
Sustainable Printed Electrodes for Energy Harvesting from Urine to
Power IoT Sensor Nodes in Smart Diapers
Muhammad Tanweer
1 a
, Raimo Sepponen
2
, I. Oguz Tanzer
1
and Kari Halonen
1
1
Department of Electronics and Nanoengineering, Aalto University, Espoo 02150, Finland
2
Department of Automation and Electrical Engineering, Aalto University, Espoo 02150, Finland
fi
Keywords:
Energy Harvesting, Green Electronics, IoT Sensors, Printed Electronics, Self-Powered, Smart Diaper,
Wearable Biomedical Devices.
Abstract:
The expansion of Internet of Things (IoT) devices is rapidly increasing across various aspects of life, notably
in wearable healthcare. With billions of already deployed IoT sensor nodes, this figure is anticipated to
escalate into the hundreds of billions in coming years. One of the most significant challenges is how
to economically power these devices by adopting sustainable and environment-friendly solutions as the
conventional power sources are inadequate to meet the demands of this vast IoT ecosystem. In recent years,
innovative approaches have emerged to design energy-optimized electronic systems, opening a pathway for
applications based on energy harvesting. In this study, a novel energy harvesting solution is proposed by
developing sustainable and disposable harvesting electrodes, leveraging the capabilities of printed electronics
technology. These electrodes are engineered to harvest energy from human urine, a readily available resource,
to power the energy-efficient wearable IoT sensor nodes of smart diapers. A comprehensive characterization
of these harvesting electrodes is conducted using pseudo-urine as an electrolyte within a controlled laboratory
environment. The results demonstrate great promise for the development of self-powered IoT sensor nodes of
smart diapers, with the capacity for overnight operations lasting up to 12 hours.
1 INTRODUCTION
Energy harvesting from saltwater, for example, salt
concentration gradient in seawater, has been a topic
of interest since the 1970s. Despite its potential,
it has historically received limited attention due to
its comparative limitations against more promising
energy sources (Muhthassim et al., 2018). However,
with advancements in integrated circuit technology,
the development of power-efficient circuits and
systems that operate at micro- and nano-watt scales
has emerged. This technological progress has
paved the way for energy harvesting from ambient
surroundings on a microscale, enabling the powering
of IoT devices.
Zinc-carbon-based dry batteries with manganese
dioxide and ammonium chloride electrolytes have
been available commercially for more than 150 years
(Kordesch and Taucher-Mautner, 2009; Linden and
Reddy, 2001). A market growth trend is expected
for zinc-carbon batteries because of their sustainable
a
https://orcid.org/0000-0001-7425-2452
and environment-friendly nature as compared to other
competitors (Reports, 2022). However, the wide
use of ammonium chloride as an electrolyte in dry
batteries still poses challenges of being hazardous for
humans (NJ-Gov, 2016; Pelner, 1956) and harmful to
aquatic life (Rani et al., 1998).
Typical human urine contains sodium, chloride,
and potassium electrolytes along with more than
150 different constituents as studied by investigators
at National Aeronautics and Space Administration
(NASA) (Putnam et al., 1971). The specific
conductivity of sodium chloride solution (1.3
k℧cm
2
/mol) is close to the specific conductivity of
ammonium chloride solution (1.5 k℧cm
2
/mol) of the
same concentration at room temperature (Murtom
¨
aki
et al., 2018). The presence of these electrolytes in
urine makes it suitable to be used as an electrolyte
for harvesting electrodes printed with materials like
zinc-carbon to harness chemical energy from urine.
In this study, a novel energy-harvesting approach is
proposed involving the design and development of
zinc-carbon-based flexible electrodes using printed
electronics technology to harvest chemical energy
Tanweer, M., Sepponen, R., Tanzer, I. and Halonen, K.
Sustainable Printed Electrodes for Energy Harvesting from Urine to Power IoT Sensor Nodes in Smart Diapers.
