
Strategic Oversight Across Real-World Health Data Initiatives in a
Complex Health Data Space: A Call for Collective Responsibility
Lotte Geys
1,2,3 a
and Liesbet M. Peeters
1,2,3 b
1
University MS Center, Pelt-Hasselt, Belgium
2
Biomedical Research Institute (BIOMED), Hasselt University, Diepenbeek, Belgium
3
Data Science Institute (DSI), Hasselt University, Diepenbeek, Belgium
Keywords: Landscaping, European Health Data Space, Interoperability Challenges, Information Scattering.
Abstract: Reusing real-world health data is useful, but challenging. Multiple initiatives exist and more are continuously
arising to overcome these challenges, but the strategic oversight across these initiatives is lacking, which leads
to a fragmented ecosystem. An overview of which initiatives that work on unlocking real-world health data,
making this data accessible for research and/or innovation and/or policy and getting an idea about which
aspect of the ecosystem the initiatives are working on would be very helpful. It could help in figuring out how
initiatives can work in synergy in order that consortia can be formed more efficiently. We tried to create an
overview, resulting in a static list, but have thereby run into many problems and difficulties and have noticed
that the information is even more scattered than expected, and often ambiguous and unclear. This paper
highlights the need for strategic oversight in our complex health data space, defines key challenges and
focuses on solutions and strategies for overcoming these challenges, and aims to guide the future of health
data research and innovation on a global scale, offering a valuable resource for stakeholders in the field.
1 REUSING REAL-WORLD
HEALTH DATA IS USEFUL,
BUT CHALLENGING
1.1 Europe Acknowledges Great
Promise in the Reuse of Health
Data
The European Commission has been working for
several years on a strategy for data sharing. In this
context, in February 2022, the Data Act, which is a
proposed regulation that clarifies who is allowed to
create value from data and under which conditions,
was adopted by the European Commission (link to
Data Act: https://digital-strategy.ec.europa.eu/en/
policies/data-act). With a specific focus on the
healthcare domain, Europe has been making
continuous efforts aiming at enhancing the
harmonization and integration of health data, which
is needed in order to be able to create a digitized and
connected healthcare system, as foreseen in the
a
https://orcid.org/0000-0003-1919-9366
b
https://orcid.org/0000-0002-6066-3899
European Health Data Space (EHDS) regulation (link
to EHDS regulation: https://health.ec.europa.eu/
publications/proposal-regulation-european-health-
data-space_en). The EHDS regulation aims at
unleashing the full potential of health data by
supporting the reuse of health data for better
healthcare delivery, better research, innovation and
policy making.
1.2 Several Socio-Technical Challenges
Are Obstructing Us from Scaling
Reusing Health Data
Several socio-technical challenges obstruct us from
reaching the true potential of reusing real-world
health data, such as the level of awareness and
understanding on the use and/or importance of real-
world health data differs between individuals,
different stakeholders have different needs, finding
and assessing health data sources is challenging and
time-consuming, limited interoperability and the
combined complexity of governance, ethical and
Geys, L. and Peeters, L.
Strategic Oversight Across Real-World Health Data Initiatives in a Complex Health Data Space: A Call for Collective Responsibility.
DOI: 10.5220/0012417700003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 2, pages 577-584
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
577

legal barriers, as well as technical challenges in data
management and analyses, impacts the execution of
large-scale initiatives. This section takes a closer
look at these problems and potential solutions.
1.2.1 Limited Awareness Leads to Limited
Engagement
The level of awareness and understanding of the use
and/or importance of real-world health data differs
between individuals, resulting in different levels of
engagement to contribute to the health data space. In
our experience, healthcare professionals or citizens
are often not motivated to consistently collect and
share health data in a structured way, and regulators
are reluctant to acknowledge evidence generated
through non-controlled observational studies. On
the other hand, individuals can be engaged but not
sufficiently educated or trained to make informed
decisions (e.g. a director of a hospital that is willing
to invest in an improved data management strategy,
but is struggling with so-called ‘analysis-paralysis’
because they have no clue where to start). Examples
of activities that can be done to overcome this
challenge include (i) Advocating the message
“Health data - why should you care?” and
showcasing the impact of reusing health data by
sharing success stories from collaborative projects,
presented in a way that resonates with a layman
audience; (ii) Develop and disseminate educational
resources (with different target audiences in mind);
(iii) Facilitate interactions and collaborations and
promote knowledge exchange by organizing
workshops, conferences and other networking
events. By raising awareness, educating, and
demonstrating the benefits of health data, we can
bridge the gap in understanding and encourage
broader engagement in the health data space.
