
Exploring the Design of Low-End Technology to Increase Patient
Connectivity to Electronic Health Records
Rens Kievit
1 a
, Abdullahi Abubakar Kawu
2 b
, Mirjam van Reisen
1 c
, Dympna O’Sullivan
2 d
and Lucy Hederman
3 e
1
Leiden Institute of Advanced Computer Science (LIACS), Leiden University, Leiden, The Netherlands
2
Technological University, Dublin, Ireland
3
School of Computer Science and Statistics, Trinity College Dublin, Dublin, Ireland
Keywords:
Patient Generated Health Data, FAIR Data, Interactive Voice Response, Home Monitoring Devices, Patient
Connectivity.
Abstract:
The tracking of the vitals of patients with long term health problems is essential for clinicians to determine
proper care. Using Patient Generated Health Data (PGHD) communicated remotely allows patients to be
monitored without requiring frequent hospital visits. Issues might arise when the communication of data
digitally is difficult or impossible due to a lack of access to internet or a low level of digital literacy as is the
case in many African countries. The VODAN-Africa project (van Reisen et al., 2021) started in 2020 and
has greatly increased the capabilities of clinics in different countries in both Africa and Asia, but currently
no systems are in place for the integration of external data from patients with long term health problems. In
this article we outline our investigation into methods to increase the connectivity of patients with long term
health problems with their clinics, and propose a solution in the form of a data pipeline prototype based on an
Interactive Voice Response (IVR) system.
1 INTRODUCTION
With the creation of the VODAN-Africa network
(van Reisen et al., 2021), the health data capabilities
of many non-western countries have been improved
tremendously. At the time of this writing, there are
a total of 67 health facilities with patient instances
spread out over 8 countries on the African continent.
The VODAN-Africa network is a novel healthcare
framework that is entirely based on the FAIR prin-
ciples. These were introduced by (Wilkinson et al.,
2016) and describe guidelines to improve the Find-
ability, Accessibility (under well-defined constraints),
Interoperability and Reproducibility of digital ob-
jects. The main goal of the development of these
guidelines was to increase the capability and action-
ability of machine algorithms to better deal with the
quickly increasing volume and complexity of data
a
https://orcid.org/0000-0003-2328-4117
b
https://orcid.org/0000-0003-2531-9539
c
https://orcid.org/0000-0003-0627-8014
d
https://orcid.org/0000-0003-2841-9738
e
https://orcid.org/0000-0001-6073-4063
in the modern world.
1
Within the context of the
VODAN-Africa project, these guidelines create the
possibility of the analysis of healthcare trends on large
geographical scales while being able to maintain data
ownership within the clinics. The former happens
through the standardization of the data flow and cre-
ation of controlled vocabularies which allows inter-
operability between clinics in various countries. Data
ownership is ensured by a smart aggregating data vis-
iting system (van Reisen et al., 2021; Plug et al.,
2022).
The data collection and registration currently only
happens within clinics, where medical staff treat the
patients and collect data, and trained data clerks in-
sert this data into the previously mentioned systems.
Traveling to, or communication with, these clinics,
however, might not always be trivial. In poorer or
more rural regions, proper access to infrastructure
such as roads or the internet might be complicated.
This can be especially difficult for patients with long
term physical-conditions such as asthma, diabetes,
1
A more detailed description of the guidelines and their
use is described at https://www.go-fair.org/
194
Kievit, R., Kawu, A., van Reisen, M., O’Sullivan, D. and Hederman, L.
Exploring the Design of Low-End Technology to Increase Patient Connectivity to Electronic Health Records.
DOI: 10.5220/0012460900003657
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 2, pages 194-200
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Copyright © 2024 by Paper published under CC license (CC BY-NC-ND 4.0)

epilepsy and high blood pressure. These are patients
that do not need to be in a clinic at all times, but their
vitals would ideally be tracked at short intervals over
a long period of time to detect developments in their
condition or predict deterioration in advance so that
proper care can be administered. To increase the con-
nectivity of this type of patient, we have developed a
proof of concept in this work for a FAIR-based data
pipeline that is capable of processing Patient Gener-
ated Health Data (PGHD) in a way that makes it inter-
operable with the existing VODAN-Africa framework
without requiring access to the internet. PGHD refers
to health data that is collected by the patient at their
homes or other settings outside the clinic environment
towards their care (Hussein et al., 2021).
