
An Insight Into Neurodegeneration: Harnessing Functional MRI
Connectivity in the Diagnosis of Mild Cognitive Impairment
Shuning Han
1,2 a
, Zhe Sun
2,3 b
Kanhao Zhao
4 c
, Feng Duan
5 d
, Cesar F. Caiafa
6 e
,
Yu Zhang
4,7 f
and Jordi Sol
´
e-Casals
1,8 g
1
Data and Signal Processing Research Group, University of Vic-Central University of Catalonia, Vic, 08500, Catalonia,
Spain
2
Image Processing Research Group, RIKEN Center for Advanced Photonics, Riken, Wako-Shi, Saitama, Japan
3
Faculty of Health Data Science, Juntendo University, Urayasu, Chiba, Japan
4
Department of Bioengineering, Lehigh University, Bethlehem, PA 18015, U.S.A.
5
Tianjin Key Laboratory of Brain Science and Intelligent Rehabilitation, Nankai University, Tianjin, China
6
Instituto Argentino de Radioastronom
´
ıa-CCT La Plata, CONICET/ CIC-PBA/ UNLP, V. Elisa 1894, Argentina
7
Department of Electrical and Computer Engineering, Lehigh University, Bethlehem, PA 18015, U.S.A.
8
Department of Psychiatry, University of Cambridge, Cambridge CB20SZ, U.K.
fi
Keywords:
Alzheimer’s Disease, Mild Cognitive Impairment, Graph Convolutional Network, Functional Magnetic
Resonance Imaging Analysis, Functional Connectivity.
Abstract:
Alzheimer’s disease is a progressive form of memory loss that worsens over time. Detecting it early, when
memory issues are mild, is crucial for effective interventions. Recent advancements in computer technology,
specifically Graph Convolutional Networks (GCNs), have proven to be powerful tools for analyzing Magnetic
Resonance Imaging (MRI) data comprehensively. In this study, we developed a GCN framework for diagnos-
ing mild cognitive impairment (MCI) by examining the functional connectivity (FC) derived from resting-state
functional MRI (rfMRI) data. Our research systematically explored various types and processing methods of
FC, evaluating their performance on the OASIS-3 dataset. The experimental results revealed several key find-
ings. On the one hand, the proposed GCN exhibited significantly superior performance over both the baseline
GCN and the Support Vector Machine (SVM) models, with statistically significant differences. It attained
the highest average accuracy of 80.3% and a peak accuracy of 88.2%. On the other hand, the GCN frame-
work obtained using individual FCs showed overall slightly better performance than the one using global FCs.
However, it is important to note that GCNs using global networks with appropriate connectivity can achieve
comparable or even better performance than individual networks in certain cases. Finally, our results also
indicate that the connectivity within specific brain regions, such as VIS, DMN, SMN, VAN, and FPC, may
play a more significant role in GCN-based MRI classification for MCI diagnosis. These findings significantly
contribute to the understanding of neurodegenerative disorders and offer valuable insights into the diverse ap-
plications of GCNs in brain analysis and disease detection.
a
https://orcid.org/0009-0004-0792-5484
b
https://orcid.org/0000-0002-6531-0769
c
https://orcid.org/0000-0002-2955-0917
d
https://orcid.org/0000-0002-2179-2460
e
https://orcid.org/0000-0001-5437-6095
f
https://orcid.org/0000-0003-4087-6544
g
https://orcid.org/0000-0002-6534-1979
1 INTRODUCTION
Alzheimer’s disease (AD) is a progressive neurode-
generative dementia Srivastava et al. (2021). The typ-
ical progression of AD comprises three stages: (early)
mild cognitive impairment (MCI), moderate demen-
tia, and severe dementia. Detecting patients in the
MCI stage is crucial, as it facilitates the implemen-
tation of effective interventions to prevent further de-
terioration of dementia.
656
Han, S., Sun, Z., Zhao, K., Duan, F., Caiafa, C., Zhang, Y. and Solé-Casals, J.
An Insight Into Neurodegeneration: Harnessing Functional MRI Connectivity in the Diagnosis of Mild Cognitive Impairment.
DOI: 10.5220/0012414600003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 656-666
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.

Due to the intricate nature of data from various
imaging modalities and the organisational complex-
ity of the human brain network, promising advances
have been observed in modelling the interactions be-
tween various brain regions. This progress is in part
due to new learning techniques that rely on graphs
derived from image data, apply graph regularisations
to the data, and employ graph embedding to repre-
sent graphs derived from recorded data. These meth-
ods show potential for capturing the fusion of infor-
mation between networks from different brain imag-
ing modalities, modelling latent spaces within high-
dimensional brain networks, and quantifying neuro-
biomarkers based on topological features (Liu et al.,
2022).
In recent years, research on magnetic resonance
imaging (MRI) has significantly contributed to our
comprehension of neuropathological mechanisms un-
derlying dementia and its clinical diagnosis (Chan-
dra et al., 2019). Functional MRI (fMRI) furnishes
valuable insights with a relatively high spatial reso-
lution (2mm isotropic) and medium temporal resolu-
tion (minutes) (Liu et al., 2015). fMRI can be cat-
egorized into two types: task-evoked fMRI (tfMRI),
collected while the subject is engaged in tasks, and
resting-state fMRI (rfMRI), collected during periods
of rest. Even in the resting state, spatial patterns of
spontaneous neural activities and metabolism persist
in the brain. The functional connectivity (FC) be-
tween various brain regions can be inferred (Bi et al.,
2020). FC serves as a reflection of the brain’s func-
tional organization, and alterations in it are believed
to be associated with psychiatric disorders (Bullmore
and Sporns, 2009).
Recently, the integration of graph theory and ma-
chine learning techniques has found extensive appli-
cation in neuroscience for the analysis of the brain
and the detection of diseases (Bi et al., 2020). A novel
domain of geometric deep learning, graph neural net-
works (GNNs), has emerged, which offers the capa-
bility to effectively process signals within the non-
Euclidean geometry of graphs. Notably, an increas-
ing number of GNNs have been introduced and em-
ployed in the analysis of brain MRI and the detec-
tion of disorders (Scarselli et al., 2008). For instance,
a graph empirical mode decomposition-based data
augmentation was presented in (Chen et al., 2022)
to generate more samples in small datasets. Parisot
et al. (2017) introduced the graph convolution net-
work (GCN) combining fMRI with non-imaging data
for brain analysis and disease diagnosis. Wang et al.
(2021) presented a connectivity based GCN architec-
ture for fMRI analysis and applied it to classifica-
tion of autistic patients from normal controls (NCs).