1.2.2 Different Stakeholders Have Different
Needs, Which Makes Large
Collaborative Efforts Complex and
Time-Consuming
Relevant stakeholders include, e.g. citizens,
healthcare professionals, researchers (private and
public), data custodians, industry and regulators.
Meeting the varied needs of different stakeholders
often requires complex coordination efforts. This
complexity can slow down decision-making
processes and the execution of large collaborative
efforts. To mitigate this, fostering a better
understanding and empathy among the various
stakeholders is crucial. This can be achieved by (i)
promoting more conversations and interactions
between different stakeholder groups, facilitating a
deeper appreciation of their unique perspectives and
requirements; (ii) documenting and disseminating
the strategies used within successful multi-
stakeholder projects in order to create a valuable
knowledge repository that can be leveraged by
others facing similar challenges.
1.2.3 Low Adoption of FAIR Guiding
Principles
As stated by Wilkinson et al. (Wilkinson M.D.,
2016), FAIRness -in which FAIR means Findable,
Accessible, Interoperable and Reusable- is a
prerequisite for proper data management and data
stewardship. It is important that real-world health
data is FAIR in the long-term as well (Holub P.,
2018). Finding and assessing health data sources is
challenging and time-consuming. Metadata
catalogues can empower end-users to assess and
compare metadata associated with health data
sources. Some examples of metadata that are of
interest include information about the (i)
organizational set-up and governance model of the
data source; (ii) type of data collected (categorizing
data sources by type, such as electronic health
records, -omics data, or medical images, allows
users to quickly identify datasets relevant to their
research or analytical needs); (iii) number of unique
patient records; (iv) detailed information about the
specific variables or data elements collected; (v)
basic information related to data quality, such as
validity, completeness, accuracy, and any quality
control measures in place. Additionally, health data
sources are heterogeneous in size, maturity and
depth, reducing their potential of reusing it.
Heterogeneous data from various resources are
difficult to integrate, thereby limiting the
interoperability. One approach to tackle this issue
involves the formulation of guidelines that serve as
a framework for standardizing data sources and
harmonizing their structure. Additionally, the
development, implementation, and widespread
adoption of data standards or common data models
are pivotal steps to ensure that health data sources
are more readily accessible and also compatible,
resulting in enhanced potential for reuse and
interconnectivity.
HEALTHINF 2024 - 17th International Conference on Health Informatics
578

1.2.4 The Combined Complexity of
Governance, Ethical and Legal
Barriers, as Well as Technical
Challenges in Health Data Handling
and Analyses, Impacts the Execution
of Large-Scale Initiatives
Some examples of potential solutions to overcome
this moving forward include: (i) Developing and
implementing advanced cutting-edge technologies
that enable secure and privacy-preserving analysis of
health data; (ii) Documenting and streamlining
governance procedures and encouraging sharing of
successful ethical and legal frameworks that have
proven effective in similar projects; (iii) Adjusting
ethical and legal frameworks in collaboration with
funding agencies, policymakers and researchers if
necessary.
2 STRATEGIC OVERSIGHT
ACROSS INITIATIVES IS
USEFUL, BUT CHALLENGING
TO ACHIEVE
Multiple 'health data initiatives’ (in short: initiatives)
exist -and more are continuously arising- to overcome
the challenges discussed in the previous section.
However, the strategic oversight across these
initiatives is lacking. In the context of this paper, the
term 'initiative' is used to encompass all plans,
projects, or studies dedicated to addressing one or
more of the socio-technical challenges listed. We
focus on initiatives that still exist today and are more
than ‘just an idea or concept note’.
