckley, J. J., Barlev,
R. A., Haff, N., and Choudhry, N. K. (2021). REin-
forcement learning to improve non-adherence for di-
abetes treatments by Optimising Response and Cus-
tomising Engagement (REINFORCE): study proto-
col of a pragmatic randomised trial. BMJ Open,
11(12):e052091.
Lee, U., Jung, G., Ma, E.-Y., Kim, J. S., Kim, H.,
Alikhanov, J., Noh, Y., and Kim, H. (2023). Toward
Data-Driven Digital Therapeutics Analytics: Litera-
ture Review and Research Directions. IEEE/CAA
Journal of Automatica Sinica, 10(1):42–66.
Li, L., Chu, W., Langford, J., and Schapire, R. E. (2010). A
contextual-bandit approach to personalized news arti-
cle recommendation. In Proceedings of the 19th inter-
national conference on World wide web, pages 661–
670, Raleigh North Carolina USA. ACM.
Liao, P., Greenewald, K., Klasnja, P., and Murphy, S.
(2020). Personalized HeartSteps: A Reinforcement
Learning Algorithm for Optimizing Physical Activity.
Proceedings of the ACM on Interactive, Mobile, Wear-
able and Ubiquitous Technologies, 4(1):1–22.
Liu, S., La, H., Willms, A., and Rhodes, R. E. (2022). A
“No-Code” App Design Platform for Mobile Health
Research: Development and Usability Study. JMIR
Formative Research, 6(8):e38737.
McInerney, J., Lacker, B., Hansen, S., Higley, K.,
Bouchard, H., Gruson, A., and Mehrotra, R. (2018).
Explore, exploit, and explain: personalizing explain-
able recommendations with bandits. In Proceedings of
the 12th ACM Conference on Recommender Systems,
pages 31–39, Vancouver British Columbia Canada.
ACM.
Mintz, Y., Aswani, A., Kaminsky, P., Flowers, E., and
Fukuoka, Y. (2020). Nonstationary Bandits with Ha-
bituation and Recovery Dynamics. Operations Re-
search, 68(5):1493–1516.
Moher, D. (2009). Preferred Reporting Items for System-
atic Reviews and Meta-Analyses: The PRISMA State-
ment. Annals of Internal Medicine, 151(4):264.
Mohr, D. C., Schueller, S. M., Montague, E., Burns, M. N.,
and Rashidi, P. (2014). The behavioral interven-
tion technology model: an integrated conceptual and
technological framework for eHealth and mHealth in-
terventions. Journal of medical Internet research,
16(6):e146. Number: 6 Publisher: JMIR Publications
Inc., Toronto, Canada.
Nahum-Shani, I., Smith, S. N., Spring, B. J., Collins, L. M.,
Witkiewitz, K., Tewari, A., and Murphy, S. A. (2018).
Just-in-Time Adaptive Interventions (JITAIs) in Mo-
bile Health: Key Components and Design Principles
for Ongoing Health Behavior Support. Annals of Be-
havioral Medicine, 52(6):446–462. Number: 6.
Nahum-Shani, I., Wetter, D. W., and Murphy, S. A. (2023).
Adapting just-in-time interventions to vulnerability
and receptivity: Conceptual and methodological con-
siderations. In Digital Therapeutics for Mental Health
and Addiction, pages 77–87. Elsevier.
Nudelman, G. and Shiloh, S. (2015). Mapping health
behaviors: Constructing and validating a common-
sense taxonomy of health behaviors. Social Science
& Medicine, 146:1–10.
Oyebode, O., Fowles, J., Steeves, D., and Orji, R. (2023).
Machine Learning Techniques in Adaptive and Per-
sonalized Systems for Health and Wellness. Inter-
national Journal of Human–Computer Interaction,
39(9):1938–1962.
Paredes, P., Giald-Bachrach, R., Czerwinski, M., Roseway,
A., Rowan, K., and Hernandez, J. (2014). PopTher-
apy: Coping with Stress through Pop-Culture. In Pro-
ceedings of the 8th International Conference on Per-
vasive Computing Technologies for Healthcare, Old-
enburg, Germany. ICST.
Pelle, T., Bevers, K., Van Der Palen, J., Van Den Hoogen, F.,
and Van Den Ende, C. (2020). Effect of the dr. Bart
application on healthcare use and clinical outcomes
in people with osteoarthritis of the knee and/or hip in
the Netherlands; a randomized controlled trial. Os-
teoarthritis and Cartilage, 28(4):418–427.
