
Influence of Arterial Occlusion at Various Cuff Pressures on Systemic
Circulation Measured by rPPG
Leah De Vos
1
, Gennadi Saiko
2
a
, Denis Bragin
3,4
b
and Alexandre Douplik
2,5
c
1
Department of Engineering, Toronto Metropolitan University, Toronto, Canada
2
Department of Physics, Toronto Metropolitan University, Toronto, Canada
3
Lovelace Biomedical Research Institute, Albuquerque, U.S.A.
4
Department of Neurology, University of New Mexico School of Medicine, Albuquerque, U.S.A.
5
iBest, Keenan Research Centre of the LKS Knowledge Institute, St. Michael’s Hospital, Canada
Keywords: Photoplethysmography, Arterial Occlusion, Microcirculation.
Abstract: Background: Arterial occlusion is a ubiquitous medical procedure, which is used in many clinical scenarios.
However, there is no standard protocol for the selection of the applied pressure. As various pressures may
trigger different physiological responses, it is important to understand these peculiarities. The aim of the
current work is to investigate if there is any difference in the systemic response to the occlusion at various
applied pressures. Methods: Hands of healthy volunteers (10 volunteers) were occluded at the wrist by
inflating the blood cuff to 150 or 200 mmHg. The remote photoplethysmography (rPPG) measurements of
control and experimental hands were taken. To assess systemic response, we have analysed the behaviour of
AC (low frequency, LF at 0.1 Hz rate) components in green and red channels during occlusion and reperfusion.
Results: We have not found a statistically significant difference in the LF spectra between occlusions at 150
and 200 mmHg pressures. Conclusions: We have performed the analysis of low-frequency (0.1 Hz)
components of remote photoplethysmography signals during arterial occlusion at 150 and 200 mmHg. Our
preliminary results show that the systemic response is similar at both levels of occlusions.
1 INTRODUCTION
Arterial occlusion is a ubiquitous medical procedure.
It is used in many clinical scenarios. Probably the
most well-known use of arterial occlusion is blood
pressure measurements using an auscultatory method,
which was originally based on a stethoscope and a
sphygmomanometer. Another important clinical use
of arterial occlusion is to detect endothelial
dysfunction in critically ill patients (Joannides et al.,
2006).
In common clinical scenarios the arterial
occlusion is caused by placing the blood pressure cuff
over the forearm or wrist and inflating it over systolic
blood pressure (Lenders et al., 1991). Common sense
would suggest that inflating the blood cuff just over
systolic pressure would be sufficient to occlude
arteries of the hand. However, it is not the case.
a
https://orcid.org/0000-0002-5697-7609
b
https://orcid.org/0000-0003-4894-0061
c
https://orcid.org/0000-0001-9948-9472
As a rule, an additional pressure of 50 mmHg is
typically considered to be a safe margin
(Kanchanathepsak et al., 2023). However, the exact
relationship between the applied pressure and the
level of occlusion is not well understood and
established in the literature. In particular, it can be
driven by multiple factors such as BMI. As such, the
applied pressure often is a tradeoff between the desire
to occlude vessels and patient’s tolerance to pain. The
latter is particularly important for a bed-side test for
endothelial dysfunction assessment, where the artery
is occluded for 3 min (Saldin, 2019). Thus, it could
be beneficial to get more insights into the differences
at various levels of the occlusion. Such investigation
can be based on observing hemodynamic response,
which can be on a local and systemic level.
Remote photoplethysmography (rPPG) has been
shown its utility in investigation of skin
De Vos, L., Saiko, G., Bragin, D. and Douplik, A.
Influence of Arterial Occlusion at Various Cuff Pressures on Systemic Circulation Measured by rPPG.
DOI: 10.5220/0012469800003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 313-318
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
313

microcirculation (Burton et al., 2023). The
predominant signal source in the PPG is the cardiac
pulsation caused by the ejection of blood from the left
ventricle during cardiac systole, which causes
distensions of blood vessels and changes in the tissue
absorption. These blood waveforms demonstrate
changes in five frequency bands, which are related to
different physiological processes. Heart rate for
normal subjects at rest varies from 60-100 beats per
minute (bpm) (Vital Signs (Body Temperature, Pulse
Rate, Respiration Rate, Blood Pressure), 2022).