2.1 Strategic Oversight Across
Initiatives Is of Utmost Importance
An overview of which initiatives that work on
unlocking real-world health data, making this data
accessible for research and/or innovation and/or
policy and getting an idea about which aspect of the
ecosystem the initiatives are working on would be
very useful, because of a variety of reasons, including
but not limited to:
(i) Guiding newcomers: One of the main reasons
for emphasizing strategic oversight across health data
initiatives is to offer a fast and comprehensive
overview of the complexity of our health data space.
This is especially invaluable for less experienced
stakeholders. Navigating this intricate landscape can
be daunting, and an overview could serve as a guiding
beacon, ensuring that those entering this complex
space find direction and fundamental understanding
rather than feeling lost.
(ii) Knowledge leveraging and preventing
redundancy: Currently, the immense challenges
posed within our health data ecosystem are often
tackled in isolated silos, leading to a redundant
‘reinventing the wheel’ paradigm. By promoting
knowledge transfer and best practices, such oversight
could ensure efficient utilization of resources.
Moreover, it could enable a collective learning
process, significantly reducing duplication of efforts.
(iii) Analysing an initiative network: A holistic
view of how initiatives interconnect and the common
challenges they face is essential. It promotes
collaboration, highlights areas where collective
solutions are needed, and fosters a sense of unity in
addressing health data challenges.
(iv) Identifying gaps: Strategic oversight can
pinpoint gaps and shortcomings in the current health
data landscape, enabling targeted efforts to fill these
voids and enhance the overall quality and coverage of
available data.
(v) Influencing policy: A comprehensive
overview facilitates the development of policy
preparatory documentation, which can influence
policy decisions and regulations that shape the health
data landscape.
(vi) Forming consortia efficiently: For efficient
consortium formation, it is essential to know which
initiatives already exist, their specific focuses, current
status, and levels of advancement. This knowledge
forms the foundation for strategic collaboration and
innovation in the health data ecosystem.
2.2 The Health Data Landscape Is
Inherently Complex. Hence,
Achieving Strategic Oversight Is
Challenging and Time-Consuming,
but at Least We Tried
We kick-started 2023 with the good intention to try to
come up with a strategic oversight of existing
initiatives using a comprehensive and multi-faceted
approach. It could help in figuring out how initiatives
can collaborate in a better way, how they can work in
synergy in order that consortia can be formed more
efficiently. Additionally, it could open the eyes of
regulators and the government, leading to policy
preparatory documentation and be able to influence
policy. A rigorous literature review and internet
scanning served as the initial screening process for
identifying existing initiatives. Between January
2023 and April 2023, several search strings in several
Strategic Oversight Across Real-World Health Data Initiatives in a Complex Health Data Space: A Call for Collective Responsibility
579

search engines were applied using different
keywords. The sources searched were PubMed,
Google Scholar, Medline NLM, Cochrane, Scopus
and Cordis. The keywords used in the literature
search were: real world data; real world data AND
infrastructure; data infrastructure; (real world) data
infrastructure AND Europe; real world data strategy;
real world data and inventory; FAIR data ecosystem;
Digitization AND health AND RWD; FAIR data
management; secondary reuse AND health data; data
science strategy; data-driven medicine; data sharing
infrastructures; Big data and health; real world data
initiative; real-world data AND health AND
initiative.
Specific inclusion and exclusion criteria were
defined for the scoping review. To be considered for
this review, studies had to meet the following
inclusion criteria: English-language articles; the
articles had to be reviews, systematic reviews or
meta-analyses. Studies that were published more than
5 years ago were excluded.
Next to this literature research, a general Google
search was performed to find initiatives that work on
unlocking real-world health data, making real-world
health data accessible for research and/or innovation
and/or policy. Additionally, information and
documents we received during the past months and
years from our network were checked and evaluated
on eligibility. This review allowed us to form a
preliminary shortlist of initiatives while gaining a
broader understanding of the field’s landscape.