This research began with an interview conducted
with a doctor, a patient and two data managers from
a clinic in Nigeria. The results and discussion sur-
rounding this interview are presented by Kawu et al.
(2024). They find that PGHD is currently already in
use, mostly for addressing white collar hypertension,
this is when patients typically have higher blood pres-
sure readings in a clinic then they do at home. How-
ever this is limited, purely on paper, and not integrated
with the rest of the electronic health records. From
the interviews, Kawu et al. (2024) define four rec-
ommendations for a system integrating PGHD with
digital health systems in low resource settings. The
system should be supportive of the economic condi-
tions of users and should be intuitive and accessible
to all users (I). The metadata surrounding the PGHD
should clearly describe both time and location when
the data is gathered (II). The data should be kept sep-
arate from the traditional clinical data to create trust
in data quality (III). The PGHD should be embedded
as part of the clinical workflow to ensure continued
engagement with patients (IV).
Following recommendation I, we find that the de-
vices to be used for both the collection and communi-
cation of PGHD should be kept both cheap and sim-
ple accounting for the low-resource setting of this re-
search. In a western and more resource-rich context,
wearables can fulfill the role of automatically collect-
ing and communicating PGHD over the internet. We
have briefly investigated the possibility of utilizing
them here, but find these to often be both too expen-
sive and too unintuitive as was also stated by Kawu
et al. (2024). Therefore we opt for a more practi-
cal approach requiring only a digital health monitor
which a patient is able to use themselves and a work-
ing cell phone. To allow the patient or their carer to
communicate the digital health monitor data, we de-
velop an Interactive Voice Response system which is
accessible over any kind of mobile phone, using the
services provided by Africa’s Talking. We include
various types of auxillary data surrounding the cre-
ation of PGHD and keep our data separate from exist-
ing data source in the VODAN-Africa network to ad-
here to respectively recommendations II and III from
Kawu et al. (2024).
Further, Kawu et al. (2023) provides a framework
which describes how PGHD can be made FAIR using
a curation tool. In this work we use The Center for
Expanded Data Annotation and Retrieval (CEDAR,
Musen et al., 2015). In the following section, we will
outline the method used to achieve patient connec-
tivity using our IVR-developed application with the
CEDAR tool.
2 IMPLEMENTATION
In this section we present the implementation details
of our proof of concept for a data pipeline from data
collection up to data storage and analytics. The imple-
mentation presented here has been developed specif-
ically for a generic blood pressure monitor that can
measure a patient’s pulse rate, systolic- and diastolic
blood pressure readings. However, this can be easily
expanded to include other types of digital health mon-
itors, or even incorporate wearable technology via the
internet if this is available. As suggested by Kawu
et al. (2023), some patients may be required to track
multiple PGHD which will require multiple devices,
hence multiple sources of PGHD should be consid-
ered in the integration with the electronic health sys-
tem. It may be the case that two PGHD devices col-
lect the same or similar data, and so, a consolidation
mechanism may need to be in place.
We present an overview of the pipeline in Fig-
ure 1. It is divided into three sections, the first two
of which are further described in the following sec-
tions. The first is the data collection & communica-
tion which contains the creation of Patient Generated
Health Data and the communication of this data to the
clinic using the procedures described in the next sub-
section. The second part is the data storage & process-
ing, which contains all the operations performed on
the data within the clinic to transform the raw PGHD
into FAIR data objects. The third and last step is the
data application & access which uses the processed
data objects stored in a clinic for prediction and visu-
alization, this will be expanded upon further in future
work. The code corresponding to the implementation
outlined is accessible online.
