
DKCDF: Dual-Kernel CNN with Dual Feature Fusion for
Lung Cancer Detection
Wariyo G. Arero
1a
, Yaqin Zhao
1b
, Longwen Wu
1c
and Yi Wang
2
1
School of Electronics and Information Engineering, Harbin Institute of Technology, Harbin, China
2
Fushan Environmental Monitoring Center, Yantai, China
Keywords: Multi Feature Fusion, CNN, Lung Cancer, HOG, LBP.
Abstract: One of the main reasons for cancer-related fatalities worldwide is lung cancer. Early diagnosis is essential
for enhancing patient outcomes and lowering mortality rates. Deep learning-based approaches have recently
demonstrated promising outcomes in medical image analysis applications, such as lung cancer identification.
In order to improve lung cancer detection, this research suggests a unique method that combines a dual-kernel
convolutional neural network (DKC) with dual-feature fusion using the Histogram of oriented gradients
(HOG) and local binary patterns (LBP). Convolutional neural networks are good at extracting and detecting
features. CNN features are built using low-level features from the first convolution layer, which might only
partially capture some local features and lead to the loss of some crucial details like edges and contours. HOG
is quite good at describing the shape of objects. LBP can record local structure and information about spatial
texture. The distribution of edge directions or local gradients in intensity can provide a good definition of an
object's shape and local appearance. The lung image is loaded with bone, air, blood, water and other
substances and appears noisy in the lung image. As a result, in this research, we favor the HOG and LBP
feature fusion for lung cancer detection.
1 INTRODUCTION
The prognosis of patients who have lung cancer can
be significantly improved by early identification,
which is a primary global health concern. The manual
analysis of medical pictures used in traditional lung
cancer screening procedures can be time-consuming
and prone to human error. Therefore, it is crucial to
create automated and reliable lung cancer detection
technologies. In recent times, the convergence of
computer vision and medical imaging has become a
promising frontier in the pursuit of diagnostic tools
that are both more accurate and efficient(Han et al.,
2019; Liang et al., 2023). Within this realm, the
amalgamation of dual kernel techniques, along with
the integration of various features like Histogram of
Oriented Gradients (HOG) and Local Binary Patterns
(LBP), has demonstrated significant promise in
elevating the sensitivity and specificity of systems
designed for detecting lung cancer. In this study, we
a
https://orcid.org/0009-0001-9074-4948
b
https://orcid.org/0000-0002-0167-0597
c
https://orcid.org/0000-0002-6914-6695
introduce a dual-kernel CNN-based method for
improving lung cancer detection by combining HOG
and LBP characteristics.
Cancer is characterized by the uncontrolled
growth of cells in the body, with lung cancer
specifically involving the formation of malignant
cells within the lungs. Especially in developing
nations, it stands out as the most prevalent cancer
among both men and women and the second most
frequently diagnosed disease. The main contributors
to lung cancer are believed to be smoking, exposure
to air pollution, and insufficient nutrition. Globally,
the number of lung cancer cases and deaths has
considerably grown (Bade & Cruz, 2020). Annually,
the American Cancer Society provides estimates for
new cancer cases and deaths in the United States by
compiling the latest data on population-based cancer
occurrences and outcomes. This information is
derived from incidence data gathered by central
cancer registries and mortality data collected by the
54
Arero, W., Zhao, Y., Wu, L. and Wang, Y.
DKCDF: Dual-Kernel CNN with Dual Feature Fusion for Lung Cancer Detection.
DOI: 10.5220/0012406100003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 54-64
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.

National Center for Health Statistics. For the year
2023, it is projected that there will be 1,958,310 new
cancer cases and 609,820 cancer-related deaths in the
United States (Siegel, Miller, Wagle, & Jemal, 2023).
As scientists explore the complexities of medical
imaging, the need for advanced algorithms capable of
discerning meaningful patterns from intricate datasets
has surged(Ma, Wan, Hao, Cai, & Liu, 2023). The
dual-kernel approach, a robust concept in machine
learning, entails utilizing multiple kernels to capture
varied facets of data representation. This paper
centers on the utilization of the dual-kernel
methodology within the realm of lung cancer
detection, seeking to harness the complementary
information inherent in different feature spaces.