Subsequently, during the period of May to August
2023, 13 semi-structured interviews were conducted
with authorities in the field of data spaces, health data
management and analyses to better understand which
information they considered critical to be gathered
from various health data initiatives. In addition, we
solicited these experts' opinions regarding how such
an exercise could become sufficiently exhaustive to
be useful, and could be kept up-to-date with minimal
effort from all stakeholders involved. The semi-
structured interviews, supported with an interview
guide that was used in a flexible way, gave much
opportunity for the respondents to speak very openly.
All interviews were done by Lotte Geys, who had no
personal connection with the experts. Interviews took
between 45 minutes and 1 hour and were recorded
(Google Meet) and transcribed verbatim.
The interviews were set-up in Google Meet and
the experts were asked the following: (i) their
opinions on the idea of setting up a living library of
existing initiatives, (ii) which questions from end
users the living library should be able to answer, (iii)
which initiatives they know about, (iv) who we could
possibly talk to in order to better carry out our
research and achieve our goal. Personal data was
collected from participants and processed in
accordance with the General Data Protection
Regulation (GDPR). This research was conducted
and seen as a task carried out in the public interest.
This study received approval from the UHasselt
Social (“Sociaal-Maatschappelijke”) ethical
committee (SMEC) (REC/SMEC/2022-2023/33).
2.3 To Date, It Is Impossible to Achieve
This Strategic Oversight Because of
Various Reasons
While striving hard for several months to achieve our
set goal towards providing strategic oversight across
initiatives, in the end, we were left deeply frustrated
because of 3 main reasons.
First of all, the information we need about the
initiatives is, most of the time, not available in the
public domain. Table 1 presents an overview of the
information deemed essential by experts for assessing
the value, impact and strategic positioning of health
data initiatives. The aim of an initiative, the data they
focus on, the way they work, what they exactly do,
etc., is often only vaguely described or not to be found
at all.
Secondly, a lot of initiatives are interlinked or
change their name and scope over time without
properly documenting these changes. It’s often very
confusing how they are linked or not clear that a
certain initiative originates from another one. It turns
out to be impossible to know for each initiative how
they originated and what it stands for. Many
initiatives started from a grant that expired after a few
years, but the initiative itself turned out to be
successful. These initiatives then often survive but
choose to work under a new name, potentially with a
new legal entity. This becomes very complex for
people who are trying to understand the ecosystem