Tang et al. (2022) proposed a contrastive learning
framework with an interpretable hierarchical signed
graph representation learning model for brain func-
tional network mining. Qu et al. (2023) proposed a
univariate neurodegeneration biomarker based GCN
semi-supervised classification framework. Neverthe-
less, there has been insufficient research dedicated to
assessing the influence of various FC on brain analy-
sis results. Moreover, the performance of GCN mod-
els in MCI detection still falls short of expectations.
Considering the points mentioned above, this
study introduces a state-of-art approach in the realm
of neurodegeneration detection using fMRI data. The
key highlights of this research are as follows:
A Novel FC Based GCN Framework for MCI
Fetection: We have designed a novel FC based GCN
framework for binary classifications utilizing rfMRI
data. The GCN framework is applied for the diagnosis
of MCI by classifying the MCI from NCs.
Impact of Different Types of FC: This study
places special emphasis on understanding the effects
of different FC types and processing methods on the
GCN framework’s performance. In this paper, FC is
regarded as a graph and is considered from two as-
pects: On one side, we compare the difference of us-
ing the global FC matrix obtained from the training
data versus the individual specific FC matrices of each
rfMRI data. On the other side, we employ different
processing methods for the FC matrices, and obtain
the k nearest neighbor (k-NN) graph and the thresh-
old graph.
An Insight Into Neurodegeneration: The study
delves deeply into the analysis of brain networks in-
cluding self-network and between-network connec-
tivity. This perspective enhances the clinical rele-
vance of neurodegeneration and impact of this study’s
findings.
The remainder of this paper is structured as fol-
lows: Section 2 provides an overview of the dataset
and methods employed. In Section 3, we present the
analysis results, which are subsequently discussed in
Section 4. Finally, Section 5 concludes this study.
2 MATERIALS AND METHODS
In this section, we initially introduce the dataset for
classification and elucidate the label assignment pro-
cess. Following that, we detail the fMRI acquisi-
tion and preprocessing methods, generation of diverse
FC, and finally the proposed GCN framework and the
baseline methods used for comparison.
An Insight Into Neurodegeneration: Harnessing Functional MRI Connectivity in the Diagnosis of Mild Cognitive Impairment
657

2.1 Data
We utilize longitudinal rfMRI series for analysis, cat-
egorizing them into NC and MCI based on the rele-
vant clinical assessments of the participants.
2.1.1 OASIS-3 Dataset
We employ the dataset from the Open Access Series
of Imaging Studies (OASIS)-3 (LaMontagne et al.,
2019) (https://www.oasis-brains.org) to vali-
date our proposed GCN framework. The dataset in-
cludes longitudinal fMRI, neuropsychological testing
and clinical information for 1098 participants. The
clinical dementia rating (CDR) scale is utilized to
evaluate the dementia status within the clinical data
of OASIS-3: CDR 0 denotes normal cognitive func-
tion, CDR 0.5 indicates very mild impairment, CDR
1 signifies mild impairment, and CDR 2 reflects mod-
erate dementia. All participants were required to have
a CDR ≤ 1 in the most recent clinical core assess-
ment, and once a participant reached CDR 2, they
were no longer eligible for continued participation in
the study.
2.1.2 MRI Scans Labelling
MRI scans can be labeled based onNotably, associ-
ated CDR values. Notably, clinical assessments were
performed on different days from the neural imaging
scans, and the time gap between the MRI scan and
clinical assessment may exceed one year in the lon-
gitudinal OASIS-3 dataset. In this study, MRI scans
are classified into two groups: NC and MCI based
on CDR values. An MRI scan is labeled as NC, if
all recorded clinical assessment results for the corre-
sponding subject are CDR = 0; an MRI scan is labeled
as MCI, if both preceding and subsequent clinical as-
sessment show CDR ≥ 0.5. For the purpose of main-
taining data balance, 503 NC and MCI rfMRI samples
are used for MCI detection, respectively.
2.2 Methods
This part provides a comprehensive exposition on the
fMRI acquisition and pre-processing methods, along
with the various FC processing methods. Following
that, we delve into a detailed description of the pro-
posed GCN framework and the baselines. Finally,
we present the specific configurations of the proposed
GCN framework.
2.2.1 fMRI Acquisition and Preprocessing
The fMRI data for each subject in each run were ac-
quired in resting state for 6 min (164 volumes) uti-
lizing 16-channel head coil in the scanners. The
acquired rfMRI data underwent preprocessing us-
ing the fMRIPrep pipeline (Esteban et al., 2019).
The T1-weighted (T1w) image underwent intensity
correction, skull-stripping, and spatial normaliza-
tion through nonlinear registration (Avants et al.,
2008). Employing FSL, brain features such as
cerebrospinal fluid, white matter, and grey matter
were segmented from the reference, brain-extracted
T1 weighted image (Zhang et al., 2000). The
fieldmap information was used to correct distortion
in low-frequency and high-frequency components of
fieldmap. Subsequently, a corrected echo-planar
imaging reference was obtained from a more ac-
curate co-registration with the anatomical reference.
The blood-oxygenation-level-dependent (BOLD) ref-
erence was then transformed to the T1-weighted im-
age with a boundary-based registration method, con-
figured with nine degrees of freedom to account for
distortion remaining in the BOLD reference (Greve
and Fischl, 2009). Head-motion parameters were es-
timated with MCFLIRT (FSL). BOLD signals were
slice-time corrected and resampled onto the partic-
ipant’s original space with head-motion correction,
susceptibility distortion’s correction, and then resam-
pled into standard space, generating a preprocessed
BOLD run in MNI152NLin2009cAsym space. Auto-
matic removal of motion artifacts using independent
component analysis (ICA-AROMA) (Pruim et al.,
2015) was performed on the preprocessed BOLD
time-series on MNI space after removal of non-
steady-state volumes and spatial smoothing.
2.2.2 FC Construction
The brain FC can be derived from rfMRI and repre-
sented as graphs, capturing the statistical time-series
correlations between brain regions of interest (ROIs).
The preprocessed BOLD-level rfMRI series are aver-
aged into 100 ROIs defined by Schaefer atlas (Schae-
fer et al., 2018) and subsequently standardized using
z-score. Ultimately, the dimension of each fMRI ses-
sion is 164 × 100 (100 regions with a length of 164
time samples each). To construct the FC matrices
for ROIs, the Pearson correlation coefficient (PCC) is
computed between the fMRI time series of every pair
of brain regions. Notably, in this study, the diagonal
elements of the FC matrices are uniformly set to 0.
There are 100 ROIs, resulting in an FC matrix with a
shape of 100 × 100.
In this paper, we analyse the impact of various
types of FC on prediction results, as depicted in Fig-
ure 1. The divergence in FC manifests in two dimen-
sions: one is the different FC matrices, individual spe-
cific FC matrices obtained from each fMRI data vs.