2
The procedures drawn with solid arrows corre-
2
https://github.com/RenVit318/ivr implementation bp
Exploring the Design of Low-End Technology to Increase Patient Connectivity to Electronic Health Records
195

Figure 1: Overview of the complete data pipeline used for the PGHD collection.
spond to procedures present in the current VODAN
implementations. The dashed arrows correspond
to the new pipeline developed in this report. The
dotted arrow leading from “Wearables” directly to
“CEDAR” represents a direct connection between a
digital wearable and CEDAR which requires a direct
internet connection and is therefore not developed in
this work. In the following sections, we will expand
on the pipeline up to and including integration with
CEDAR.
2.1 Data Collection & Communication
The first step in any data pipeline is the collection of
the data to be processed. In the current version of the
pipeline this collection has to be performed manually
by the patient or their caregiver. They do this by ei-
ther using a digital blood pressure monitor they have
at home and reading off their vitals from its screen,
or by simply reading these off from a wearable that
continuously monitors them. In both cases the pa-
tient will have to memorize or write down their pulse
rate, systolic blood pressure and diastolic blood pres-
sure. Once they have this data, they call a cellphone
number connected to our Interactive Voice Response
(IVR) system, which we have set up using a service
from Africa’s Talking (AT).
Interactive voice response (IVR) is a technology
that allows people to call and converse with a pro-
gram that uses a synthetic human voice to communi-
cate information and ask questions. The human inter-
acting with this service can respond using the number
keys on their cell phone.
3
This technology is ubiq-
uitous in telephone systems for hospitals, where you
are normally first placed inside a queue and need to
interact with an IVR program first to be redirected
3
In some modern implementations of IVR users can
also respond by speaking which is then translated into text
by a machine learning algorithm. However this is naturally
less reliable than manual key press of numbers on the mo-
bile phone, and is also not currently offered as a possibility
by Africa’s Talking.
to the correct department or physician. Our decision
to utilize IVR for this implementation mainly stems
from its accessibility; there is no requirement for liter-
acy as the data requests are purely conveyed through
sound and key presses. Of course a certain level of
technical literacy is required to utilize both the digi-
tal blood pressure monitor and the cellphone, but that
is inescapable. In addition to this, the IVR service
provided by AT in Nigeria is also the cheapest option
which means less financial strain on the clinics.
We have created a custom application written in
Python that runs using Flask (Grinberg, 2018) and uti-
lizes the Africa’s Talking IVR service.
4
Our applica-
tion communicates with this using Hypertext Transfer
Protocol (HTTP) requests and provides instructions
written in Extensible Markup Language (XML) inter-
pretable by their Application Programming Interface
(API) which describe what to do during a call with a
patient, an example of this XML code is presented in
Figure 3. The full procedure is outlined in Figure 2. It
begins with the PGHD being collected using the BP
monitor (step 1) and then calling our virtual phone
number registered with Africa’s Talking (step 2). The
patient is then greeted and asked for their identifica-
tion number. In future implementation work this can
be used together with the incoming phone number to
authenticate the patient and deduce which type of data
needs to be requested. After this the medical data is
requested field-by-field (steps 3-6), the patient can re-
spond by inserting the corresponding measurements
using the number pad on their phone. Then some ad-
ditional auxiliary information surrounding the collec-
tion of the data is requested by providing numbered
options. Currently implemented are the position of
the patient during data collection (sitting, standing,
laying), location during the collection (at home, or
not at home), and the person collecting the data (the
patient themselves, or a caregiver). The decision to
use these fields is based on the results of Kawu et al.