Conservatively extending the lower bound to 50 bpm
to consider lower resting heart rates that can occur in
certain people, such as athletes (Doyen et al., 2019),
then the corresponding frequency range is 0.83-1.67
Hz. The normal respiration rate for a healthy subject
is 12 to 20 breaths per minute, corresponding to a
frequency range of 0.20-0.33 Hz (Vital Signs, n.d.).
This PPG signal further contains oscillations in 0.01-
0.02 Hz, 0.02-0.06 Hz, 0.06-0.15 Hz ranges
corresponding to endothelial related metabolic,
neurogenic, and myogenic activities, respectively (Li
et al., 2006). Myogenic range also contains Mayer
waves, which are oscillations in blood pressure that
typically occur at a frequency of 0.1 Hz (Julien, 2006).
The mechanism for Mayer waves is subject to active
debate, but recent findings advocate that the
oscillations are produced by a sympathetic
baroreceptor response to hemodynamic disturbances
(Julien, 2006). The ability to capture Mayer waves by
a smartphone camera was demonstrated in (Burton et
al., 2022). As occlusion and/or reperfusion may trigger
hemodynamic disturbance we hypothesize that this
disturbance can depend on the severity of
occlusion/reperfusion and characterize this difference.
The aim of the current work is to investigate the
differences in systemic physiological response to the
occlusion/reperfusion at various applied pressures.
We used rPPG to analyze skin microcirculation in an
arterial occlusion model with 2 different applied
pressures. We have selected 150 mmHg as a pressure
which is just marginally higher than the systolic
pressure, and 200 mmHg, as a higher pressure with
50 mmHg safety margin. As we aim to investigate the
systemic response, we have analyzed the rPPG signal
in both experimental and control hands.
2 METHODS
2.1 Data Collection
The experimental setup, as seen in Figure 1, includes
the subject sitting with their hands placed side by side
on a raised platform in the prone position. An iPhone
14 camera (Apple, Cupertino, CA, USA) is held by a
tripod above the hands positioned directly above. As
a light source, two rectangular video light panels with
600 LEDs on each panel (NEEWER LED Video
Light, Shenzhen, China) were positioned on either
side of the camera illuminating the hands (light colour
was set to 4600K to maximize the green channel
signal and 100% intensity was set to ensure maximum
signal to noise ratio). A white circle of 1 cm diameter
is also placed in the frame next to the hands for colour
normalization during processing. In each iteration of
data collection, one hand is designated as the
experimental hand, occluded throughout the data
collection, and the other acts as a control. On the arm
of the experimental hand, a pressure cuff is worn
around the wrist to apply pressure during the data
collection.
Figure 1: Experimental setup. (From top to bottom) LED
panels, iPhone 14, pressure cuff and hands placed on
staging table.
Once the setup is established, the data collection
procedure is as follows:
1. The subject's initial blood pressure is
measured from the experimental arm.
2. The subject places both hands on the platform,
in the prone position. At this point, the iPhone
starts recording video at 60 fps.
3. Baseline measurements are recorded for 1
minute (baseline interval).
BIOIMAGING 2024 - 11th International Conference on Bioimaging
314

4. Arterial occlusion is then applied on the
experimental hand for 3 minutes by applying
150 mmHg of pressure (occlusion interval).
5. The pressure is then released and no pressure
is applied to the arm for 4 minutes (reperfusion
interval).
6. At the 8-minute mark data collection
concludes and the iPhone recording video and
the muscle oxygenation monitor are both
turned off.
A rest period of 5 minutes is taken and then the
process is repeated for steps 2-6 with the opposite arm
acting as the experimental hand and with 200 mmHg
of pressure being applied instead of 150 mmHg.
For this work, data from 10 subjects were
acquired (age range: 20-60 years old, 4 males, 6
females) each with no evidence of cardiovascular
disease and no extreme BMI values. The Toronto
Metropolitan Research Ethical Board approved this
study.
2.2 Image Processing
The recorded video footage from the data collection
is then processed and analysed using MATLAB
2023b (Mathworks, Natick, MA, USA). First, to
improve processing time, the video is cut into three
segments each representing sequentially no pressure,
arterial occlusion (150 mmHg or 200 mmHg) and
pressure release. The following processing is applied
to each video segment. A Gaussian filter is applied to
each video frame to reduce the amount of noise in the
video and high-frequency components in the video.