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
658
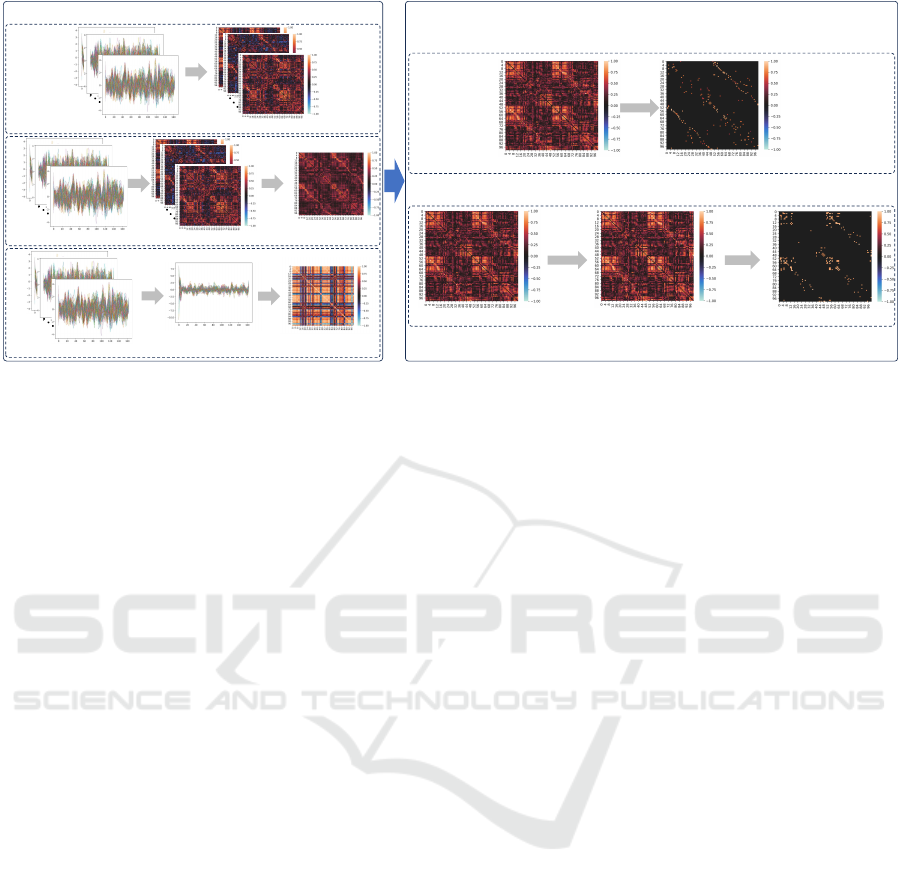
FC-of-avgROI
Average
PCC
avgFC
PCC
PCC
k-NN
Absolute FC k-NN graph
Absolute FC Threshold graphNAFC
Approaches for Obtaining Different Types of FC
a.
b.
c.
d.
e.
Normalize
Different Graph Processing Methods
Threshold
Average
fMRI samples
Training fMRI samples
Individual specific FC
Training fMRI samples
Figure 1: FC construction. Left part: a. Approach for individual specific FC; b. Approach for global FC-of-avgROI; c.
Approach for global avgFC; Right part: d. Method for k-NN graph; e. Method for threshold graph.
global FC matrix obtained from the training data for
both training and testing data; the other is the different
processing methods employed to derive the FC matri-
ces. In Han et al. (2024), an extensive and compre-
hensive analysis is carried out, delving into a wider
range of aspects to provide a global understanding.
This extended analysis includes the examination of
two additional forms of FC, namely top-p and the p-
MST (Minimum Spanning Tree). The exploration of
these specific types of FC aims to capture a more de-
tailed perspective of the intricate network dynamics
within the brain. In addition, the research extends its
focus by addressing the classification of at-risk de-
mentia versus normal control subjects. This compar-
ative analysis offers valuable insights into the unique
neural signatures associated with this particular con-
dition when contrasted with the normal control group.
• Obtain global FC matrix from training data
Individual specific FC matrices can be derived
from each fMRI data by directly computing PCC, as
illustrated in Figure 1a. While, a global FC matrix
for both training and testing fMRI samples is derived
from training samples using distinct approaches. In
the first approach for global FC, the FC-of-avgROI
matrix is obtained from the standardized average data
of the training fMRI samples, as illustrated in Fig-
ure 1b. In the second approach, we regard the average
PCC of all PCC matrices from the training fMRI sam-
ples, denoted as avgFC, as the global FC matrix, as
shown in Figure 1c.
Special to note is that: (i) matrix averaging oper-
ations are performed across subjects; (ii) the global
avgFC was also utilized in the baseline GCN; (iii) the
final obtained FC matrices are further processed using
the methods in “Different graph processing meth-
ods”, depicted in the right part of Figure 1.
• Different graph processing methods
To investigate the impact of different graph types
on the classification results of GCN, we employ vari-
ous processing methods to individual or global FC, as
shown in the right part of Figure 1. In the first method,
we take the k largest values in each row of the absolute
FC matrix as the k-NN graph, as shown in Figure 1d.
In the second method, we normalize the absolute FC
values to the range [0− 1] for consistent thresholding,
denoted as the normalized absolute FC (NAFC), and
then the thresholding is applied on the NAFC matrix
(denoted as threshold graph), as illustrated in Figure
1e.
2.2.3 Graph Convolutional Network (GCN)
Graphs (Zhou et al., 2020) represent a non-Euclidean
data structure comprising nodes and edges, where
nodes denote objects and edges signify relation-
ships between these objects. Brain FC can be
modeled as graphs, with nodes representing ROIs
and edges corresponding to activity correlations be-
tween these ROIs (Hanik et al., 2022; Sporns et al.,
2005; Suprano, 2019). GCNs (Scarselli et al., 2008;
Micheli, 2009) have gained widespread popularity
in machine learning for graph analysis, owing to
their persuasive performance. The GCN architec-
tures effectively integrate node features and graph
topology to construct distributed node representations
with graph convolutional layers (GCLs) (Errica et al.,
2020). In this work, we introduce a novel GCN frame-
work for binary classification of fMRI data, where the
fMRI time series of brain ROIs are directly regarded
as the node features and the FC serves as the graph
topology.