(2024) but could change in the process of future im-
4
https://africastalking.com/voice
HEALTHINF 2024 - 17th International Conference on Health Informatics
196
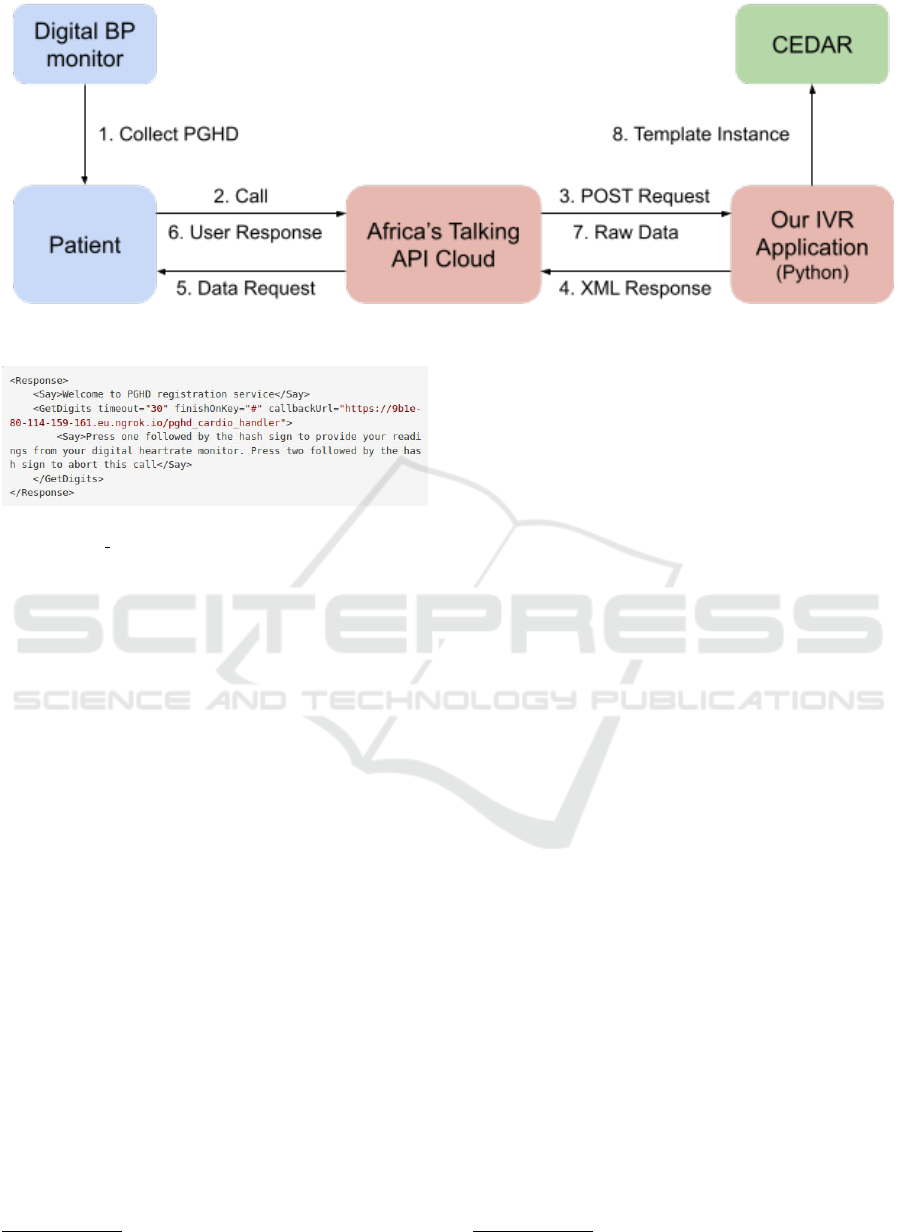
Figure 2: IVR communication protocol used in this work described in more detail in text.
Figure 3: XML Response given when sending a POST re-
quest to /pghd handler.
plementation research. A field that is currently empty
is some identifier of the device used for collecting
data, this could be the International Mobile Equip-
ment Identity (IMEI) number which should be known
in the clinic and related to the patient ID.
The call is ended when the application has col-
lected all data and stored it in a local JSON file. This
file is sent using an HTTP POST request to CEDAR
using its REST API accessed through application/json
(steps 7 and 8 in Figure 2). In this step the data is con-
verted into FAIR objects and saved as an instance of
our CEDAR template which we will describe in more
detail in the next subsection. Once the data is suc-
cessfully sent it is immediately deleted from our IVR
application and it is ready for the next caller. In a real-
life implementation our application would be hosted
on a web-server, but for the purposes of testing this
prototype we temporarily host it online using ngrok.