Next, three regions of interest (ROI) are manually
selected from the video file, one on the experimental
hand, one on the control hand and one around the
white circle. For each ROI, each video frame’s pixels
are averaged for each colour channel (red, green and
blue (RGB)) resulting in a single red, green and blue
value per frame. This creates signals representing a
time series for each colour channel. To normalize the
time series for the experimental and control hands the
following equations are used,
𝑒
,
255
𝐸
,
𝑅
,
(1)
𝑐
,
255
𝐶
,
𝑅
,
(2)
Where i refers to the colour channel (i = red,
green, and blue), j refers to the frame’s number (j = 1
- N, where N is the number of frames), E represents
the experimental hand time series, C represents the
control hand times series and R represents the white
circle time series.
Figure 2: Experiment hand placements, and highlighted
regions of interest in red.
2.3 Data Analysis
The DC component of the signal represents the
average intensity of the signal over time and the AC
component represents changes in the signal
associated with the cardiac cycle. For this work, we
considered the AC component of the signal. As such,
for AC component of the rPPG signal, changes in
amplitude over time due to arterial occlusion events
were analysed, specifically amplitude drop relative to
baseline condition when occlusion is applied and the
amplitude overshoot relative to baseline when the
pressure is released. The calculations performed for
each component are as follows:
2.3.1 AC Component
For the AC component, the low frequency range in
the red and green channels were analysed. To retrieve
the LF spectral range, each channel was run through
a bandpass filter with a frequency range of 0.05-5 Hz.
After the signals are filtered to this frequency range,
the continuous wavelet transforms (CWT) is applied
on the signals calculating the mean power of the
results. After this the power spectral density (PSD) in
the LF range for both the red and green channels can
be calculated. To express the percent drop of the LF
frequency of the experimental hand relative to the
control hand when pressure is applied and the
overshoot when pressure is released, the following
equation is used,
Influence of Arterial Occlusion at Various Cuff Pressures on Systemic Circulation Measured by rPPG
315
% 𝑐ℎ𝑎𝑛𝑔𝑒
𝑣𝑎𝑙𝑢𝑒
𝑏𝑎𝑠𝑒𝑙𝑖𝑛𝑒
𝑏𝑎𝑠𝑒𝑙𝑖𝑛𝑒
𝑥 100
(3)
Where i represents the segment number (i = 2-3),
value
i
represents the PSD value at 0.1 Hz on
occlusion (i=2) or reperfusion (i=3) segment, and
baseline represents the PSD value at 0.1 Hz on
baseline (i=1) segment. The above equation was
performed on both the red and green channels for
each segment, and for experimental and control
hands.
2.3.2 Statistical Analysis
To assess the statistical significance of the differences
between results, i.e. comparing between AC
components, two-tailed t-tests were performed. In
this case the null hypothesis was that the mean of data
1 is equal to the mean of data 2, essentially that there
is no significant difference between the two datasets.
The alternative hypothesis is that the mean of data 1
is not equal to the mean of data 2 denoting a
significant difference between the two datasets. To
perform the t-test, the following process is followed.
First the t-test statistic is calculated as,
𝑡
𝑥̅
𝑥̅
(4)
Where 𝑥̅
and 𝑥̅
are the means of data 1 and data
2 respectively,𝑠
and 𝑠
are the standard deviations of
data 1 and data 2 respectively, and 𝑛
and 𝑛
and the
sample sizes of data 1 and data 2 respectively. Then
the p-value is determined by first calculating the
degree of freedom by,
𝑑𝑓
/
/
(5)
Once 𝑑𝑓 is calculated then the p-value can be
found as,
𝑝𝑣𝑎𝑙𝑢𝑒 𝑃𝑇𝑡 | 𝑑𝑓
(6)
Where T is the random variable following a t-
distribution with df and t is the test statistic. From
there if the p-value < α (significance level set as α =
0.05) the null hypothesis is rejected and if the p-value
≥ α the null hypothesis is accepted. For this study the
computations were performed in MATLAB.
3 RESULTS
We have analysed the behaviour of the AC
components at LF spectral range in green and red
channels during occlusion and overshoot. The results
are depicted in Figure 3 and Figure 4, respectively.
The results are also summarized in Table 1 for the
experimental hand and Table 2 for the control hand.