and trying to get an overview of what is going on. To
give a concrete example: the Population Health
Information Research Infrastructure (PHIRI)
initiative allows for better coordinated European
efforts across national and European stakeholders.
PHIRI aims to generate the best available evidence
for research on health and well-being of populations
as impacted by COVID-19 to underpin decision-
making. It was born from two former initiatives:
BRIDGE Health and the Joint Action on Health
Information (InfAct) projects, which both have a
whole history. On top of that, PHIRI launched a
“spin-off initiative”: its Health Information Portal,
which is a one-stop shop facilitating access to
HEALTHINF 2024 - 17th International Conference on Health Informatics
580
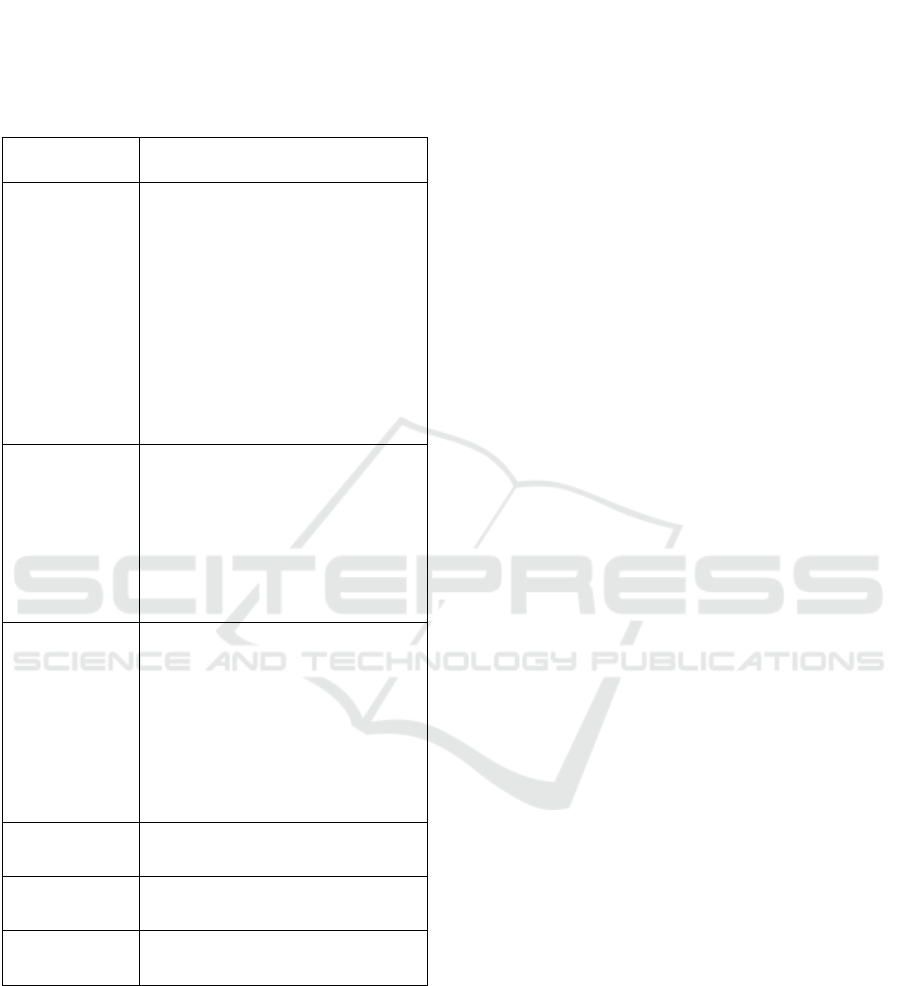
Table 1: Essential Information for evaluating Health Data
Initiatives. Through semi-structured interviews with
experts in the field, we inquired which information they
considered critical to be gathered from various health data
initiatives. This table presents an overview of the
information deemed essential by experts for assessing the
value, impact and strategic positioning of health data
initiatives.
Category
More specifically
Data Data type
Granularity
Centralized vs federated
Coding
Numbers
Data quality
Missing data
Link to prospective data
Standards
Category of health data
How to apply for data access
How to contribute
Stakeholders Which stakeholders are involved?
Who is it accessible to? Also for
industry? Also for commercial
purposes?
Looking for partners? How many
and type of partners?
Who is the initiator, who are
participating organizations?
Scope of the
initiative
Objectives
What are their strengths?
What specific socio-technical
challenges are they focusing on?
(E.g. standardization, infrastructure,
legal point of view, business
modeling)?
Geographical scope (E.g. Europe,
global vs specific region)
Costs/Financial
aspect
Free of charge or not
Funding
Governance of
the initiative
Links with other initiatives
General
information
Publications
Contact details
population health and healthcare data, information
and expertise in Europe. Unfortunately, PHIRI ended
in November 2023, and it could be that they will
continue to exist with another name, putting it at risk
of complicating it even more for people to
understand. This is just one initiative, but considering
that we found 67 initiatives
(10.5281/zenodo.10451144) and there are even more,
one might understand that it becomes impossible to
keep track of, especially when their websites are not
very detailed either.
And last, but not least, we were continuously
haunted by the question ‘where to start and where to
stop’. Initially, we wanted to include as many
initiatives as possible. But the more we progressed, the
more overwhelmed we were. The term “initiative” can
be interpreted in many different ways, making it
difficult to define precise inclusion/exclusion criteria.