A critical element of our proposed approach
revolves around merging two distinct texture
descriptors, namely HOG and LBP. The HOG
descriptor excels at capturing the spatial arrangement
of pixel intensities, emphasizing gradient information
crucial for delineating structural nuances in medical
images. Conversely, LBP, renowned for its ability to
encode local texture patterns, contributes a
supplementary layer of information, enhancing the
overall feature representation.
The rationale behind this fusion strategy is
grounded in the notion that different imaging
modalities may highlight diverse aspects of the
underlying pathology. By combining the strengths of
HOG and LBP within a dual kernel framework, our
goal is to construct a more comprehensive and
discriminative feature set, thereby bolstering the
robustness of our lung cancer detection system.
Additionally, the diagnosis is typically made at an
advanced stage, when there is no longer hope for
treatment (Soerjomataram et al., 2023). In order to
enhance overall survival, detect lung cancer in its
earliest stages while successful therapies are still
viable, and lower side effects associated with
systemic treatments, it is vital to develop novel
diagnostic techniques that boost the accuracy of early
diagnosis (Shah, Malik, Muhammad, Alourani, &
Butt, 2023). Examining computed tomography (CT)
images is one of the crucial steps in the pre-diagnosis
of lung cancer. The pre-diagnosis process that follows
X-ray or Computed Tomography (CT) scanning takes
the radiologist a lot of time and energy. Additionally,
this scanning procedure calls for a very high level of
focus and proficiency. In particular, if interpretation
is heavily reliant on prior expertise, less experienced
radiologists have extremely variable detection rates,
which accelerates the speed of false positive detection
(S. Shen, Han, Aberle, Bui, & Hsu, 2019).
Low-dose helical Computed Tomography
(LDCT) (Fang Lei, 2019) (Fedewa et al., 2021) is
currently being used as a method for lung cancer
screening (Jonas et al., 2021). To increase the
diagnostic accuracy for the classification of lung
cancer detection, several efforts are being made to
develop computer-assisted diagnosis and detection
systems. The development of computer-aided
systems was motivated by the requirement for
trustworthy and impartial analysis. The purpose of
this research is to identify whether a picture is
cancerous or not and to extract features for detection
(Ani Brown Mary & Dejey, 2018).
The identification, segmentation, and
classification of benign and malignant pulmonary
nodules are the core topics of research on deep
learning-based lung imaging approaches. To enhance
the performance of deep learning models, researchers
mainly concentrate on creating new network
architectures and loss functions. Review papers on
deep learning approaches have lately been published
by a number of research groups (Mandal & Vipparthi,
2021) (Hamedianfar, Mohamedou, Kangas, &
Vauhkonen, 2022) (Highamcatherine &
Highamdesmond, 2019). However, deep learning
techniques have advanced quickly, and every year,
numerous new approaches and applications appear.
This study has topics that earlier studies were unable
to cover.
Early detection of lung cancer patients can
considerably improve their prognosis, which is a
serious global health concern. Traditional lung cancer
screening methods include the manual examination of
medical images, which can be time-consuming and
prone to human error. Therefore, developing
automated and trustworthy lung cancer detection
methods is essential. In this article, we combine the
HOG and LBP feature fusion mechanisms to present
a dual-kernel CNN-based technique for enhancing
lung cancer identification. The following is our
work's primary contribution:
1. We propose dual paths CNN with different
receptive fields (dual-kernel).
2. We propose that HOG and LBP features are
fused with the output of our dual-kernel CNN to
supplement the edge, profile information and
spatial texture information of lung images.
3. We fix the problem of class imbalance by using
data augmentation.
In the subsequent sections, we delve into the
technical foundations of our methodology, which
involves dual kernels and the fusion of multiple
features. We illustrate its potential through
experimental results and comparative analyses. As we
DKCDF: Dual-Kernel CNN with Dual Feature Fusion for Lung Cancer Detection
55

navigate through the intricacies of this innovative
approach, it becomes evident that the amalgamation
of diverse features and dual kernel processing not
only enhances the accuracy of lung cancer detection
but also provides a more nuanced understanding of
the disease at the pixel level. To conclude, this paper
introduces an innovative advancement in medical
image analysis by highlighting the effectiveness of a
dual kernel framework combined with the fusion of
HOG and LBP features for improved lung cancer
detection. Our study emphasizes the significance of
harnessing diverse information sources and
showcases how advanced machine learning
techniques have the potential to reshape the landscape
of early cancer diagnosis.