5
2.2 Data Storage & Processing
The second step in the data pipeline commences once
the data communication procedure described above is
completed. As described in more detail above, the
collected data is sent to the CEDAR metadata center
as an instance of the Patient Generated Health
Data: Blood Pressure metadata template created
for this project based on the existing outpatient data
5
https://ngrok.com/
(OPD) template. Figure 4 shows a filled-in example
of respectively the JSON file and the CEDAR tem-
plate. The JSON file snippet (left) is how the data
is stored during the data collection phase and what
is sent to the CEDAR API in step 8 of Figure 2,
the CEDAR template (right) shows the same infor-
mation in the CEDAR webview. Each CEDAR field
is described in the JSON file by its field name, e.g.
hasPulseRate; this is not the same as the preferred
label shown in the template (”The Pulse of the client”)
which is more verbose to improve human readability.
Within the curly brackets the value (@value) and con-
straint on allowed values (@type) are given. For the
three collection detail fields (CollectionPosition,
CollectionLocation, CollectionPerson) the in-
formation is represented as a URI (@id) which is re-
lated to the ontology made for this project that will be
discussed further later in this section.
Here the PGHD recorded by the patient is stored
along with the date and time of collection, pa-
tient identification number and a new field indicating
whether IVR was used to communicate the data to
CEDAR. This final field is included for possible meta
analyses in future work. The date of collection of the
PGHD is currently assumed to be the same as the date
of the phone call. However in reality this might not al-
ways be the case. Nevertheless due to possible issues
that might arise in entering a long form value such as
date using IVR we leave the exploration of this prob-
lem to future work.
The fields in the CEDAR template related to
the measurement of blood pressure data are linked
through Uniform Resource Identifiers (URI) to the
Semantic Mining of Activity, Social, and Health data
(SMASH) ontology (Phan et al., 2016).
6
This ontol-
ogy describes concepts related to activity, social and
health data. In this work we only utilize the pulse rate,
6