3 OUR RESULTS AND
HIGHLIGHTED INITIATIVES
FROM OUR IMPERFECT
VENTURE
While we acknowledge that achieving a complete
overview is impossible (see reasons above in 2.3), we
believe the list of initiatives v2023
(10.5281/zenodo.10451144) serves as a valuable
starting point for those navigating the complex
landscape of health data initiatives. For each
initiative, details about the covered regions,
associated countries, website links, and whether the
initiative is specific to healthcare or encompasses
multiple domains are presented, as available. We
share it with the hope of assisting others in their quest
for clarity. Important Disclaimer: we acknowledge
that this list is neither exhaustive nor free from bias,
influenced by their geographical location and their
research emphasis on chronic disorders. However,
this list offers a starting point to address the issues
discussed in this position paper, aiming to provide
readers with insights gained after extensive online
exploration. The list resulting from our work can
assist individuals seeking clarity on the evolving
landscape of health data initiatives. While a
comprehensive overview remains elusive, this list
serves as a valuable resource to navigate the intricate
ecosystem. In this section, we will provide more
details on some highlighted initiatives to explain
some interesting emerging trends we have noticed
during our extensive landscaping exercise.
3.1 Initiatives Focused on Tackling
Data Heterogeneity - Data
Standards and Common Data
Models
As mentioned earlier, health data is heterogeneous in
size, maturity and depth, reducing their potential of
reusing it (limited interoperability). To reduce
Strategic Oversight Across Real-World Health Data Initiatives in a Complex Health Data Space: A Call for Collective Responsibility
581

heterogeneity, the health informatics community is
focusing on the development and adoption of data
standards (e.g. Logical Observation Identifiers
Names and Codes (LOINC), International
Classification of Diseases (ICD), and SNOMED
Clinical Terms (CT)) and common data models
(CDMs). Different initiatives are developing and/or
adopting different CDMs, potentially obstructing the
interconnection of these initiatives over time.
OMOP is an abbreviation of ‘Observational
Medical Outcomes Partnership’ and is a common data
model for observational healthcare data managed by
the Observational Health Data Sciences and
Informatics community (OHDSI: OHDSI.org). More
than 810 million unique patient records have been
mapped to the OMOP CDM, clearly showcasing the
wide adoption of this CDM. The OMOP CDM is
implemented within two important European large-
scale collaborative efforts: the European Health Data
and Evidence Network (EHDEN.eu) and the Data
Analysis and Real-world Interrogation Network in
the European Union (DARWIN.org). EHDEN,
established in 2018, aims to build a network and
infrastructure that uses harmonized health data to gain
real-world evidence. It currently compasses 187 data
partners from 29 different countries. DARWIN, a
federated data coordination network established by
the European Medicine Agency, aspires to deliver
real-world evidence from across Europe on diseases,
populations and the uses and performance of
medicines (pharmacovigilance studies).
3.2 Initiatives Focused on Education
and Awareness Raising
Data Saves Lives (datasaveslives.eu) is a multi-
stakeholder initiative with the aim of raising wider
patient and public awareness about the importance of
health data, improving understanding of how it is
used and establishing a trusted environment for multi-
stakeholder dialogue about responsible use and good
practices across Europe. It is led by European
Patients’ Forum (eu-patient.eu) and European
Institute for Innovation through Health Data (i-
HD.eu). Data Saves Lives aspires to share relevant
information and best practice examples about the use
of health data and generate easy-to-use materials
about the basic concept related to the data journey.
The portal of data.europe.eu, aiming to be the central
point of access to European open data, educates
citizens and organizations about the opportunities that
arise from the availability of open data with their
“Academy tab”. An inspiring new trend within
recently approved programmes within Horizon
Europe (HE) and Innovative Medicine and Health
Initiative (IHI/IMI) is to disseminate lessons learned
more broadly to the public (e.g. EHDEN Academy
(academy.ehden.eu) and other initiatives within the
Big Data For Better Outcomes roadmap (bd4bo.eu)).
3.3 Initiatives Focused on a Specific Set
of Data Types
Distinct categories of data require tailed technical
solutions for data management, storage and analyses.