The remainder of this paper is organized as follows:
we describe related works in part 2, the method and
dataset in part 3, next experiment in part 4, ablation
study in part 5 and finally conclusion in part 6.
2 RELATED WORK
Image processing methods have been studied in the
past to detect lung cancer (Gurcan et al., 2002). The
field of medical imaging has recently seen the
adoption of neural networks and deep learning
techniques (Fakoor, Ladhak, Nazi, & Huber, 2013)
(Greenspan, Van Ginneken, & Summers, 2016) (D.
Shen, Wu, & Suk, 2017). In order to categorize and
diagnose lung cancer using machine learning and
neural networks, a number of researchers (Cai et al.,
2015) (Al-Absi, Belhaouari, & Sulaiman, 2014)
(Gupta & Tiwari, 2014) (Penedo, Carreira, Mosquera,
& Cabello, 1998) (Taher & Sammouda, 2011)
(Kuruvilla & Gunavathi, 2014) have made an effort.
Deep learning methods have not been used frequently
to identify lung cancer. This is due to the dearth of a
sizable dataset of medical photographs, particularly
those of lung cancer. Urine samples are used by
Shimizu et al. (Shimizu et al., 2016) to identify lung
cancer.
When the literature is searched, a sizable number
of research are discovered that help with the quick
detection of lung cancer. Wang and colleagues (Wang
et al., 2018) suggested a new CNN-based
methodology to categorize cancerous or non-
cancerous tissue. In the suggested model, full-slide
imaging (WSI) is typically one megapixel. Hence,
considerably smaller picture patches recovered from
WSI are frequently employed as input. Each 300x300
pixel image patch from lung adenocarcinoma (ADC)
WSIs was employed in this 2018 study. The
suggested model had a success percentage of 89.8%.
A deep convolutional neural network-based
pulmonary nodule identification technique is
proposed by Deng and Chen (DENG & CHEN,
2019), which ingeniously includes the deep
supervision of incomplete CNN layers. (S. Chen,
Han, Lin, Zhao, & Kong, 2020) uses balanced CNN
with traditional candidate detection to create a
computer-aided detection (CADe) strategy. A
convolutional neural network-based automatic
pulmonary nodule identification and classification
system with only four convolutional layers is
proposed by Masud et al. (Masud et al., 2020).
The DSC (dice coefficient) for nodule segmenta-
tion is 73.6%, according to Tong et al.'s (Tong, Li,
Chen, Zhang, & Jiang, 2018) proposed pulmonary
nodule segmentation algorithm, which is based on an
upgraded U-Net and adds a residual network.
(Guo et al., 2014) suggests using a convolution
neural network to create a lung cancer prediction
system, which resolves the problems with manual
cancer prediction. During this procedure, CT scan
images are gathered and processed using a layer of
neural network that automatically extracts image
features. These features are then processed using deep
learning to predict the features associated with cancer
using a large volume of images.
The system the authors developed assists in
decision-making while analysing the patient's CT scan
report. With the aid of convolution neural networks,
lung nodules from CT scan pictures were predicted (El-
Baz et al., 2013). In order to effectively classify lung
cancer-related features as benign and malignant, LIDC
IDRI database images are gathered and put into the
stack encoder (SAE), convolution neural network
(CNN), and deep neural network (DNN). A technique
developed by the author provides an accuracy of up to
84.32%. In our work, we introduce dual-kernel CNN,
which is used for local and global range dependencies
because most deep learning networks are limited to
fixed receptive field size; we also propose a feature
fusion mechanism, which is HOG and LBP, which are
used for obtaining more comprehensive lung feature
and improve the ability to describe and identify lung
cancer image.
3 METHODS
3.1 Data Set and Pre-Processing
We take advantage of the Kaggle Data Science Bowl
2017 (KDSB, 2017) (Kaggle, 2017) database of
medical images. The data set includes 2101 images
that have been labelled with 0 for patients without
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
56
cancer and 1 for patients with cancer. Digital Imaging
and Communications in Medicine, or DICOM, is the
format used for the image. This dataset has a label of 0
for 70% of the data and a label of 1 for the remaining
30%. The CT scan for each patient consists of a
variable number of images (often 100–400; each image
is a 2-D axial slice) with a resolution of 512x512
pixels. Nodules in this dataset are not labelled.