DOI: 10.5220/0012424100003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 65-70
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
65

from urine in diapers. The electrodes are printed
directly onto the diaper back-sheets, aiming to
offer a sustainable, environmentally friendly, and
cost-effective disposable solution. This innovation
intends to eliminate the need for batteries, which are
not only hazardous but also challenging to recycle.
The primary objective is to harvest the chemical
energy to supply power to the energy-optimized
circuits of IoT sensor nodes and integrate the printed
harvesting electrode seamlessly into smart diapers.
The materials used to develop the printed
harvesting electrodes, the preparation of pseudo
urine, the experimental setup, and the various
measurement scenarios are discussed in section 2 of
this article. Section 3 includes a discussion of the
obtained measurement results with the help of plots.
Finally, section 4 concludes the work by discussing
the findings and limitations along with the potential
prospects of the research.
2 MATERIALS AND METHODS
The rolled sheets and printable inks of zinc and
carbon materials are used to develop the electrodes to
harvest chemical energy from indigenously developed
pseudo-urine. The design and development of
electrodes, formulation of pseudo-urine, and
the details of measurement setup for various
measurements are discussed in the following
subsections.
2.1 Energy Harvesting Mechanism
Off-the-shelf flexible rolled sheets of carbon
material and zinc metal are used initially to develop
the harvesting electrodes for the preliminary
measurements to establish the proof-of-concept. A
sheet of pure conductive carbon holding a thickness
of 0.1 mm (Fly Fiber, 2022) is used to develop the
cathode having a size of 7 cm x 7 cm. The anode
of the same size is developed with an off-the-shelf
flexible rolled sheet of 99.99% pure zinc metal
(Tools Store, 2022) with a thickness of 0.2 mm. Both
electrodes have a surface area of 98 cm
2
each with
49 cm
2
on either side of the electrode. The electrical
connections for the carbon cathode are implemented
using a small patch of copper tape with conductive
adhesive (3M, StPaul, Minnesota, USA) and for the
zinc anode by directly soldering the copper wire to
the sheet surface.
The printed harvesting electrodes of 7 cm x 7 cm
size are developed using zinc and carbon inks on the
inner side of the diaper back sheet. The Figure. 1(a)
Figure 1: Printed electrode design for energy harvesting;
(a) carbon ink cathode, (b) zinc ink anode over the current
collector layer of carbon ink.
depicts the cross-section of the cathode electrode with
printed carbon ink on the single side of the diaper
back sheet and 1(b) shows the cross-section of the
anode with printed zinc ink on top of the carbon-based
current collector layer.
2.1.1 Fabrication of Printed Electrodes
The cathode (reducing electrode) with the same
geometry as described in Figure 1 is fabricated
by deposition and curing of electrically conductive
carbon ink (Saral Carbon 700A, by Saralon GmbH).
Consecutive two layers of carbon ink with a
sheet resistance of 30 Ω/□/25 µm are deposited
sequentially and each layer is thermally dried in a
95 °C preheated oven (ProtoFlow E, by LPKF Laser
& Electronics) at 100 °C for 10 minutes. The
electrical connections are implemented using a small
patch of copper tape with conductive adhesive (3M,
StPaul, Minnesota, USA) on the dried carbon ink
layer. The water-resistant tape is employed to make
the connections waterproof, preventing short circuits.
The anode (oxidizing electrode) is fabricated by
depositing and curing the electrically resistive zinc
ink (Saral Zinc 700, by Saralon GmbH). Consecutive
two layers of Zinc ink with a thickness of 100
microns are deposited sequentially and each layer is
thermally dried in a 95 °C preheated oven (ProtoFlow
E, by LPKF Laser & Electronics) at 100 °C for 10
minutes. Two consecutive layers of carbon ink are
printed as a current collector under the zinc layer as
Figure 2: Fabricated flexible harvesting electrodes on diaper
back sheets; (a) carbon ink cathode, (b) zinc ink anode.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
66

shown in Figure 1(b) because of the low conductive
properties of zinc ink. The finished version of the
printed cathode and anode are shown in Figure 2.