InterRAI (interrai.org) is a partnership of researchers
and practitioners in more than 35 countries committed
to improving healthcare for people in long-term care
by tackling the challenges that arise with handling so-
called ‘resident assessment instruments’ (RAI). RAIs
are scientifically validated instruments enabling an
assessment of the degree of dependency and the care
needs of individuals. The Health Outcome
Observatory (H20; health-outcomes-observatory.eu)
aspires to create a standardized data governance and
infrastructure system across Europe with a specific
focus on patient-reported information. Within the
European Strategy Forum on Research
Infrastructures (ESFRI.eu) roadmap, two initiatives
are focusing on tackling two specific sets of data
types: ELIXIR (elixir-europe.org), focusing on -
omics data (e.g. genomics, proteomics) and
EBRAINS (ebrains.eu) with a specific focus on brain-
related data (e.g. neuroimaging data). Interestingly,
ESFRI is currently working on a landscape analysis
which will provide an overview of the European
transnational research infrastructure ecosystem. The
Landscape Analysis will include research
infrastructure services, technology, instrumentation
and data aspects, as well as societal and economic
impact; it covers national, European and global scales
and will be published online in December 2023.
3.4 Initiatives Focused on a Specific
Disease Area of Interest
While the existence of broader, disease-independent
initiatives is undoubtedly advantageous, the
importance of preserving disease-specific initiatives
cannot be overstated. These specialized initiatives are
indispensable because of their domain expertise and
knowledge needed to meet disease-specific
requirements. Additionally, these initiatives are
crucial to improve the disease-specific community
engagement, and communication and collaboration
between stakeholders involved. Some interesting
initiatives showcasing this are: the Multiple Sclerosis
Data Alliance (msdataalliance.com); PIONEER
HEALTHINF 2024 - 17th International Conference on Health Informatics
582

focusing on prostate cancer (prostate-pioneer.eu), the
European Platform on Rare Disease Registration, the
Haematological Outcomes Network in Europe
(HONEUR, portal.honeur.org) and the European
Society for Blood and Marrow Transplantation
(EBMT.org).
3.5 Initiatives Focused on Streamlining
Governance Principles
The project entitled ‘Towards European Health Data
Space’ (TEHDAS; tehdas.eu) plays a pivotal role in
addressing the challenge of streamlining governance
principles within and across member states in the
context of the EHDS regulation. TEHDAS helps EU
member states and the European Commission to
develop and promote concepts for the secondary use
of health data to benefit public health and health
research and innovation in Europe. At the level of the
member states, national data authorities have already
been installed to act as the single-point-of-entry
responsible for orchestrating the reuse of health data
in a specific country. Examples include the Finnish
Social and Health Data Permit Authority (findata.fi)
and the French Health Data Hub (health-data-hub.fr).
In October 2022, the HealthData@EU Pilot project
started (ehds2pilot.eu), bringing together 17 partners
including health data access bodies, health data
sharing infrastructures and European agencies. The
HealthData@EU Pilot project is a two-year-long
European project co-financed by the EU4Health
programme. It will build a pilot version of the EHDS
infrastructure for the secondary use of health data.
3.6 Initiatives not Focusing Specifically
on Health, but that Could Deliver
Interesting Insights to Be
Leveraged to the Health Domain
The socio-technical challenges that we face in the
health data space are similar in other domains.
Therefore, it is interesting to follow and align with
some initiatives that have a broader scope. Examples
funded by the European Commission include: the
Data Spaces Support Center (DSSC, dssc.eu), the
European Open Science Cloud (EOSC; eosc-
portal.eu) and Open Digitising European Industries
(opendei.eu). The DSSC explores the needs of data
space initiatives, defines common requirements and
establishes best practices to accelerate the formation
of sovereign data spaces as a crucial element of digital
transformation in all areas. One of the key objectives
of the DSSC is to establish a Network of Stakeholders
that aims to build a strong and innovative data
ecosystem in Europe through the development of
common data spaces in strategic economic sectors
and domains. OpenDEI focuses on “Platforms and
Pilots” to support the implementation of next-
generation digital platforms in four basic industrial
domains: manufacturing, agriculture, energy and
healthcare. The ambition of EOSC is to provide
European researchers, innovators, companies and
citizens with a federated and open multi-disciplinary
environment where they can publish, find and reuse
data, tools and services for research, innovation and
educational purposes. EOSC ultimately aims to
develop a Web of FAIR Data and services for science
in Europe upon which a wide range of value-added
services can be built. These range from visualization
and analytics to long-term information preservation
or the monitoring of the uptake of open science
practices.