An Insight Into Neurodegeneration: Harnessing Functional MRI Connectivity in the Diagnosis of Mild Cognitive Impairment
659

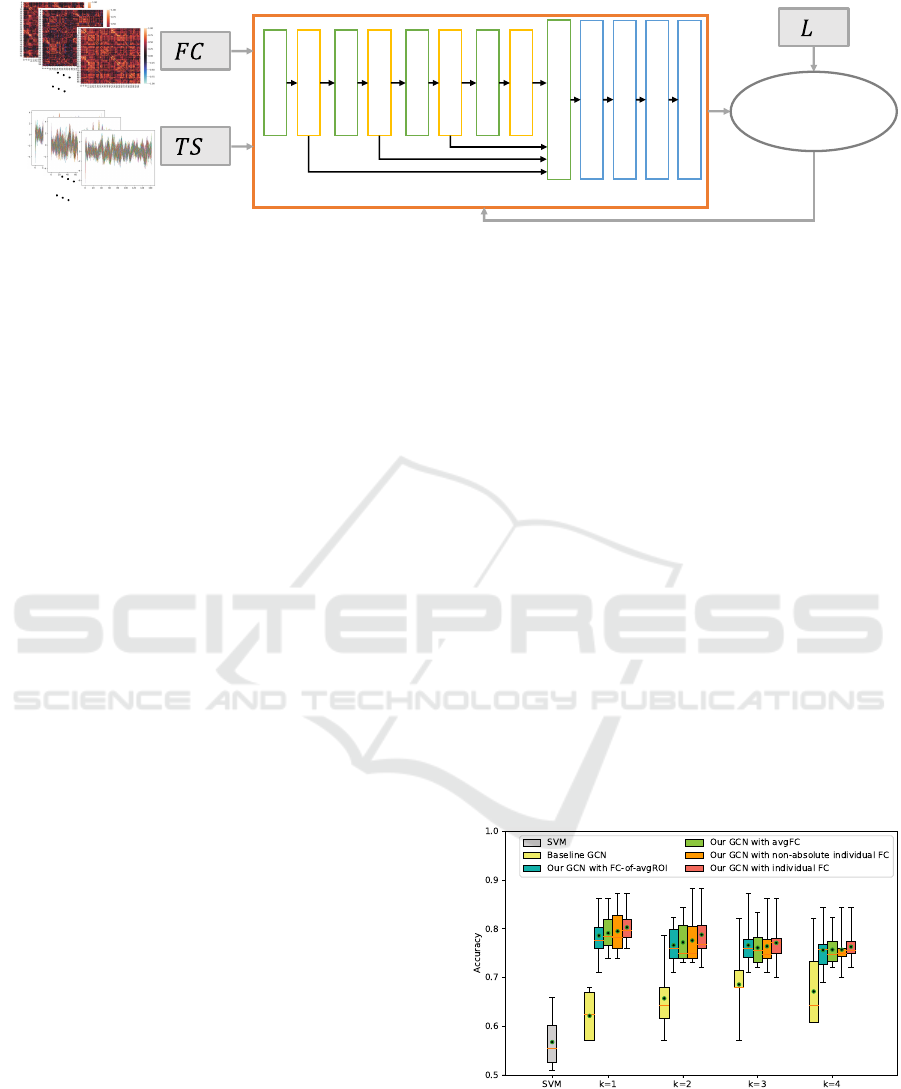
• The Proposed GCN
The proposed GCN framework is implemented
using the Pytorch Geometric (PyG) library (Fey and
Lenssen, 2019). This library encompasses a vari-
ety of GNN models and graph preprocessing meth-
ods to easily build and train GNNs. The designed
GCN framework, illustrated in Figure 2, comprises
five GCLs based on GraphConv (Morris et al., 2019).
The nonlinear activation function Rectified Linear
Unit (ReLU) (Nair and Hinton, 2010) layer defined
as f (x) = max(0, x) follows after each of the first 4
GCLs. The input of the last GCL layer is the outputs
of the first 4 layers. The fifth GCL includes a batch
normalization layer, enhancing the speed and stabil-
ity of the GCN framework. Subsequently, the global
mean pool or global average pool is followed to avoid
overfitting and enhance the robustness of the frame-
work. To further prevent overfitting, a dropout layer
is implemented, randomly setting output data to zero
with a specified probability.
In the presented GCN framework, the optimiza-
tion is carried out using the Adam algorithm (Kingma
and Ba, 2015), and the loss function employed is the
cross-entropy loss. The proposed GCN framework
is applied for two types of classification: one with
global FC and the other with individual FC. For the
classification with individual FC, as illustrated in Fig-
ure 2, the inputs consist of individual time series of
ROIs with corresponding FC and labels. While, in the
classification with global FC, the inputs of the GCN
framework are individual time series of ROIs with the
same global FC and labels of each sample.
• Baselines
For the purpose of comparison, we establish
two baselines: the Support Vector Machine (SVM)
employing the radial basis function (RBF) kernel
(Burges, 1998), and the GCN architecture for fMRI
analysis developed by Wang et al. (Wang et al., 2021)
in 2021.
The baseline GCN architecture using avgFC con-
sisted of 5 convolutional layers, one recurrent neu-
ral network (RNN) layer and a Softmax layer. In the
original study, the GCN was applied for autism spec-
trum disorder (ASD) classification, attaining the best
average accuracy of 70.7% (max 79.0%, min 66.7%)
when k = 3 (among 3, 5, 10, and 20) using 10-fold
cross validation.
2.2.4 Configurations
We reimplement the baseline GCN architecture and
apply it to the OASIS-3 dataset using all recom-
mended parameters from the original paper. In the
current study, a 10-fold cross validation strategy is
adopted to evaluate the performance of the GCN
framework which is set to be the same when apply-
ing the baseline GCN to OASIS-3 dataset.
Code of the proposed GCN framework is imple-
mented based on Python, and the GCN structure is re-
alized by PyTorch based on PyG. In our experiment,
the output dimension of each convolutional layer is
128; the learning rate is set to 0.001; the dropout rate
is set to 0.5; and the model is trained for 100 epochs
with a batch size of 8.
To assess the impact of variations in the FC ma-
trix on the outcomes, we varied the number of nearest
neighbors k (1, 2, 3, and 4) and the threshold value
(0.7, 0.8, 0.9, 0.95, and 0.99) for FC matrix process-
ing. To evaluate the significant differences in clas-
sification results between the proposed GCN and the
baseline GCN, as well as those between GCN with
different types of FC, the independent t-test method
was employed in this study. The brain networks of
different global graphs in one fold are visualized with
Brainnet viewer (Xia et al., 2013). The brain net-
works are grouped into seven canonical functional
networks defined by the 7 Yeo networks (Buckner
et al., 2011): visual network (VIS), somatomotor net-
work (SMN), dorsal attention network (DAN), ven-
tral attention network (VAN), limbic network (LIM),
frontoparietal control network (FPC), default mode
network (DMN).