2.1.2 Pseudo-Urine as Electrolyte
The pseudo-urine is used as an electrolyte to
replace the hazardous electrolyte ammonium chloride
(NH
4
Cl) which is used in commercial zinc-carbon
dry batteries. The reference concentration of sodium
electrolytes in human urine exhibits a range of 80
to 240 mmol/l and the concentration of chloride
electrolytes has a range of 85 to 260 mmol/l for
adults, as reported by the Laboratory of Helsinki
University Hospital and FimLabs of Finland (HUS,
2023; FimLab, 2023). In this study, the chemical
composition of pseudo-urine is formulated at Aalto
University labs using sodium chloride (NaCl) at
an electrolyte concentration of 220 mmol/l. The
designed concentration falls within the referenced
concentrations for sodium and chloride electrolytes
in the human urine. It is noteworthy that a one-mole
solution of NaCl consists of a molar mass of 23 g/mol
for sodium and 35.5 g/mol for chloride, as outlined in
a reference source (Lide, 2009).
2.2 Measurement Setup
A galvanic cell is established as a vertical container,
holding the pseudo-urine of one liter as electrolyte.
The harvesting electrodes are deployed on the inner
side of the container in a parallel orientation. The
salt bridge of pseudo-urine connects the oxidation
and reduction reactions of these half cells. Figure 3
depicts the established measurement setup to evaluate
the harvested energy. Various measurement scenarios
are employed to analyze the harvested voltage level
and amount of energy from pseudo-urine. The
multimeter (73 III, Fluke) is used to measure
the open circuit voltages (OCV) of the developed
galvanic cell. A direct current (DC) energy
analyzer and power profiler instrument Otii Arc Pro
(Qoitech AB, Sweden) is employed with Otii Battery
Toolbox (Qoitech AB, Sweden) to characterize the
zinc-carbon flexible electrodes for energy harvesting
from pseudo-urine as an electrolyte.
In the first measurement scenario, the rolled
sheet-based zinc-carbon harvesting electrodes with
a surface area of 98 cm
2
are deployed in the
galvanic cell with an inter-electrode distance of 7
cm. The pseudo-urine-based electrolyte is poured
inside the cell and the immersed depth of electrodes
is increased by 1 cm in each measurement hence
increasing the immersed electrode area by 14 cm
2
for each measurement to analyze the effect of the
Figure 3: Measurement setup to harvest energy from
pseudo-urine.
variations in the partially immersed electrode area
on harvested energy. The voltage level and the
amount of harvested energy are analyzed to emulate
the frequent urination events when the electrodes are
partially immersed in small urine quantities inside
diapers. The second measurement scenario uses
fully immersed zinc-carbon electrodes in a one-liter
pseudo-urine electrolyte of the galvanic cell and the
inter-electrode distance is varied from 5 mm to 30 mm
with an increment of 5 mm for each measurement.
In the third measurement scenario, the printed
zinc-carbon flexible electrodes are fully immersed
in one liter of pseudo-urine electrolyte of the
galvanic cell in parallel orientation at a distance
of 7 cm to evaluate the change in voltages and
current flow when a constant power of 800 uW is
continuously withdrawn. The fourth measurement
scenario evaluates the intermittent energy harvesting
sessions every 30 minutes where the same printed
zinc-carbon electrodes are fully immersed in the
pseudo-urine electrolyte of a galvanic cell for 11
hours to understand the possibility of powering the
IoT sensor node overnight to perform measurements
for multiple urination events inside the same diaper.