https://bioportal.bioontology.org/ontologies/SMASH
Exploring the Design of Low-End Technology to Increase Patient Connectivity to Electronic Health Records
197
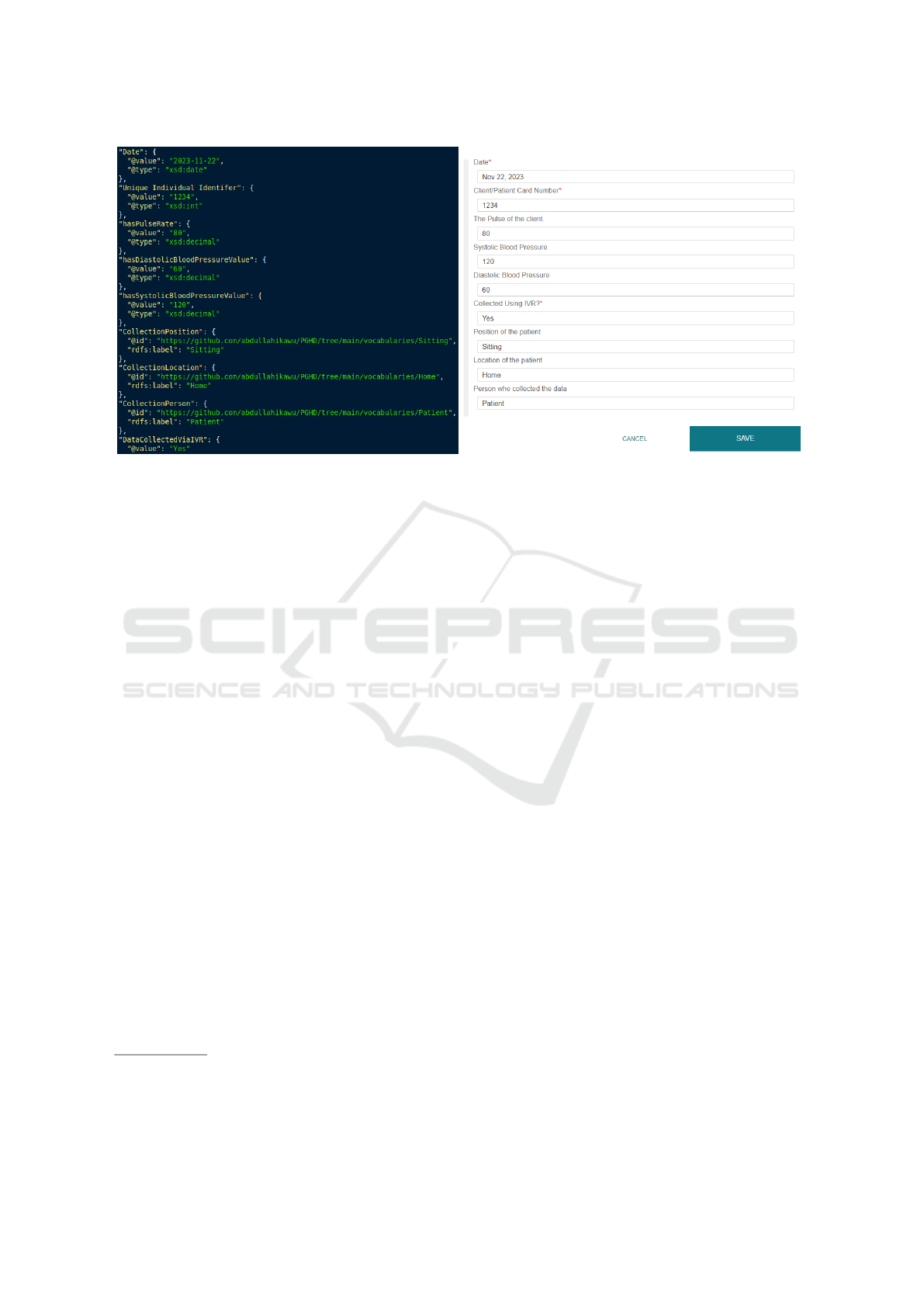
Figure 4: left: Snippet of the JSON package sent to CEDAR. right: Filled-In CEDAR template for collecting Patient Gener-
ated Health Data from a digital blood pressure monitor as used for this project.
systolic- and diastolic blood pressure field, however
future work could incorporate fields such as those re-
lated to whether or not a patient does physical activity.
The patient number is directly linked to the Unique
Individual Identifier from the VODANTERMS on-
tology for interoperability with existing OPD records
that contain information such as the age, gender, di-
agnoses and other physical information of the pa-
tient which could be used for additional monitoring
(Van Reisen et al., 2022).
7
The fields related to the
auxiliary information surrounding the measurement
of blood pressure data are linked to a custom ontol-
ogy hosted on BioPortal named PGHD-BP.
8
We have
opted to create our own ontology for this to allow for
more flexibility in describing the measurements based
on current thoughts and later feedback by patients and
clinicians using our system. The PGHD-BP ontology
is specifically scoped to describe additional informa-
tion surrounding BP data collected outside a clinical
setting. It currently consists of two broad classes. The
first is CommunicationDetail describing the ancil-
lary information regarding the data communication
which currently only describes whether or not the
data was collected via the IVR service. The second
is CollectionDetail which contains the auxiliary
information surrounding data collection described in
subsection 2.1.
7
https://bioportal.bioontology.org/ontologies/VODAN
A-GENERAL
8
https://bioportal.bioontology.org/ontologies/PGHD-B
P
3 DISCUSSION
We have presented a prototype of an implementation
for Patient Generated Health Data with the VODAN-
Africa architecture through CEDAR using Interactive
Voice Response. The implementation presented here
fully adheres to recommendations I (any PGHD inte-
gration system should be conscious of the economic
conditions of its users) and III (PGHD data should
be kept separate from clinical data) by Kawu et al.