3 RESULTS
As mentioned earlier, to better understand the impact
of different FC, the proposed GCN utilizes the graphs
of global FC (avgFC or FC-of-avgROI) or individual
FC, and then the graphs are processed as k-NN graph
and threshold graph, respectively. In this section,
we provide the classification results for MCI vs. NC
using GCN with k-NN graphs or threshold graph.
3.1 Results with k-NN Graph
We implement the MCI vs. NC classification of the
proposed GCN with k-NN graphs obtained from
individual FC, non-absolute individual FC, global
FC of avgFC and FC-of-avgROI. Especially, we
also utilize the non-absolute individual FC here to
demonstrate the superiority of GCN with absolute FC
over that with non-absolute FC. It should be noted
that absolute FC are used as the default in this article.
We compare the performance of our proposed GCN
with the baseline GCN framework and SVM method.
The experimental results are shown in Figure 3,
which illustrates that:
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
660
𝑇𝑆
Cross-entropy
loss
𝑇𝑆
𝐹𝐶
𝑇𝑆
𝐹𝐶
𝐹𝐶
GraphConv
GraphConv
ReLU
GraphConv
ReLU
GraphConv
ReLU
GraphConv
ReLU
BatchNorm
Global Mean Pool
Dropout
Linear
GNN
Figure 2: GCN classification with individual FC. T S
i
denotes the ith individual time series of ROIs; FC
i
and L
i
denote the
corresponding ith individual FC and label. In the classification with global FC, we utilize one same global FC for each
individual time series of ROI.
1) The proposed GCN outperforms both the
baseline GCN (best average accuracy of 68.6% when
k = 3) and SVM (average accuracy of 56.8%) in
terms of accuracy. The t-test outcomes in Table 1
show significant differences between the results of
proposed GCN and the baseline GCN with different
values of k at the 5% significance level. Our proposed
GCN with k-NN graphs achieves the best average
accuracy of 80.3% (max 87.3%, min 76.0%) with
absolute-individual FC when k = 1.
2) The proposed GCN with k-NN graphs exhibits
differently compared to the baseline GCN. While the
accuracy of the baseline GCN increases as k increases
and achieves the best average accuracy at k = 3 (the
same as in ASD classification in the baseline paper),
our proposed GCN’s performance with individual or
global FC declines as k increases.
3) The proposed GCN with absolute individual
FC demonstrates a slight performance improvement
compared to its counterpart with non-absolute indi-
vidual FC.
4) Our proposed GCN with individual FC per-
forms slightly better than that with global FC. The
use of global avgFC or FC-of-avgROI exhibits neg-
ligible differences for the proposed GCN with k-NN
graphs.Besides, the t-test results in Table 2 indicate
that there are no significant differences between the
outcomes of the individual and the two types of global
FC across various values of k at the 5% significance
level.
Brain networks of k-NN graphs of avgFC or FC-
of-avgROI are displayed as Figure 4. It is important
to note that the k-NN graphs are non-symmetrical
matrices and cannot guarantee full connectivity. In
this figure, there are only 50 and 45 edges in avgFC
and FC-of-avgROI as k = 1, respectively. From the
brain networks, it can be observed that:
1) Increasing k leads to more edges in both avgFC
and FC-of-avgROI brain networks.
This highlights an important finding that ex-
cessive connectivity can have a detrimental effect
on improving classification performance, which is
evidenced by the diminishing performance results as
the number of connectivity (k) increases.
2) The brain networks of k-NN avgFC and
FC-of-avgROI show little difference for each value
of k, which can explain the negligible difference of
the accuracy between avgFC and FC-of-avgROI with
the same k.
3) The brain networks of avgFC involve a slightly
larger number of ROIs than FC-of-avgROI when k =
1, 2, and the average accuracy of GCN with avgFC
are marginally higher than that of GCN with FC-of-
avgROI. This finding illustrates that graphs involving
a greater number of nodes may contain more valuable
information for GCN classification.
Figure 3: MCI vs. NC results of the proposed GCN frame-
work with k-NN graph. The black dot in the box presents
the average value in the 10-fold cross validation results.
An Insight Into Neurodegeneration: Harnessing Functional MRI Connectivity in the Diagnosis of Mild Cognitive Impairment
661

Table 1: The probability results in t-test between baseline methods and our proposed GCNs with k-NN graph.
t − test k = 1 k = 2 k = 3 k = 4
Baseline GCN vs. our GCN with individual FC 1.72 × 10
−8
5.19 × 10
−5
3.51 × 10
−3
2.97 × 10
−3
Baseline GCN vs. our GCN with avgFC 7.10 × 10
−8
1.20 × 10
−4
6.48 × 10
−3
4.43 × 10
−3
Baseline GCN vs. our GCN with FC-of-avgROI 3.32 × 10
−7
1.99 × 10
−4
5.43 × 10
−3
7.35 × 10
−3
SVM vs. our GCN with individual FC 4.47 × 10
−10
1.04 × 10
−8
1.87 × 10
−8
9.00 × 10
−9
SVM vs. our GCN with avgFC 1.54 × 10
−9
1.19 × 10
−8
1.80 × 10
−8
9.84 × 10
−9
SVM vs. our GCN with FC-of-avgROI 6.96 × 10
−9
1.73 × 10
−8
2.78 × 10
−8
5.73 × 10
−8
Table 2: t-test results between our proposed GCNs with k-NN graph of different types (individual, avgFC, FC-of-avgROI).
P denotes the probability and CI denotes the confidence interval in the t-test.
t-test avgFC vs. individual FC FC-of-avgROI vs. individual FC FC-of-avgROI vs. avgFC
k = 1
P 0.47 0.36 0.79
CI [-0.046,0.022] [-0.055,0.021] [-0.044,0.034]
k = 2
P 0.45 0.30 0.76
CI [-0.059,0.027] [-0.064,0.021] [-0.045,0.034]
k = 3
P 0.61 0.81 0.80
CI [-0.049,0.030] [-0.047,0.037] [-0.035,0.045]
k = 4
P 0.70 0.70 0.95
CI [-0.038,0.026] [-0.045,0.031] [-0.038,0.036]
k = 1 k = 2 k = 3 k = 4
avgFC
FC-of-
avgROI
VIS SMN DAN VAN LIM FPC DMN
Figure 4: The brain networks of k-NN avgFC or FC-of-
avgROI. The seven-colored nodes are indicative of seven
grouped networks (VIS, SMN, DAN, VAN, LIM, FPC,
DMN), while color-coded links denote self-network con-
nectivity and grey links denote the between-network con-
nectivity.