3 RESULTS AND DISCUSSION
In the evaluation measurements, the OCV level of
1032 mV is measured for the developed single
galvanic cell and 2060 mV when the two galvanic
cells are connected in series. The harvested voltage
level of the single galvanic cell, with fully immersed
electrodes, drops to 875 mV when connected to
a constant power load of 100 uW and further
drops to 831 mV when the load is increased to
800 uW. The effect of variation in the immersed
electrode area out of the total area on the amount of
harvested energy from the first measurement scenario
is depicted in Figure 4. It is observed that the amount
of harvested energy is linearly proportional to the
immersed surface area of the harvesting electrode
with a variation of 1.9 uWh/cm
2
to 4.3 uWh/cm
2
when the percentage of the immersed surface area
of the single electrode increases from 14% to 100%.
Sustainable Printed Electrodes for Energy Harvesting from Urine to Power IoT Sensor Nodes in Smart Diapers
67
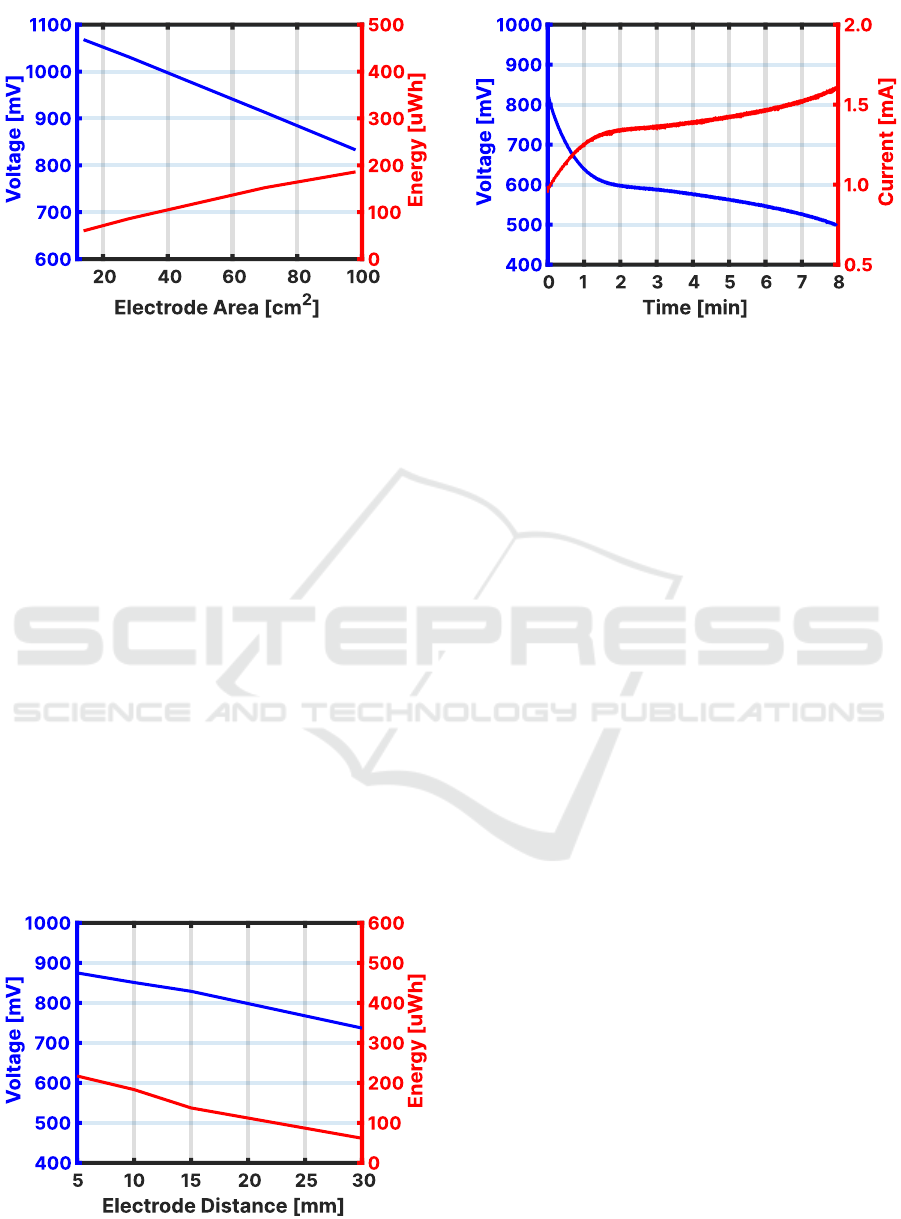
Figure 4: The effect of surface area on the harvested energy
and voltages.