(2024). It also partially adheres to recommendation II
(PGHD should be accompanied with contextual meta-
data) insofar as there are rough descriptions surround-
ing the data collection, and a date is included with the
communicated data. As mentioned in subsection 2.2
this date might not be the same as the real date of data
collection, which is an issue to be tackled in future
work. Recommendation IV (PGHD should be em-
bedded in the clinical workflow) was not taken into
consideration in this work and is also left for future
implementation research.
It is important to note that the work presented in
section 2 provides a complete workflow or pipeline
for the implementation of a PGHD integration sys-
tem, and a fully functional and tested prototype from
data collection up to and including the storage on
(a local version of) CEDAR. However integration
with the VODAN-Africa architecture is not yet imple-
mented, and would require further real-world testing.
However once this integration is deployed, PGHD
data could be included in the existing VODAN-Africa
dashboard, both at an in-clinic level where clinicians
can track the vitals of their patients in ‘real-time’ and
HEALTHINF 2024 - 17th International Conference on Health Informatics
198

aggregated on a national/global level to track the us-
age of PGHD across clinics.
Another crucial aspect that is still lacking in this
pipeline is a proper method of access control. Allow-
ing patients to send data to clinics remotely can in-
troduce a range of issues from both data mismanage-
ment and impersonation perspectives. To tackle these
issues, secure authentication mechanisms would have
to be implemented (Jati et al., 2022). These might
make use of some combination of incoming phone
number, patient ID and date of birth to make sure the
reported PGHD are assigned to the correct patients.
Despite some of these shortcomings, the pipeline
presented in this work provides a strong basis for the
integration of PGHD in low-resource settings which
has not been developed before to the authors knowl-
edge and can serve as a great tool in further improv-
ing the capacities of healthcare in these regions that
require it most.
4 CONCLUSIONS
We have developed a pipeline for the collection and
communication of Patient Generated Health Data
(PGHD) that is functional from the point of data col-
lection to the storage on a (local) CEDAR installation.
The communication of PGHD is performed using
the Interactive Voice Response service provided by
Africa’s Talking in which an automated voice prompts
the user to insert the readings of vitals that they have
gathered themselves along with auxiliary informa-
tion surrounding the measurements. The implemen-
tation presented here is specifically made for an at-
home blood pressure monitor that is able to measure
a patient’s pulse rate and both systolic- and diastolic
blood pressure. However expansion to more types of
measurement devices is relatively simple by design.
Upon data collection in CEDAR the provided PGHD
is automatically FAIRified and enriched with meta-
data among which fields describing the date when the
data was submitted and a flag indicating that the data
was communicated using the IVR service.
While the prototype outlined here is not yet ready
for direct implementation with the VODAN-Africa
framework, once expanded, the inclusion of PGHD
with existing Outpatient Data objects could form a
cornerstone in further care for patients suffering from
long term health problems in low resource settings
such as the one considered in this work. We therefore
emphasize the importance of future work to evaluate
the acceptability and usability of this system with the
patients that need it most.
ACKNOWLEDGEMENTS
We would like to thank the students in Fieldlab 5
from the 2023 Leiden University course ’Data Sci-
ence in Practice’ taught by Prof.dr.Mirjam van Reisen
for their hard work and valuable insights in develop-
ing the pipeline presented in this work.
This work was conducted with the financial sup-
port of the Science Foundation Ireland Centre for
Research Training in Digitally-Enhanced Reality (d-
real) under Grant No. 18/CRT/6224. For the pur-
pose of Open Access, the author has applied a CC
BY public copyright licence to any Author Accepted
Manuscript version arising from this submission
REFERENCES
Grinberg, M. (2018). Flask web development: develop-
ing web applications with python. ” O’Reilly Media,
Inc.”.