3.2 Results with Threshold Graph
The MCI vs. NC classification performances of the
proposed GCN with threshold graph derived from
individual FC, global FC of avgFC or FC-of-avgROI
are reported in Figure 5. It can be observed that:
1) As the threshold value increases, the accuracy
of GCN with FC-of-avgROI performs differently
from that of GCN with individual FC or avgFC. The
t-test outcomes in Table 3 show significant differ-
ences between the results of the FC-of-avgROI and
individual FC or avgFC with threshold = 0.7, 0.8, 0.9
or 0.95 at the 5% significance level
2) The average accuracy of GCN with FC-
of-avgROI graphs are notably lower compared
to that of GCN with individual FC or avgFC as
threshold ≤ 0.95, and then exhibits a significant rise
when the threshold reaches 0.99.
3) The average accuracy of GCN with individual
and avgFC graphs show a gradual increase and attain
an optimal average accuracy at threshold = 0.95
and threshold = 0.90, respectively. Nonetheless,
the accuracy of both decrease when threshold = 0.99.
4) In some cases (avgFC with all threshold val-
ues and FC-of-avgROI as threshold = 0.99), the GCN
with global FC achieves comparable or even supe-
rior performance compared with GCN using individ-
ual FC. Moreover, the t-test outcomes in Table 3 indi-
cate that there are no significant differences between
the results of the individual and avgFC across vari-
ous threshold values, and between the results of the
individual and FC-of-avgROI as threshold = 0.99 at
the 5% significance level. Overall, the proposed GCN
with threshold graphs achieves the best average accu-
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
662

Figure 5: MCI vs. NC results of the proposed GCN frame-
work with threshold graph.
racy of 80.0% (max 88.2%, min 74.0%) with FC-of-
avgROI graphs when threshold = 0.99.
The brain networks of avgFC and FC-of-avgROI
with different threshold values are displayed in
Figure 6. It can be observed that:
1) Increasing threshold results in fewer edges in
both networks. However, FC-of-avgROI contains
much more edges than avgFC when threshold > 0.7.
When few edges remain in the graph, there are
self-network edges of SMN, DMN, FPC, and VIS
in avgFC (threshold = 0.9 or threshold = 0.95);
while, there are both self-network and between-
network edges of SMN and VAN in FC-of-avgROI
(threshold = 0.99).
2) The GCN with global FC that have very
few edges in the graph obtained a higher average
accuracy, which emphasizes the observation in k-NN
graphs that excessive connectivity can negatively
affect classification performance.
3) The slight decrease in average GCN accu-
racy with avgFC when there is only one edge left
(threshold = 0.99) shows that a minimum number of
edge information can cause a slight decrease in accu-
racy, although the ROI series contain a large amount
of information. Furthermore, with suitable connec-
tivity, the GCN with global FC can achieve compa-
rable or superior performance compared with GCN
with individual FC. These findings highlight the sig-
nificance of suitable edge information for achieving
performance, rather than the threshold itself.
4 DISCUSSION
In this study, we developed an FC based GCN frame-
work for fMRI binary classifications to detect MCI
thr = 0.7 thr = 0.8 thr = 0.9 thr = 0.95 thr = 0.99
avgFC
FC-of-
avgROI
VIS SMN DAN VAN LIM FPC DMN
Figure 6: The brain networks of threshold avgFC or FC-of-
avgROI. The size of nodes reflects the degree of the graph,
and the nodes in k-NN graph have the same size. Other
annotations are identical to Figure 4.
from NCs on the longitudinal OASIS-3 dataset. Be-
sides, we explored the impact of different types and
processing methods of FC on the GCN classification
performance.
The results of our experiments revealed several
important findings. First, our proposed GCN signifi-
cantly outperformed both the baseline GCN and SVM
in terms of accuracy, indicating its effectiveness for
MCI diagnosis. The proposed GCN achieved the best
average accuracy of 80.3% (11.7 % higher than the
baseline GCN and 23.5% higher than SVM) and the
highest accuracy of 88.2%. This highlights the poten-
tial of deep learning techniques, specifically the GCN
framework, for analyzing rfMRI data and detecting
neurodegenerative disorders.
Second, we compared the effects of different types
of FC utilized in the GCN. The proposed GCN with
absolute individual FC performed slightly better than
non-absolute individual FC. In this study, we found
that the GCN framework with individual FC per-
formed slightly better than that with global FC gen-
erally, which is consistent with most of the current
studies (Parisot et al., 2017). This suggests that
individual-specific FC may contain valuable informa-
tion for classification tasks, and incorporating them
into the GCN model can improve its performance.
However, GCN using global graphs with appropriate
connectivity can achieve equivalent or superior per-
formance to individual graphs in some cases. This as-
sertion is supported by t-test results indicating no sig-
nificant differences between the individual and global
FC in some cases at the 5% significance level.
Furthermore, we investigated different processing
methods for FC matrices, including the k-NN graphs
and threshold graphs. The results suggest that the
choice of FC type and graph construction method can
influence GCN classification performance. The GCN
with k-NN graphs achieved the best average accuracy
when k is set to 1, indicating that considering only the
An Insight Into Neurodegeneration: Harnessing Functional MRI Connectivity in the Diagnosis of Mild Cognitive Impairment
663
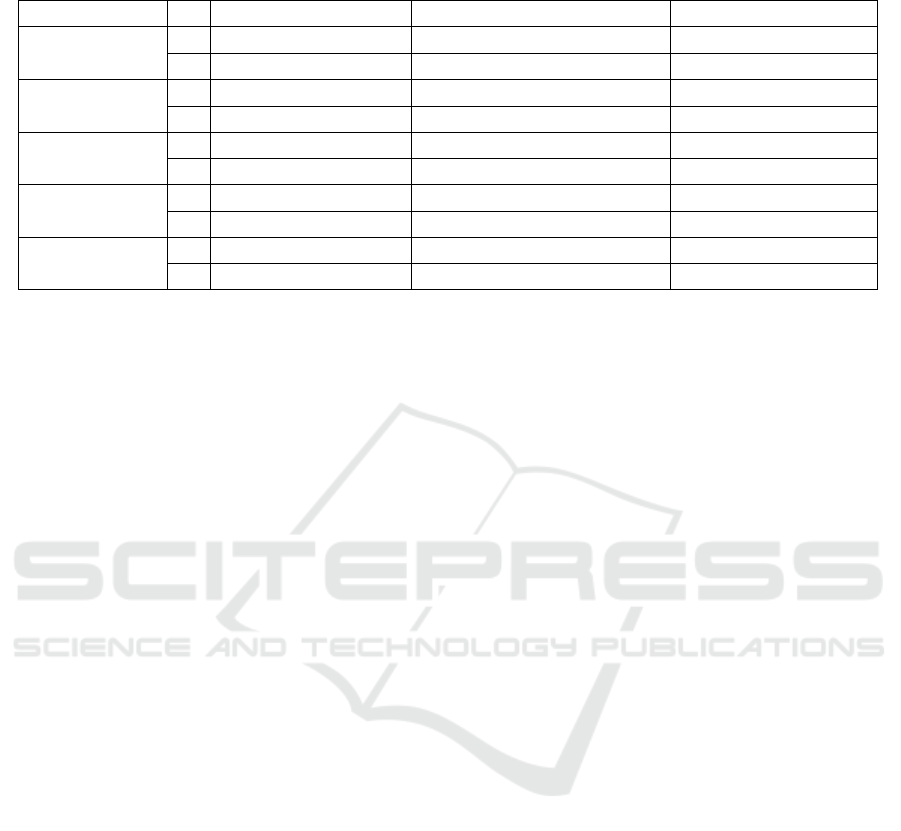
Table 3: t-test results between our proposed GCNs with threshold graph of different types (individual, avgFC, FC-of-avgROI).