Pelle, T., Bevers, K., Van Der Palen, J., Van Den Hoogen,
F. H., and Van Den Ende, C. H. (2019). Development
and evaluation of a tailored e-self-management inter-
vention (dr. Bart app) for knee and/or hip osteoarthri-
tis: study protocol. BMC musculoskeletal disorders,
20(1):398. Number: 1.
Perski, O., Blandford, A., West, R., and Michie, S. (2017).
Conceptualising engagement with digital behaviour
change interventions: a systematic review using prin-
ciples from critical interpretive synthesis. Transla-
tional Behavioral Medicine, 7(2):254–267.
Philipp, P., Merkle, N., Gand, K., and Gißke, C. (2019).
Continuous support for rehabilitation using machine
learning. it - Information Technology, 61(5-6):273–
284. Number: 5-6.
Piette, J. D., Newman, S., Krein, S. L., Marinec, N., Chen,
J., Williams, D. A., Edmond, S. N., Driscoll, M.,
LaChappelle, K. M., Kerns, R. D., Maly, M., Kim,
H. M., Farris, K. B., Higgins, D. M., Buta, E., and
Heapy, A. A. (2022). Patient-Centered Pain Care Us-
ing Artificial Intelligence and Mobile Health Tools: A
Randomized Comparative Effectiveness Trial. JAMA
Internal Medicine, 182(9):975.
Piette, J. D., Thomas, L., Newman, S., Marinec, N., Krauss,
J., Chen, J., Wu, Z., and Bohnert, A. S. B. (2023).
An Automatically Adaptive Digital Health Interven-
tion to Decrease Opioid-Related Risk While Conserv-
ing Counselor Time: Quantitative Analysis of Treat-
ment Decisions Based on Artificial Intelligence and
Patient-Reported Risk Measures. Journal of Medical
Internet Research, 25:e44165.
HEALTHINF 2024 - 17th International Conference on Health Informatics
244

Rabbi, M., Aung, M. H., , M., and Choudhury, T. (2015).
MyBehavior: automatic personalized health feedback
from user behaviors and preferences using smart-
phones. In Proceedings of the 2015 ACM Interna-
tional Joint Conference on Pervasive and Ubiquitous
Computing, pages 707–718.
Rabbi, M., Aung, M. S., Gay, G., Reid, M. C., and
Choudhury, T. (2018). Feasibility and Acceptabil-
ity of Mobile Phone–Based Auto-Personalized Physi-
cal Activity Recommendations for Chronic Pain Self-
Management: Pilot Study on Adults. Journal of Med-
ical Internet Research, 20(10):e10147.
Sporrel, K., Wang, S., Ettema, D. D. F., Nibbeling, N.,
Krose, B. J. A., Deutekom, M., De Boer, R. D. D., and
Simons, M. (2022). Just-in-Time Prompts for Run-
ning, Walking, and Performing Strength Exercises in
the Built Environment: 4-Week Randomized Feasibil-
ity Study. JMIR Formative Research, 6(8):e35268.
Sutton, R. S. and Barto, A. G. (2018). Reinforcement
learning: an introduction. Adaptive computation and
machine learning series. The MIT Press, Cambridge,
Massachusetts, second edition edition.
Tomkins, S., Liao, P., Klasnja, P., and Murphy, S. (2021).
IntelligentPooling: practical Thompson sampling for
mHealth. Machine Learning, 110(9):2685–2727.
Tong, X., Mauriello, M. L., Mora-Mendoza, M. A., Prabhu,
N., Kim, J. P., and Paredes Castro, P. E. (2023).
Just Do Something: Comparing Self-proposed and
Machine-recommended Stress Interventions among
Online Workers with Home Sweet Office. In Proceed-
ings of the 2023 CHI Conference on Human Factors in
Computing Systems, pages 1–20, Hamburg Germany.
ACM.
Tragos, E., O’Reilly-Morgan, D., Geraci, J., Shi, B., Smyth,
B., Doherty, C., Lawlor, A., and Hurley, N. (2023).
Keeping People Active and Healthy at Home Using a
Reinforcement Learning-based Fitness Recommenda-
tion Framework. In Proceedings of the Thirty-Second
International Joint Conference on Artificial Intelli-
gence, pages 6237–6245, Macau, SAR China. Inter-
national Joint Conferences on Artificial Intelligence
Organization.