Another interesting observation is that the voltage
level gets as high as 1068 mV when the percentage
of the submerged surface area is reduced to 14% and
the galvanic cell is connected to a constant power load
of 100 uW.
The effect of variations in the inter-electrode
distance on the amount of harvested energy is
analyzed in the second measurement scenario.
The galvanic cell with fully immersed zinc-carbon
electrodes in the pseudo-urine electrolyte is loaded
with 100 uW for the initial 10 seconds. The load
is increased to 500 uW power afterward and the
harvested energy is recorded until the voltage level
drops to 500 mV, the lower threshold of Otii Battery
Toolbox (Qoitech AB, Sweden). The measurement
is repeated by increasing the inter-electrode distance
by 5 mm for each measurement using the same
electrodes and electrolyte. The results of harvested
energy and starting voltage levels with inter-electrode
distance variations are presented in Figure 5. It
is observed that the amount of harvested energy
reduces linearly with an increase in the inter-electrode
Figure 5: The effect of inter-electrode distance on the
harvested energy and voltages.
Figure 6: The output voltage and output current response of
printed harvesting electrodes.
distance and drops 25% when the distance is doubled.
The non-linearity at the inter-electrode distance of 15
mm is observed due to measurement errors because
each measurement was taken only once. The starting
voltage level of the galvanic cell has also seen a drop
of 130 mV because the redox process deteriorates
the surface of the harvesting electrodes over the
harvesting time period (Singh et al., 2021).
The third measurement scenario involves the
utilization of printed zinc-carbon electrodes on the
inner side of the diaper back sheet having 50% surface
area (49 cm
2
) compared to sheet-based electrodes.
The printed electrodes are fully immersed in the
one-liter pseudo-urine electrolyte and connected to
the constant power load of 800 uW. The trend of
voltage level and current flow is observed over time
until the voltage level drops to 500 mV. The voltage
and current response of the printed zinc-carbon
electrodes is depicted in Figure 6. It is observed
that the single cell of single-side printed zinc-carbon
electrodes is capable of supporting output current
flow up to 1.6 mA.
The same setup of printed zinc-carbon electrodes
from the previous measurement scenario is further
used in the fourth measurement scenario to evaluate
the performance of harvesting electrodes when fully
immersed in the same electrolyte for a longer period.
A fixed power amount of 800 uW is withdrawn in
each harvest session until the voltage level reduces to
500mV and the session is repeated every 30 minutes
for up to 11 hours. The results of the harvested
energy and the starting voltage level of the single
galvanic cell are shown in Figure 7. It is observed
that the starting voltage level and the amount of the
harvested energy from the same electrodes in the
same electrolyte drop linearly for each subsequent
harvesting session. It is evident that even after
11 hours the printed zinc-carbon electrodes are still
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
68

Figure 7: The harvested energy and voltages of the printed
electrode over a longer time in the electrolyte.
capable of providing 25% of the energy amount from
the first harvesting session. The total drop in single
cell starting voltage level is measured as low as 110
mV after 22 harvesting sessions.
The proposed novel chemical energy harvesting
solution using printed zinc-carbon electrodes in
a pseudo-urine electrolyte has yielded promising
results. These electrodes may be adopted with a
power-optimized on-chip system for smart diapers
as developed by (Tanweer et al., 2023b) interfaced
with printed coplanar capacitive sensors developed
by (Tanweer et al., 2023c) to detect wet diapers and
quantify voided volumes in diapers.