P denotes the probability and CI denotes the confidence interval in the t-test.
t-test avgFC vs. individual FC FC-of-avgROI vs. individual FC FC-of-avgROI vs. avgFC
threshold = 0.7
P 0.28 2.08 × 10
−6
3.04 × 10
−7
CI [-0.021,0.068] [-0.165,-0.088] [-0.190,-0.110]
threshold = 0.8
P 0.61 5.01 × 10
−8
3.94 × 10
−8
CI [-0.027,0.045] [-0.207,-0.128] [-0.217,-0.135]
threshold = 0.9
P 0.96 8.44 × 10
−9
4.85 × 10
−8
CI [-0.042,0.040] [-0.202,-0.132] [-0.205,-0.127]
threshold = 0.95
P 0.82 6.34 × 10
−7
4.76 × 10
−6
CI [-0.051,0.041] [-0.191,-0.107] [-0.191,-0.097]
threshold = 0.99
P 0.97 0.47 0.53
CI [-0.047,0.049] [-0.028,0.057] [-0.032,0.060]
nearest neighbors in the graph can be beneficial for
classification.
Lastly, we analyzed the brain networks derived
from the graphs used in the GCN framework. We
observed that excessive connectivity or between-
network connectivity in the networks could negatively
impact the GCN classification performance. This dis-
covery aligns with the current research status that few
studies have demonstrated significant associations be-
tween disturbed self-network connectivity and cogni-
tive impairments in MCI or AD (Huijser, 2021). The
results indicate that the self-network connectivity in
VIS, DMN, SMN, VAN and FPC may play a more
significant role in GCN classification. These findings
are in line with the findings in (Zheng et al., 2017) that
disturbed FC of rest state was seen in the DMN and
VIS in AD patients. Li et al. (2015) found that both
MCI and AD patients showed hyperactivation fell in
frontoparietal, VAN, DMN and SMN relative to NCs.
Katsumi et al. (2023) observed that increased base-
line atrophy in the FPC and DMN was related to a
higher risk of progression to dementia.
5 CONCLUSIONS
In this study, we formulated an FC based GCN frame-
work for binary classifications of fMRI data. Specif-
ically, we applied this framework for the detection
of MCI from NCs using the longitudinal OASIS-
3 dataset. Additionally, we systematically explored
the influence of various FC types (individual FC,
avgFC, FC-of-avgROI) and processing methods (k-
NN and threshold) on the GCN classification perfor-
mance. The proposed GCN framework exhibits sig-
nificantly superior performance compared with the
baseline GCN and SVM. The outcomes of our in-
vestigation offer valuable insights into the applica-
tion of FC-based graphical approaches in brain anal-
ysis and disease detection. These findings signifi-
cantly contribute to the understanding of neurodegen-
erative disorders, presenting potential clinical appli-
cations in the detection and management of neurode-
generative diseases, particularly in the context of MCI
or AD. and potential clinical applications in MCI or
AD detection and management of neurodegenerative
diseases. Further research can explore additional FC
measures and refine the GCN framework to improve
classification performance and expand its applicabil-
ity in clinical settings.
ACKNOWLEDGEMENTS
This work was carried out as part of the doctoral
programm in Experimental Sciences and Technol-
ogy at the University of Vic - Central University of
Catalonia. F.D. work was supported in part by the
Tianjin Science and Technology Plan Project (No.
22PTZWHZ00040). C.F.C work was partially sup-
ported by grants PICT 2020-SERIEA-00457 and PIP
112202101 00284CO (Argentina). J.S.-C. work was
partially supported by the University of Vic-Central
University of Catalonia grant R0947.
REFERENCES
Avants, B. B., Epstein, C. L., Grossman, M., and Gee, J. C.
(2008). Symmetric diffeomorphic image registration
with cross-correlation: evaluating automated labeling
of elderly and neurodegenerative brain. Medical im-
age analysis, 12(1):26–41.
Bi, X., Zhao, X., Huang, H., Chen, D., and Ma, Y.
(2020). Functional brain network classification for
Alzheimer’s disease detection with deep features and
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
664

extreme learning machine. Cognitive Computation,
12(3):513–527.
Buckner, R. L., Krienen, F. M., Castellanos, A., Diaz, J. C.,
and Yeo, B. T. (2011). The organization of the hu-
man cerebellum estimated by intrinsic functional con-
nectivity. Journal of neurophysiology, 106(5):2322–
2345.
Bullmore, E. and Sporns, O. (2009). Complex brain net-
works: graph theoretical analysis of structural and
functional systems. Nature Reviews Neuroscience,
10(3):186–198.
Burges, C. J. (1998). A tutorial on support vector machines
for pattern recognition. Data mining and knowledge
discovery, 2(2):121–167.
Chandra, A., Dervenoulas, G., and Politis, M. (2019). Mag-
netic resonance imaging in Alzheimer’s disease and
mild cognitive impairment. Journal of Neurology,
266(6):1293–1302.
Chen, X., Li, B., Jia, H., Feng, F., Duan, F., Sun, Z., Ca-
iafa, C. F., and Sol
´
e-Casals, J. (2022). Graph em-
pirical mode decomposition-based data augmentation
applied to gifted children MRI analysis. Frontiers in
Neuroscience, 16:866735.
Errica, F., Podda, M., Bacciu, D., and Micheli, A. (2020).
A fair comparison of graph neural networks for graph
classification. In International Conference on Learn-
ing Representations (ICLR 2020).
Esteban, O., Markiewicz, C. J., Blair, R. W., Moodie, C. A.,
Isik, A. I., Erramuzpe, A., Kent, J. D., Goncalves, M.,
DuPre, E., Snyder, M., et al. (2019). fMRIPrep: a ro-
bust preprocessing pipeline for functional MRI. Na-
ture methods, 16(1):111–116.