Hussein, R., Crutzen, R., Gutenberg, J., Kulnik, S., Sare-
ban, M., and Niebauer, J. (2021). Patient-Generated
Health Data (PGHD) Interoperability: An Integrative
Perspective, volume 281, page 228–232. Studies in
health technology and informatics.
Jati, P. H. P., van Reisen, M., Flikkenschild, E., Oladipo,
F., Meerman, B., Plug, R., and Nodehi, S. (2022).
Data Access, Control, and Privacy Protection in
the VODAN-Africa Architecture. Data Intelligence,
4(4):938–954.
Kawu, A. A., Kievit, R., Abubakar, A., van Reisen, M.,
O’Sullivan, D., and Hederman, L. (2024). Explor-
ing the integration of a patient generated health data
in a fair digital health system in low-resourced set-
tings: A user-centered approach. In Proceedings of the
4th African Human Computer Interaction Conference,
AfriCHI ’23, page 215–220, New York, NY, USA. As-
sociation for Computing Machinery.
Kawu, A. A., O’Sullivan, D., Hederman, L., and Reisen, M.
(2023). Fair4pghd: A framework for fair implementa-
tion over pghd. FAIR Connect, 1:35–40.
Musen, M. A., Bean, C. A., Cheung, K.-H., Dumontier, M.,
Durante, K. A., Gevaert, O., Gonzalez-Beltran, A.,
Khatri, P., Kleinstein, S. H., O’Connor, M. J., Pouliot,
Y., Rocca-Serra, P., Sansone, S.-A., Wiser, J. A., , and
the CEDAR team (2015). The center for expanded
data annotation and retrieval. Journal of the American
Medical Informatics Association, 22(6):1148–1152.
Phan, N. H., Dou, D., Wang, H., Kil, D., and Piniewski,
B. (2016). Ontology-based deep learning for human
behavior prediction with explanations in health social
networks (information sciences - if: 4.832). Informa-
tion Sciences, 384.
Plug, R., Liang, Y., Basajja, M., Aktau, A., Jati, P., Amare,
S., Taye, G., Mpezamihigo, M., Oladipo, F., and van
Reisen, M. (2022). Fair and gdpr compliant popula-
tion health data generation, processing and analytics.
Exploring the Design of Low-End Technology to Increase Patient Connectivity to Electronic Health Records
199

van Reisen, M., Oladipo, F., Stokmans, M., Mpezamihgo,
M., Folorunso, S., Schultes, E., Basajja, M., Aktau,
A., Amare, S. Y., Taye, G. T., Purnama Jati, P. H.,
Chindoza, K., Wirtz, M., Ghardallou, M., van Stam,
G., Ayele, W., Nalugala, R., Abdullahi, I., Osigwe, O.,
Graybeal, J., Medhanyie, A. A., Kawu, A. A., Liu, F.,
Wolstencroft, K., Flikkenschild, E., Lin, Y., Stocker,
J., and Musen, M. A. (2021). Design of a fair digital
data health infrastructure in africa for covid-19 report-
ing and research. Advanced Genetics, 2(2):e10050.
Van Reisen, M., Oladipo, F. O., Mpezamihigo, M., Plug,
R., Basajja, M., Aktau, A., Jati, P. H. P., Nalugala,
R., Folorunso, S., Amare, S. Y., Abdulahi, I., Afo-
labi, O. O., Mwesigwa, E., Taye, G. T., Kawu, A.,
Ghardallou, M., Liang, Y., Osigwe, O., Medhanyie,
A. A., and Mawere, M. (2022). Incomplete COVID-
19 Data: The Curation of Medical Health Data by the
Virus Outbreak Data Network-Africa. Data Intelli-
gence, 4(4):673–697.
Wilkinson, M. D., Dumontier, M., Aalbersberg, I. J., Apple-
ton, G., Axton, M., Baak, A., Blomberg, N., Boiten,
J.-W., da Silva Santos, L. B., Bourne, P. E., and et al.
(2016). The fair guiding principles for scientific data
management and stewardship. Scientific Data, 3(1).
HEALTHINF 2024 - 17th International Conference on Health Informatics
200