Due to tumours in the lung tissue, the lung image
consists of unimportant parts that must be removed
through segmentation. These unimportant parts
include bone, air, blood, water, and other substances
that must be excluded due to their effects on data noise
and nodule learning. The Hounsfield (HU), a unit of
radio density and representative of CT scan radio
densities, is the measurement used in CT scans.
Diverse researchers employ several segmentation
techniques to weed out irrelevant data, including
clustering (Rao, Pereira, & Srinivasan, 2016), k-means
(Gurcan et al., 2002), watershed (Ronneberger,
Fischer, & Brox, 2015), and thresholding (Alakwaa et
al., 2017). In our work, we used thresholding with a
filter value of -600 to our 2D image.
Initially, the pixel values of each CT scan are
transformed into Hounsfield Units (HU), a
quantitative metric used to express the radio density
of substances in lung CT images. Notably, the lung,
bone, blood, kidney, and water exhibit radio density,
values of -500 HU, 700 HU, 0 HU, 30 HU, and 0 HU
respectively. Following this conversion, each CT
scan comprises multiple slices, with pixel values
corresponding to HU and falling within the range of
[-1024, 3071].
Table 1: Typical Radio densities in HU of Various
Substances in a CT scan (Alakwaa, Nassef, & Badr, 2017).
Substance Radio density(HU)
Ai
r
-1000
Lung tissue -500
Water and Bloo
d
0
Bone 700
The subsequent step involves the removal of
specific tissues, a process commonly addressed by
scholars through methods described above. In our
study, we opt for thresholding. To accomplish this, a