The harvested energy from the proposed printed
harvesting electrodes is sufficient to power on-chip
circuits fundamental for most of the front-end sensor
interface electronics such as 0.39–3.56 µW wide
dynamic range universal multi-sensor interface circuit
by (Moayer et al., 2020) and 462 nW 2-axis gesture
sensor interface based on capacitively controlled ring
oscillators (Pulkkinen et al., 2017). It can also power
energy-optimized wireless communication blocks of
the IoT sensor nodes such as a low-power wireless
transceiver with a 67 nW differential pulse-position
modulation (DPPM) transmitter (Pulkkinen et al.,
2020). The on-chip sensor-end electronics can also
be combined with the low-power DPPM transmitter
to develop a self-powered IoT sensor node for smart
diapers running on harvested energy from urine
with sustainable and economical printed harvesting
electrodes.
4 CONCLUSIONS
This research introduces an innovative approach to
creating printed electrodes that can harvest energy
from pseudo-urine, offering a sustainable solution for
powering IoT sensors in smart diapers. The study
explores the potential of using urine as a renewable
energy source to operate these sensors and interfaces,
emphasizing the importance of eco-friendly practices
in the development of wearable technology. By
utilizing zinc and carbon materials, these electrodes
are printed directly onto the back sheets of diapers,
a process that aligns well with current diaper
manufacturing techniques. The primary focus of the
research is to test the effectiveness of these disposable
zinc-carbon electrodes in generating power from
urine. This breakthrough presents for the first time
a significant step towards developing smart diaper
sensors that operate overnight without the need for
traditional batteries, promoting sustainability and
energy efficiency.
In the future, an on-chip power management
unit might be incorporated with the single cell
of proposed flexible printed harvesting electrodes
to harness energy from urine and to ensure the
continuous supply of regulated voltage to the on-chip
front-end sensor interface circuits, control systems,
and communication interfaces of an IoT sensor node
developed for smart diapers. The changes in the
level of generated voltages from printed harvesting
electrodes might also be further studied to provide
an additional parameter together with measurement
data from the printed coplanar capacitive sensors
for reliable and precise quantification of the voided
volume inside the diaper which is otherwise affected
by body weight on the wet diaper as discussed by
(Tanweer et al., 2023a).
ACKNOWLEDGEMENTS
Technical assistance towards the printed harvester
electrodes from Mr. Muhammad Qaisar Nadeem is
gratefully acknowledged. The authors would also like
to thank Dipesh C. Monga, Aalto University, Finland
for his valuable review and comments.
REFERENCES
FimLab (2023). dU-Kloridi (2077 dU-Cl ) — vshp.fi. https:
//www.vshp.fi/medserv/klkemi/fi/ohjekirja/2077.htm.
[Accessed 02-11-2023].
Fly Fiber (2022). Fly-Fiber 10 Stück
High Pure Flexible Graphite Sheet Folien
Graphite Film Conductive Graphitpapier
250x200x0.1 mm : Amazon.de: Küche,
Haushalt & Wohnen — amazon.de.
h t t p s : / / w w w. a m a z o n . d e / d p / B 0 8 3 L X K 9 G 6.
[Accessed 06-11-2023].
Sustainable Printed Electrodes for Energy Harvesting from Urine to Power IoT Sensor Nodes in Smart Diapers
69

HUS (2023). HUSLAB - Natrium, vuorokausivirtsasta —
huslab.fi. https://huslab.fi/ohjekirja/2376.html.
[Accessed 02-11-2023].
Kordesch, K. and Taucher-Mautner, W. (2009). Primary
batteries – aqueous systems — leclanch
´
e and
zinc–carbon. In Garche, J., editor, Encyclopedia
of Electrochemical Power Sources, pages 43–54.
Elsevier, Amsterdam.
Lide, D. R., editor (2009). CRC handbook of chemistry and
physics, 90th edition. CRC Press, Boca Raton, FL, 90
edition.