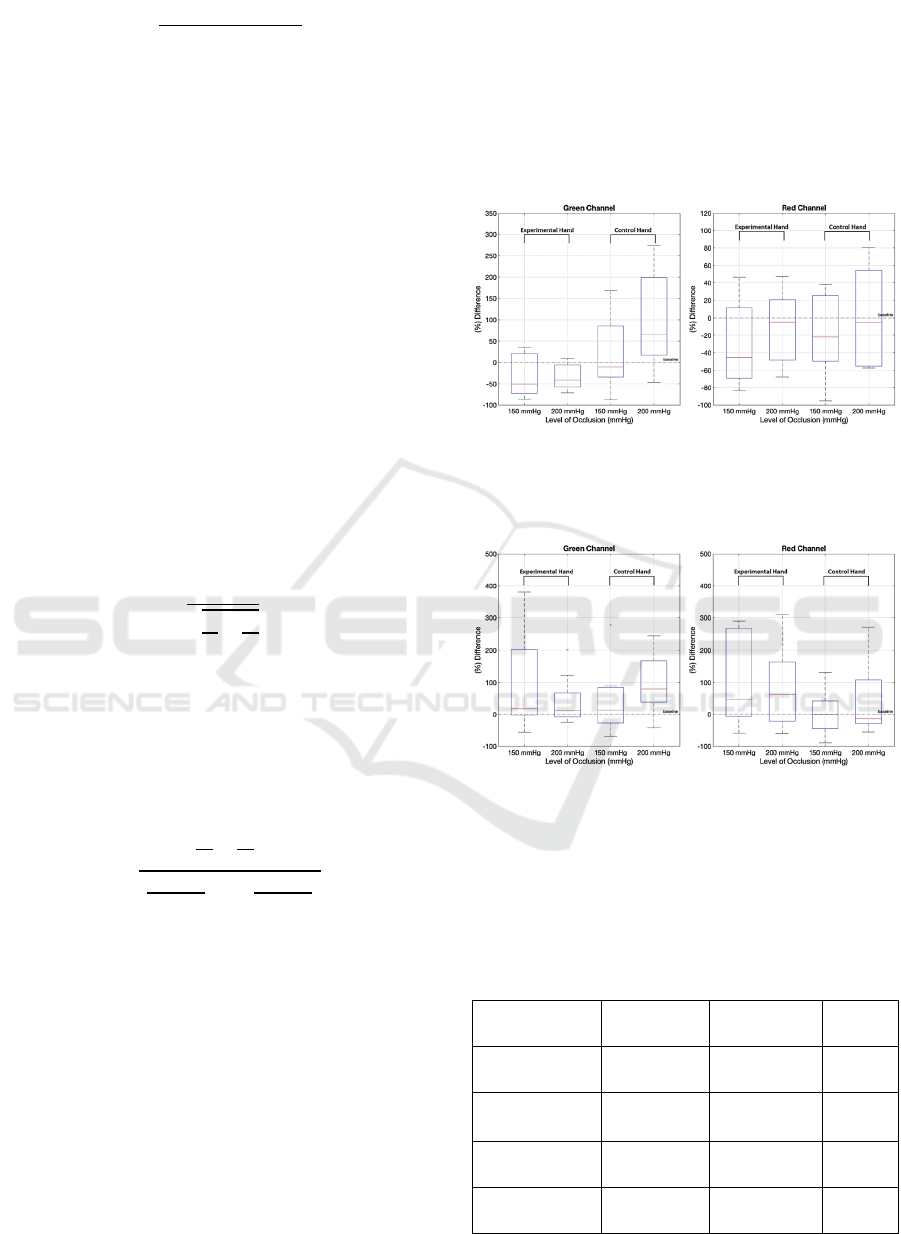
Figure 3: The behaviour of AC components in green (left
panel) and red (right panel) channels during occlusion. The
drop has been calculated as a relative change in AC
component on the 2nd interval compared to the baseline.
Figure 4: The behaviour of AC components in green (left
panel) and red (right panel) channels during reperfusion.
The overshoot has been calculated as a relative change in
AC component on the 3rd interval compared to the baseline.
Table 1: Comparison of different metrics at 150 and 200
mmHg on the experimental hand. Specifically mean,
standard deviation (in brackets), and p-value (with
significance noted by (ns), no statistical significance, when
p > 0.05).