There are some interesting arising initiatives that
focus mainly on some of the more technical
challenges requiring privacy-preserving
decentralized storage and analytics like federated
learning. Besides the already previously mentioned
health-specific initiatives ELIXIR, EHDEN, OHDSI
and EBRAINS, SOLID (solidproject.org) and GAIA-
X (gaia-x.eu) are more general initiatives worth
considering to learn more about in this area. Solid is
a technical specification that allows citizens to store
their data securely in decentralized, private data
stores called “pods”. Gaia-X strives towards
developing and implementing a federated system
linking many cloud service providers and users
together in a transparent environment that will drive
the European data economy. Within the Gaia-x
initiative, the International Data Spaces (IDS;
internationaldataspaces.org) initiative aims at cross-
sectoral data sovereignty and data interoperability.
4 CONCLUSIONS AND
CALL-TO-ACTIONS TO THE
ECOSYSTEM
Our health data landscape is a mess and that is a
problem. Strategic oversight across initiatives is
crucial, because it could provide valuable guidance to
newcomers, promote efficient resource utilization,
identify common challenges, help fill gaps, influence
policy making and facilitate and speed-up consortium
formation. Striving to accomplish this strategic
oversight has been a challenging journey that left us
deeply frustrated. Although we did not expect it to be
easy, we have noticed that the information is even
Strategic Oversight Across Real-World Health Data Initiatives in a Complex Health Data Space: A Call for Collective Responsibility
583

more scattered than expected and is often ambiguous
and unclear.
However, we are hopeful that together we can
overcome some of these challenges moving forward.
To accomplish this, we suggest concrete call-to-
actions for different actors:
• Specific for the actors involved in the set-up
and implementation of the European Health
Data Space: Install multiple teams (e.g. at least
one per country) that safeguards the strategic
oversight within a member state and make sure
that orchestration and knowledge leveraging
across member states is facilitated. Libraries
or scientific reports providing strategic
oversight are only useful when they are kept
up-to-date and the efforts required to
accomplish that should not be underestimated.
• Specific for funders of large-scale
collaborative efforts: Regularly scan the
landscape for existing initiatives and
encourage initiatives that request funding to
continue to work on top of previously
delivered results and successes (ideally
without pushing them to change their name).
Currently, innovation appears to be meaning
that you have to do something ‘new’, pushing
consortia to be ‘unique’ and again start from
scratch, leading to the ‘reinventing the wheel’
paradigm we see happening at the moment.
We believe that we can only truly scale-up
health data research if we start focusing on the
adoption and implementation of existing
solutions and principles instead of
continuously developing new solutions.
• Specific for the individuals leading these
initiatives: Make sure that at least your
websites are providing detailed information
about the what, (for) who, why and how of the
initiatives, as well as whether the initiative is
a still ongoing effort, whether or not acting
under a new name. In addition, be open to
understanding more about and learning from
other initiatives, even if they appear to be (at
first glance) in competition with your own
aspirations.
Together with the acquired experience resulting from
this landscaping exercise, we hope that the static list
we compiled (10.5281/zenodo.10451144) can
contribute to a start in creating policy preparation
documentation that will lead to clear guidelines on
how to proceed and work together in synergy and
move the data ecosystem in the right direction.
ACKNOWLEDGEMENTS
We would like to thank all the experts who
generously contributed their insights and expertise
during the interviews. Their valuable input has been
instrumental in shaping our thoughts formulated in
this position paper. This work was supported by
Research Foundation - Flanders (FWO) for ELIXIR
Belgium (I000323N).
REFERENCES
Wilkinson M. D., Dumontier M., Aalbersberg I. J.,
Appleton G., Axton M., Baak A., et al. (2016). The
FAIR Guiding Principles for scientific data
management and stewardship. In Scientific Data,
3:160018.
Holub P., Kohlmayer F., Prasser F., Mayrhofer M. T.,
Schlünder I., Martin G. M., et al. (2018). Enhancing
Reuse of Data and Biological Material in Medical
Research: From FAIR to FAIR-Health. In Biopreserv
Biobank., 16(2):97-105.
HEALTHINF 2024 - 17th International Conference on Health Informatics
584