Linden, D. and Reddy, T. (2001). Handbook of batteries.
Handbook Series. McGraw-Hill Professional, New
York, NY, 3 edition.
Moayer, M. M., Salomaa, J., and Halonen, K. A. I. (2020).
A 0.39–3.56-µW Wide-Dynamic-Range Universal
Multi-Sensor Interface Circuit. IEEE Sensors Journal,
20(20):12262–12273.
Muhthassim, B., Thian, X. K., and Hasan, K. N. M.
(2018). Energy harvesting from salinity gradient. IOP
Conference Series: Earth and Environmental Science,
140:012045.
Murtom
¨
aki, L., Kallio, T., Lahtinen, R., and Ikonen, M.
(2018). Fundamental electrochemistry: An interactive
e-book with quizzes in mycourses learning platform.
NJ-Gov (2016). nj.gov. https://www.nj.gov/health/eoh/rtkw
eb/documents/fs/0013.pdf. [Accessed 02-11-2023].
Pelner, L. (1956). TOXIC EFFECTS OF AMMONIUM
CHLORIDE. Journal of the American Medical
Association, 162(1):63.
Pulkkinen, M., Haapala, T., Salomaa, J., and Halonen, K.
(2020). Low-power wireless transceiver with 67-nW
differential pulse-position modulation transmitter.
IEEE Transactions on Circuits and Systems I: Regular
Papers, 67(12):5468–5481.
Pulkkinen, M., Salomaa, J., Moayer, M. M., Haapala,
T., and Halonen, K. (2017). 462-nW 2-axis gesture
sensor interface based on capacitively controlled ring
oscillators. In 2017 IEEE International Symposium on
Circuits and Systems (ISCAS). IEEE.
Putnam, D., Company-West, M. D. A., Center, L. R.,
Aeronautics, U. S. N., and Administration, S. (1971).
Composition and Concentrative Properties of Human
Urine. NASA contractor report. National Aeronautics
and Space Administration.
Rani, E. F., Elumalai, M., and Balasubramanian, M. P.
(1998). Water, Air, and Soil Pollution, 104(1/2):1–8.
Reports, W. M. (2022). Global Zinc Carbon
Battery Market – Market Reports
World — marketreportsworld.com. h t t p s :
//www.marketreportsworld.com/global- zinc- c
ar b o n- b at te r y- m ar ke t- 2 10 62 60 4. [Accessed
31-10-2023].
Singh, A. K., Pahlevaninezhad, M., Yasri, N., and Roberts,
E. P. L. (2021). Degradation of carbon electrodes in
the all-vanadium redox flow battery. ChemSusChem,
14(9):2100–2111.
Tanweer, M., Gillan, L., Sepponen, R., Tanzer, I. O.,
and Halonen, K. A. (2023a). Evaluation of printed
coplanar capacitive sensors for reliable quantification
of fluids in adult diaper. In MEDICON & CMBEBIH
2023. Springer International Publishing.
Tanweer, M., Monga, D. C., Gillan, L., Sepponen, R.,
Tanzer, I. O., and Halonen, K. A. (2023b). Smart
diaper with printed capacitive sensors and integrated
front-end to monitor voided fluid volume. IEEE
Sensors Journal [submitted 2023].
Tanweer, M., Sepponen, R., Tanzer, I. O., and Halonen,
K. A. (2023c). Development of capacitive sensors
to detect and quantify fluids in the adult diaper.
In Bio-inspired Information and Communications
Technologies, pages 237–245. Springer Nature
Switzerland.
Tools Store, T. L. o. (2022). 5 Stück
reines Zinkblech, hohe Reinheit 99,99
für wissenschaftliches Labor, 140 x
140 x 0,2 mm. : Amazon.de: Gewerbe,
Industrie & Wissenschaft — amazon.de.
https://www.amazon.de/dp/B096SDJS9K. [Accessed
06-11-2023].
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
70