Gaussian filter is applied, and pixel values are
normalized to fit within the [0, 1] range, utilizing a
threshold of -600. Figure 2 depicts a CT scan slice of
a patient alongside its segmentation outcome based
on thresholding.
To enable the utilization of the proposed network,
we convert the HU values of each slice into UINT8,
signifying that the initial raw data, ranging from [-
1024, 3071], undergo linear transformation to [0,
255]. Subsequently, the mask employed for lung
tissue segmentation is multiplied by these values,
with substances outside the mask set to 170,
representing a standard tissue luminance.
We used the thresholding technique to segment
the CT scan image. The unnecessary parts of the
lungs with their typical radio densities of different
parts of the CT scan are shown in Table 1; as shown
in Table 1, the pixels near -1000 and greater than -320
are masked. The resampled image with thresholding
-600 of sample patients with 3D plotting is shown in
Figure 2.
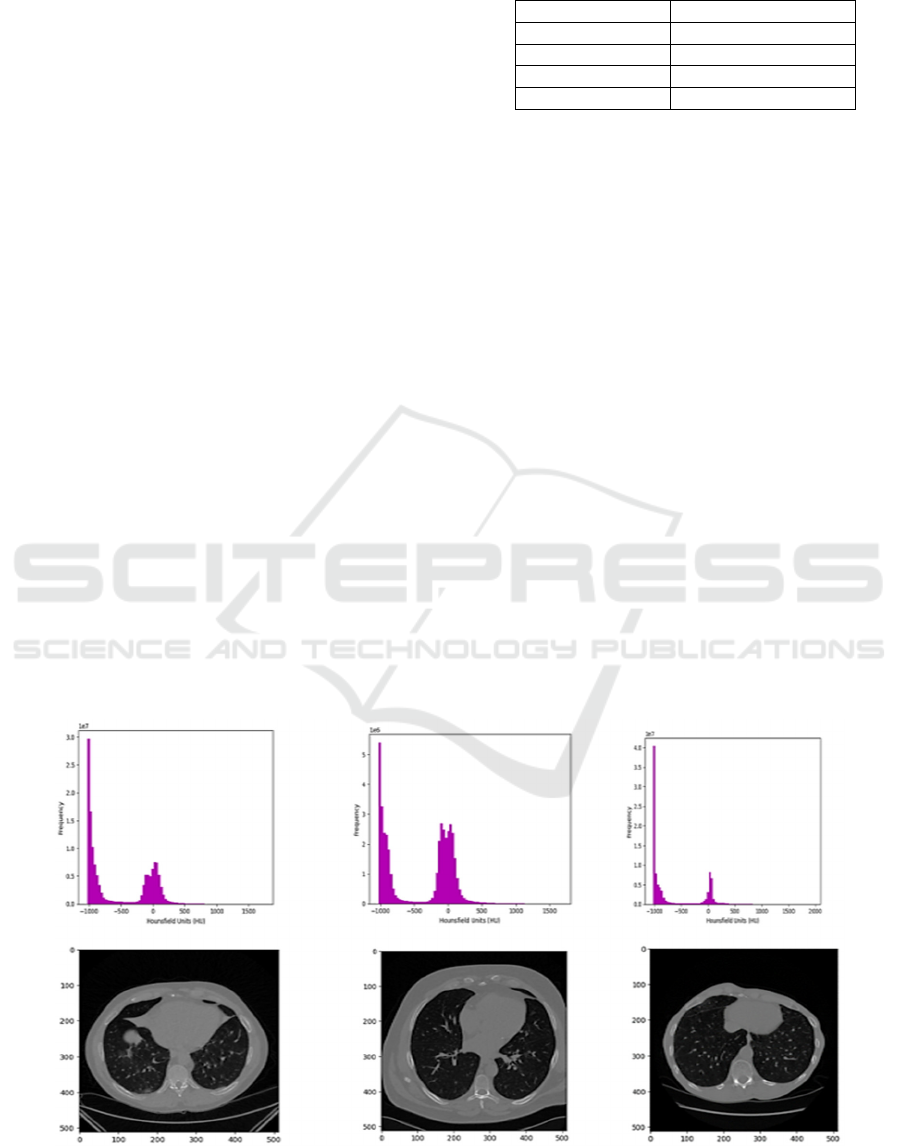
Figure 1: Histogram of pixel values in HU (Hounsfield) for patient 601 at 180 slices, patient 801 at 70 slices, and patient 1001
at slice 120, respectively and with corresponding 2D axial.
DKCDF: Dual-Kernel CNN with Dual Feature Fusion for Lung Cancer Detection
57
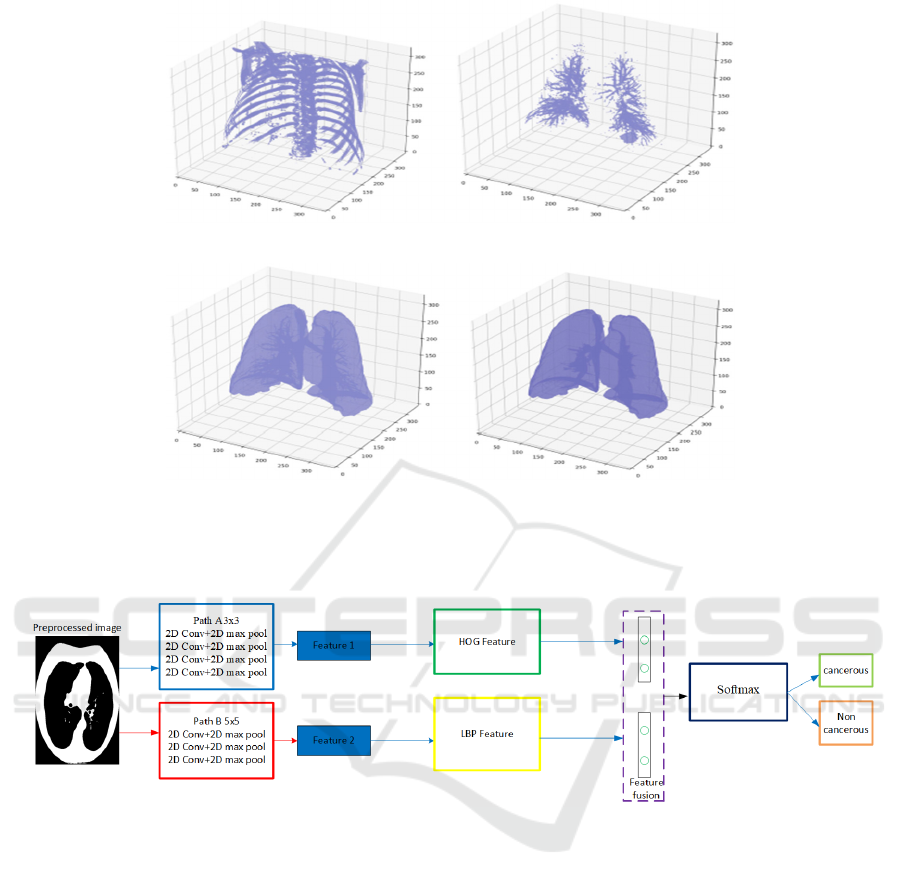
(a) (b)
(c) (d)
Figure 2: 2(a) resample of a 3D image with 600-pixel value HU uncover the bone segment 2(b) resample of sample patient
including lung bronchioles 2(c) resample sample patient performing mask with air 2(d) resample sample patient with
bronchioles included as a terminal mask.