Wang, S., Sporrel, K., Van Hoof, H., Simons, M., De Boer,
R. D. D., Ettema, D., Nibbeling, N., Deutekom, M.,
and Kr
¨
ose, B. (2021a). Reinforcement Learning to
Send Reminders at Right Moments in Smartphone
Exercise Application: A Feasibility Study. Interna-
tional Journal of Environmental Research and Public
Health, 18(11):6059.
Wang, S., Zhang, C., Kr
¨
ose, B., and Van Hoof, H. (2021b).
Optimizing Adaptive Notifications in Mobile Health
Interventions Systems: Reinforcement Learning from
a Data-driven Behavioral Simulator. Journal of Medi-
cal Systems, 45(12):102.
Watkins, C. J. and Dayan, P. (1992). Technical Note: Q-
Learning. Machine Learning, 8(3/4):279–292.
Webster, J. and Watson, R. T. (2002). Analyzing the past
to prepare for the future: Writing a literature review.
MIS quarterly, pages xiii–xxiii. Publisher: JSTOR.
Wei, Y., Zheng, P., Deng, H., Wang, X., Li, X., and Fu, H.
(2020). Design Features for Improving Mobile Health
Intervention User Engagement: Systematic Review
and Thematic Analysis. Journal of Medical Internet
Research, 22(12):e21687.
Weimann, T. G., Schlieter, H., and Brendel, A. B. (2022).
Virtual Coaches: Background, Theories, and Future
Research Directions. Business & Information Systems
Engineering, 64(4):515–528.
White, J. M. (2013). Bandit algorithms for website op-
timization. Developing, deploying, and debugging.
O’Reilly, Sebastopol, California.
Yom-Tov, E., Feraru, G., Kozdoba, M., Mannor, S., Ten-
nenholtz, M., and Hochberg, I. (2017). Encouraging
physical activity in patients with diabetes: interven-
tion using a reinforcement learning system. Journal
of medical Internet research, 19(10):e338.
Zhou, M., Fukuoka, Y., Mintz, Y., Goldberg, K., Kamin-
sky, P., Flowers, E., and Aswani, A. (2018a). Eval-
uating Machine Learning–Based Automated Person-
alized Daily Step Goals Delivered Through a Mo-
bile Phone App: Randomized Controlled Trial. JMIR
mHealth and uHealth, 6(1):e28.
Zhou, M., Mintz, Y., Fukuoka, Y., Goldberg, K., Flow-
ers, E., Kaminsky, P., Castillejo, A., and Aswani, A.
(2018b). Personalizing mobile fitness apps using rein-
forcement learning. In CEUR workshop proceedings,
volume 2068. NIH Public Access.
Zhou, T., Wang, Y., Yan, L. L., and Tan, Y. (2023).
Spoiled for Choice? Personalized Recommenda-
tion for Healthcare Decisions: A Multiarmed Ban-
dit Approach. Information Systems Research, page
isre.2022.1191.
Zhu, F., Guo, J., Li, R., and Huang, J. (2018a). Ro-
bust Actor-Critic Contextual Bandit for Mobile Health
(mHealth) Interventions. In Proceedings of the
2018 ACM International Conference on Bioinformat-
ics, Computational Biology, and Health Informatics,
pages 492–501, Washington DC USA. ACM.
Zhu, F., Guo, J., Xu, Z., Liao, P., Yang, L., and Huang, J.
(2018b). Group-Driven Reinforcement Learning for
Personalized mHealth Intervention. In Frangi, A. F.,
Schnabel, J. A., Davatzikos, C., Alberola-L
´
opez, C.,
and Fichtinger, G., editors, Medical Image Computing
and Computer Assisted Intervention – MICCAI 2018,
volume 11070, pages 590–598. Springer International
Publishing, Cham. Series Title: Lecture Notes in
Computer Science.
Zhu, F., Liao, P., Zhu, X., Yao, J., and Huang, J. (2018c).
Cohesion-driven Online Actor-Critic Reinforcement
Learning for mHealth Intervention. In Proceedings
of the 2018 ACM International Conference on Bioin-
formatics, Computational Biology, and Health Infor-
matics, pages 482–491, Washington DC USA. ACM.
Zhu, J., Dallal, D. H., Gray, R. C., Villareale, J., Onta
˜
n
´
on,
S., Forman, E. M., and Arigo, D. (2021). Personal-
ization Paradox in Behavior Change Apps: Lessons
from a Social Comparison-Based Personalized App
for Physical Activity. Proceedings of the ACM on
Human-Computer Interaction, 5(CSCW1):1–21.
Unleashing the Potential of Reinforcement Learning for Personalizing Behavioral Transformations with Digital Therapeutics: A Systematic
Literature Review
245