Metric
Mean @ 150
mmHg
Mean @ 200
mmHg
p-value
Reperfusion
Red Channel
155.26
(268.91)
84.69
(122.72)
0.46
(ns)
Reperfusion
Green Channel
92.18
(158.02)
38.60
(72.34)
0.34
(ns)
Occlusion Red
Channel
-15.91
(82.94)
-10.51
(43.26)
0.86
(ns)
Occlusion
Green Channel
-32.80
(47.77)
-34.30
(27.79)
0.93
(ns)
BIOIMAGING 2024 - 11th International Conference on Bioimaging
316

Table 2: Comparison of different metrics at 150 and 200
mmHg on the control hand. Specifically mean, standard
deviation (in brackets), and p-value (with significance
noted to (ns), no statistical significance, when p > 0.05).
Metric
Mean @ 150
mmHg
Mean @ 200
mmHg
p-value
Reperfusion
Red Channel
3.70
(67.42)
68.15
(155.25)
0.244
(ns)
Reperfusion
Green Channel
35.05
(99.15)
118.91
(129.85)
0.12
(ns)
Occlusion Red
Channel
-6.18
(72.96)
24.19
(111.01)
0.48
(ns)
Occlusion
Green Channel
20.57
(80.03)
93.23
(102.29)
0.09
(ns)
4 DISCUSSION
Here, we present an initial pilot investigation of the
microvasculature hemodynamic during
occlusion/reperfusion captured by a smartphone
camera. Data was collected as described from ten
healthy subjects. We have performed the analysis of
remote photoplethysmography signals in the low
frequency (0.1 Hz) range for two levels of occlusion:
mild occlusion (150 mmHg) and occlusion with a
safety margin (200 mmHg).
As we aimed to investigate the systemic response,
we analyzed the rPPG signal in both the experimental
and control hands.
The behaviour of the LF component is very
similar for both levels of occlusion. In particular, we
have not found any statistically significant
differences between distributions caused by 150 and
200 mmHg either during occlusion or reperfusion.
During occlusion, AC amplitudes in the
experimental hand in the 0.1 Hz range drop for both
150 and 200 mmHg pressures (see Figure 3). The
drop distributions are characterized by very similar
means and standard deviations. It holds true for both
the green and red channels.
During occlusion, AC amplitudes in the control
hand in the 0.1 Hz range increase for both 150 and
200 mmHg pressures in the green channel (see Figure
3). The increase distributions are characterized by
very similar means and standard deviations. It holds
true for both green and red channels.
During reperfusion, the behaviour of the 0.1 Hz
range component in the experimental hand in 150 and
200 mmHg cases are also characterized by very
similar means. However, standard deviations for 150
and 200 mmHg pressures are quite different in both
red and green channels (see Figure 4).
Moreover, during reperfusion, one can see that
there is a substantial (by 50-70% in red channel)
increase in 0.1 Hz oscillations in the experimental
hand.
We hypothesize that these low-frequency
oscillations can be attributed to Mayer waves. While
Mayer waves share the same frequency range as
myogenic activities (0.06-0.15Hz), their origins are
different. Mayer waves are the sympathetic activity
with baroreflex activation. Myogenic oscillations are
local and independent of the sympathetic nervous
vasoconstriction.
Our conclusion regarding the origin of the 0.1 Hz
amplitude increase is based on two observations.
Firstly, we see similar results during reperfusion in
both experimental and control hands. Thus, it speaks
in favour of systemic response. Secondly, the results
are quite similar for red and green channels, which
have different sampling depth. Thus, similarity in
these responses also points in favour of Mayer waves,
as local regulation should demonstrate some
differences between capillary network (sampled by
the green channel) and deep vascular plexus (sampled
by the red channel)
Thus, we can conclude that both lower and higher
pressures are probably triggering a similar systemic
response in the form of a sympathetic baroreceptor
response to hemodynamic disturbances.
The work has certain limitations. Firstly, the
measurements were performed on just 10
participants. Thus, larger studies are required to
generalize the results. Secondly, the short time
interval (5 min) between the measurements on left
(150 mmHg occlusion) and right (200 mmHg
occlusion) hands was taken. While quite a significant
amount of time was allowed for baseline and
reperfusion measurements (1 and 4 min,
respectively), it potentially still may impact the blood
flow in the control (left) hand during 200 mmHg
occlusion of the right hand. To mitigate this risk,
more time (e.g. 10 min) needs to be allowed between
experiments in future.