Figure 3: Our proposed dual-kernel with dual-feature fusion model.
3.2 Dual-Kernel CNN
Because most researchers only use one receptive field
within a single path, this affects the nearby pixel and
high-level information during feature extraction. To
address this challenge, we propose two dual CNNs
with different receptive field sizes with dual-feature
fusion mechanisms. We named our paths as path A
and path B with receptive field sizes 3x3 and 5x5,
respectively. The output of path A from the fourth
convolution layer is concatenated with the first
convolution layer of the second path. Path A has four
convolution layers and four max pooling layers.
Three convolution layers and three maximum pooling
layers are present in the second path. We only paid
attention to the indicated kernel sizes and two paths.
In order to study the problems described. Table 2
contains the model parameters. Furthermore, in the
proposed model, we incorporate a feature fusion
7strategy. Figure 3 shows the network of the proposed
model.
3.3 Feature Fusion
The objective of feature extraction is typically to
portray the raw data as a condensed set of features that
more accurately captures its essential characteristics
and attributes. By doing so, we can lower the original
input's dimensionality and train pattern recognition
and classification algorithms using the new features
as input. In our study, we make use of two types of
features, HOG and LBP, which we will go through
individually below.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
58
3.3.1 Histogram of Gradients (HOG)
Feature Fusion
The HOG description highlights an object's structure
or shape, distinguishing it from the edge features used
in photo extraction. While edge features focus solely
on determining if a pixel is part of an edge, HOG goes
further by providing information on edge direction.
This involves extracting gradients and orientations
(magnitude and direction) of edges, dividing the
entire image into smaller sections, and determining
gradients and orientation for each region.
Subsequently, HOG generates separate histograms
for each zone. The term "Histogram of Oriented
Gradients" denotes the histograms produced from
pixel values' gradients and orientations. In a dense
grid, the HOG approach (Dalal & Triggs, 2005)
evaluates locally normalized histograms of image
gradient origins, effectively characterizing an object's
form and local appearance through edge distribution
or local intensity gradients. This method proves
valuable in discerning lung characteristics,
particularly in identifying lung cancer, as it provides
orientation information about the lung boundary and
texture details in the surrounding area. The lung,
containing extraneous elements like air, bone, tissue,
and water in the low-attenuation region, benefits from
the nuanced information provided by the HOG
feature extraction approach.
The HOG feature extraction method (Dalal &
Triggs, 2005) evaluates locally normalized
histograms of picture gradient origins in a dense grid.
Edge distribution or local intensity gradients can
efficiently describe an object's form and local
appearance. The lung is filled with extraneous
components, including air, bone, tissue, and water
and appears in a low-attenuation region. Therefore,
the only information offered is orientation
information of the lung boundary and texture
information of the surrounding area. Therefore, in this
study, we favor the HOG characteristic for
identifying lung cancer. We show a sample image
with HOG feature fusion in Figure 4.
() ( )( )
() ( )( )
,,1,1
,1,1,
x
y
Grc Irc Irc
Grc Ir c Ir c
=+−−
=−−+
(1)
After calculating
x
G
and
Gy
, the magnitude and
angle of each pixel are calculated using the formulae
mentioned below.