Fey, M. and Lenssen, J. E. (2019). Fast graph represen-
tation learning with PyTorch Geometric. In ICLR
Workshop on Representation Learning on Graphs and
Manifolds.
Greve, D. N. and Fischl, B. (2009). Accurate and robust
brain image alignment using boundary-based registra-
tion. Neuroimage, 48(1):63–72.
Han, S., Sun, Z., Zhao, K., Duan, F., Caiafa, C. F., Zhang,
Y., and Sol
´
e-Casals, J. (2024). Early prediction of de-
mentia using fMRI data with a graph convolutional
network approach. Journal of Neural Engineering.
Submitted, under review.
Hanik, M., Demirtas¸, M. A., Gharsallaoui, M. A., and
Rekik, I. (2022). Predicting cognitive scores with
graph neural networks through sample selection learn-
ing. Brain Imaging and Behavior, 16(3):1123–1138.
Huijser, D. (2021). Functional connectivity changes and
cognitive deficits in Alzheimer’s disease: A review.
Katsumi, Y., Quimby, M., Hochberg, D., Jones, A., Brick-
house, M., Eldaief, M. C., Dickerson, B. C., and
Touroutoglou, A. (2023). Association of regional cor-
tical network atrophy with progression to dementia in
patients with primary progressive aphasia. Neurology,
100(3):e286–e296.
Kingma, D. P. and Ba, J. L. (2015). Adam: A method for
stochastic optimization. pages 1–13.
LaMontagne, P. J., Benzinger, T. L., Morris, J. C., Keefe,
S., Hornbeck, R., Xiong, C., Grant, E., Hassenstab, J.,
Moulder, K., Vlassenko, A. G., et al. (2019). OASIS-
3: longitudinal neuroimaging, clinical, and cogni-
tive dataset for normal aging and Alzheimer’s disease.
MedRxiv.
Li, H.-J., Hou, X.-H., Liu, H.-H., Yue, C.-L., He, Y., and
Zuo, X.-N. (2015). Toward systems neuroscience in
mild cognitive impairment and Alzheimer’s disease:
A meta-analysis of 75 fMRI studies. Human brain
mapping, 36(3):1217–1232.
Liu, F., Zhang, Y., Rekik, I., Massoud, Y., and Sol
´
e-Casals,
J. (2022). Graph learning for brain imaging. Frontiers
in Neuroscience, 16:1001818.
Liu, S., Cai, W., Liu, S., Zhang, F., Fulham, M., Feng,
D., Pujol, S., and Kikinis, R. (2015). Multimodal
neuroimaging computing: a review of the applica-
tions in neuropsychiatric disorders. Brain Informatics,
2(3):167–180.
Micheli, A. (2009). Neural network for graphs: A con-
textual constructive approach. IEEE Transactions on
Neural Networks, 20(3):498–511.
Morris, C., Ritzert, M., Fey, M., Hamilton, W. L., Lenssen,
J. E., Rattan, G., and Grohe, M. (2019). Weisfeiler and
leman go neural: Higher-order graph neural networks.
In Proceedings of the AAAI Conference on Artificial
Intelligence, volume 33, pages 4602–4609.
Nair, V. and Hinton, G. E. (2010). Rectified linear units
improve restricted boltzmann machines. In ICML.
Parisot, S., Ktena, S. I., Ferrante, E., Lee, M., Moreno,
R. G., Glocker, B., and Rueckert, D. (2017). Spectral
graph convolutions for population-based disease pre-
diction. In International Conference on Medical Im-
age Computing and Computer-assisted Intervention,
pages 177–185. Springer.
Pruim, R. H., Mennes, M., van Rooij, D., Llera, A., Buite-
laar, J. K., and Beckmann, C. F. (2015). Ica-aroma:
A robust ica-based strategy for removing motion arti-
facts from fMRI data. Neuroimage, 112:267–277.
Qu, Z., Yao, T., Liu, X., and Wang, G. (2023). A graph con-
volutional network based on univariate neurodegen-
eration biomarker for Alzheimer’s disease diagnosis.
IEEE Journal of Translational Engineering in Health
and Medicine.
Scarselli, F., Gori, M., Tsoi, A. C., Hagenbuchner, M.,
and Monfardini, G. (2008). The graph neural net-
work model. IEEE Transactions on Neural Networks,
20(1):61–80.
Schaefer, A., Kong, R., Gordon, E. M., Laumann, T. O.,
Zuo, X.-N., Holmes, A. J., Eickhoff, S. B., and Yeo,
B. T. (2018). Local-global parcellation of the human
cerebral cortex from intrinsic functional connectivity
MRI. Cerebral cortex, 28(9):3095–3114.
Sporns, O., Tononi, G., and K
¨
otter, R. (2005). The hu-
man connectome: a structural description of the hu-
man brain. PLoS computational biology, 1(4):e42.
Srivastava, S., Ahmad, R., and Khare, S. K. (2021).
Alzheimer’s disease and its treatment by different ap-
proaches: A review. European Journal of Medicinal
Chemistry, 216:113320.
Suprano, I. (2019). Cerebral connectivity study by func-
An Insight Into Neurodegeneration: Harnessing Functional MRI Connectivity in the Diagnosis of Mild Cognitive Impairment
665

tional and diffusion MRI in intelligence. PhD thesis,
Universit
´
e de Lyon.
Tang, H., Ma, G., Guo, L., Fu, X., Huang, H., and Zhan, L.
(2022). Contrastive brain network learning via hier-
archical signed graph pooling model. IEEE Transac-
tions on Neural Networks and Learning Systems.
Wang, L., Li, K., and Hu, X. P. (2021). Graph convolu-
tional network for fMRI analysis based on connectiv-
ity neighborhood. Network Neuroscience, 5(1):83–95.
Xia, M., Wang, J., and He, Y. (2013). Brainnet viewer:
a network visualization tool for human brain connec-
tomics. PloS one, 8(7):e68910.
Zhang, Y., Brady, J. M., and Smith, S. (2000). Hidden
markov random field model for segmentation of brain
mr image. In Medical Imaging 2000: Image Process-
ing, volume 3979, pages 1126–1137. SPIE.
Zheng, W., Liu, X., Song, H., Li, K., and Wang, Z. (2017).
Altered functional connectivity of cognitive-related
cerebellar subregions in Alzheimer’s disease. Fron-
tiers in aging neuroscience, 9:143.
Zhou, J., Cui, G., Hu, S., Zhang, Z., Yang, C., Liu, Z.,
Wang, L., Li, C., and Sun, M. (2020). Graph neu-
ral networks: A review of methods and applications.
AI Open, 1:57–81.
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
666