In future work we plan to compare rPPG with
contact PPG, which also measures microcirculation in
skin, and investigate other frequency ranges.
5 CONCLUSIONS
We have performed the analysis of low-frequency (0.1
Hz) components of remote photoplethysmography
signals during arterial occlusion and reperfusion at 150
and 200 mmHg. Our preliminary results show that the
systemic response is similar at both levels of
occlusions.
Influence of Arterial Occlusion at Various Cuff Pressures on Systemic Circulation Measured by rPPG
317

ACKNOWLEDGEMENTS
We would like to thank the volunteers who
participated in our study, without whom our work
would not have been possible. The authors
acknowledge funding from NSERC Alliance (A.D),
NSERC Personal Discovery (A.D. and G. S.), and
Toronto Metropolitan University Faculty of Science
Discovery Accelerator program (G.S.).
REFERENCES
Burton, T., Saiko, G., Cao, M., & Douplik, A. (2023).
Remote photoplethysmography with consumer
smartphone reveals temporal differences between
glabrous and nonglabrous skin: Pilot in vivo study.
Journal of Biophotonics, 16(1), e202200187.
https://doi.org/10.1002/jbio.202200187
Burton, T., Saiko, G., &Douplik, A. (2022). Remote PPG
Imaging by a Consumer-grade Camera under Rest and
Elevation-invoked Physiological Stress Reveals Mayer
Waves and Venous Outflow: Proceedings of the 15th
International Joint Conference on Biomedical
Engineering Systems and Technologies, 153–159.
https://doi.org/10.5220/0010883100003123
Doyen, B., Matelot, D., & Carré, F. (2019). Asymptomatic
bradycardia amongst endurance athletes. The Physician
and Sportsmedicine, 47(3), 249–252.
https://doi.org/10.1080/00913847.2019.1568769
Joannides, R., Bellien, J., &Thuillez, C. (2006). Clinical
methods for the evaluation of endothelial function – a
focus on resistance arteries. Fundamental & Clinical
Pharmacology, 20(3), 311–320.
https://doi.org/10.1111/j.1472-8206.2006.00406.x
Julien, C. (2006). The enigma of Mayer waves: Facts and
models. Cardiovascular Research, 70(1), 12–21.
https://doi.org/10.1016/j.cardiores.2005.11.008
Kanchanathepsak, T., Pukrittayakamee, N. C.,
Woratanarat, P., Tawonsawatruk, T., &
Angsanuntsukh, C. (2023). Limb occlusion pressure
versus standard tourniquet inflation pressure in minor
hand surgery: A randomized controlled trial. Journal of
Orthopaedic Surgery and Research, 18(1), 539.
https://doi.org/10.1186/s13018-023-04000-3
Lenders, J., Janssen, G. J., Smits, P., &Thien, T. (1991).
Role of the wrist cuff in forearm plethysmography.
Clinical Science (London, England: 1979), 80(5), 413–
417. https://doi.org/10.1042/cs0800413
Li, Z., Leung, J. Y., Tam, E. W., &Mak, A. F. (2006).
Wavelet analysis of skin blood oscillations in persons
with spinal cord injury and able-bodied subjects.
Archives of Physical Medicine and Rehabilitation,
87(9), 1207–1212; quiz 1287.
https://doi.org/10.1016/j.apmr.2006.05.025
Saldin, T. (2019). Assessing Endothelial Dysfunction
Estimating the Differences Between 3 Minute and 5
Minute Reactive Hyperemia. Master’s Theses.
https://doi.org/10.15368/theses.2019.4
Vital Signs (Body Temperature, Pulse Rate, Respiration
Rate, Blood Pressure). (2022, June 14).
https://www.hopkinsmedicine.org/health/conditions-
and-diseases/vital-signs-body-temperature-pulse-rate-
respiration-rate-blood-pressure
Vital Signs: How to Check My Vitals at Home. (n.d.).
Cleveland Clinic. Retrieved November 21, 2023, from
https://my.clevelandclinic.org/health/articles/10881-
vital-signs
BIOIMAGING 2024 - 11th International Conference on Bioimaging
318