22
1
()
() tan
xy
y
x
Magnitude G G
G
Angle
G
μ
θ
−
=−
=
(2)
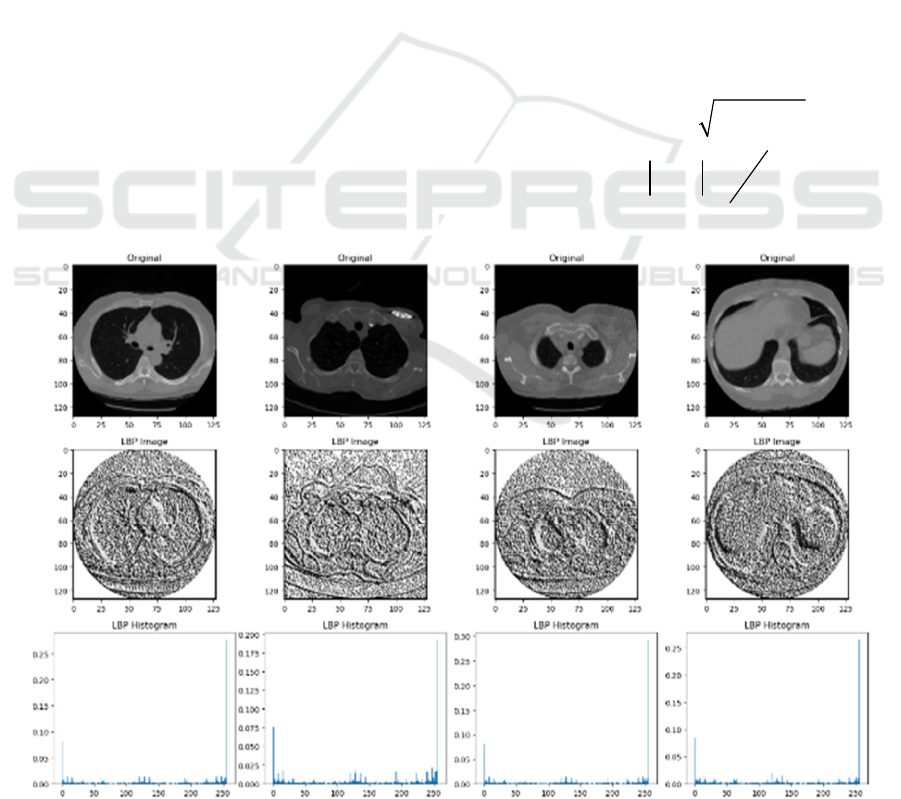
Figure 4: Sample of Lung image by LBP feature with its Histogram.
DKCDF: Dual-Kernel CNN with Dual Feature Fusion for Lung Cancer Detection
59
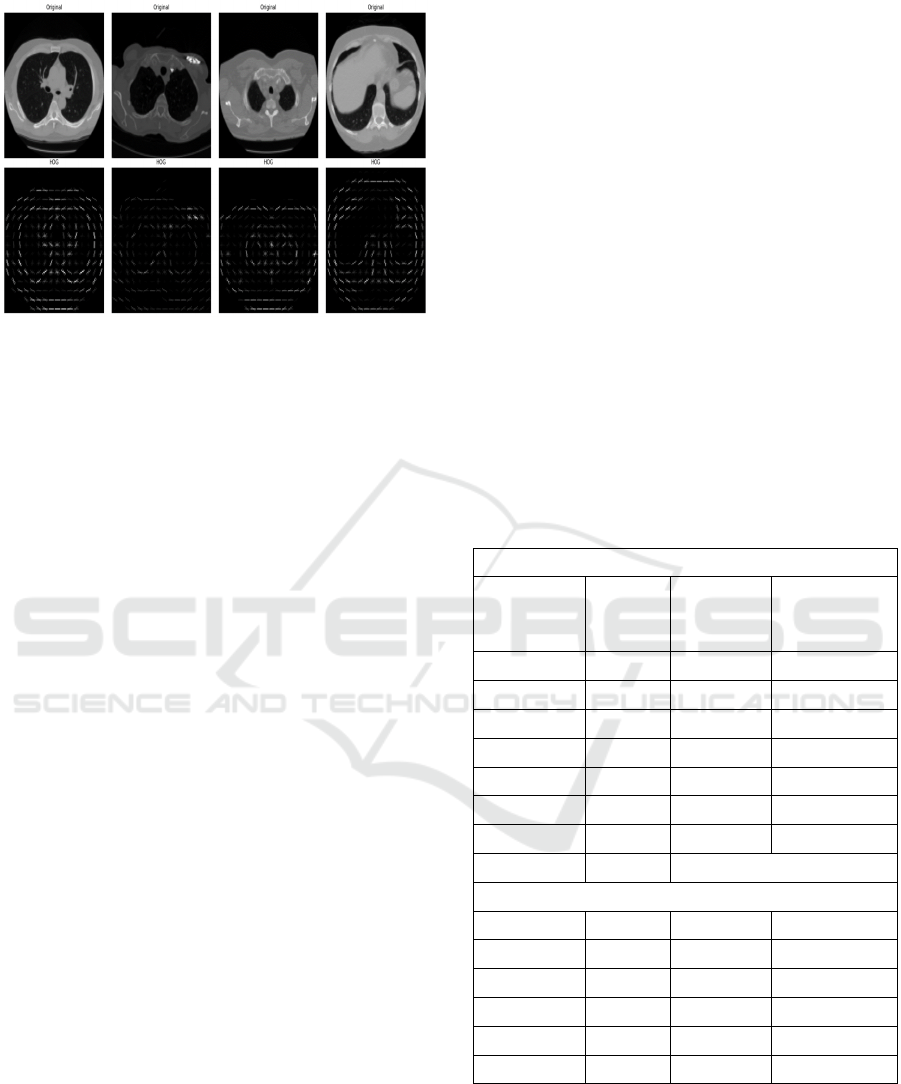
Figure 5: A sample lung image with HOG feature.
3.3.2 Local Binary Feature (LBP)
In biomedical image analysis, two-dimensional
texture analysis is incredibly crucial. A practical and
multiresolution method for processing a grayscale
image is LBP (Ojala, Pietikäinen, & Harwood, 1996).
It is a rotation-invariant texture descriptor built using
nonparametric sample discrimination and local
binary patterns. There are two different sorts of
distinguishing information for the lung imaging sign:
(1) edge orientation and grayscale gradient
information and (2) backdrop texture. The edge
information of the lung is only auxiliary and generic
for class differentiation because the lung itself is
packed with extraneous components like air, bone,
tissue, water, and other substances. The background
information of a lung cancer imaging sign is crucial
for its recognition, making the LBP helpful texture for
lung cancer diagnosis.
()
()
1
,
0
,2
P
p
PR c c p c
p
LBP x y s g g
−
=
=−
(3)
()
10
00
x
sx
x
≥
=
<
(4)
where
c
x
and
c
y
are the coordinates of the center
pixel, p are circular sampling points, P is the number
of sampling points or neighborhood pixels,
p
g
is the
grayscale value of p,
c
g
is the center pixel, and s or
sign is threshold function. For classification purposes,
the LBP values are represented as a histogram, as we
show in Figure 5.
A variety of applications, including face
recognition (Ahonen, Hadid, & Pietikainen, 2006)
and medical picture analysis (Tian, Fu, & Feng,
2008), have made extensive use of the LBP (Ojala et
al., 1996), a potent tool for characterizing texture
properties. The first LBP operator which was first
presented in (Ojala et al., 1996) by Ojala et al. By
comparing the points of, for instance, 3×3
neighboring pixels with respect to the value of the
central pixel, LBP is a straightforward approach that
creates binary codes. If the neighboring pixel's value
is less than the center pixel's, it produces the binary
code 0. If not, it produces the binary code 1. The LBP
code is created by multiplying the binary codes by the
respective weights and adding the results. This value
is determined using Eq. (1) as follows:
() ( )
1
,
0
,2
P
i
PR i c
i
LBP x y s g g
−
=
=−
(5)
()
1
0
ic
ic
if g g
Sg g
else
≥
−=
(6)
Table 2: The parameter of our multi-kernel model.
Path A
Layers
w. size/
#weight
Activation Input
Conv 3×3/32 ReLu 128×128×1
Max pool 2×2 32×126×126
Conv 3×3/32 ReLu 32×125×125
Max pool 2×2 32×123×123
Conv 3×3/32 ReLu 32×122×122
Max pool 2×2 32×120×120
Conv 3×3/32 ReLu 32×119×119
Max pool 4×4 32 ×115×115
Path B
Conv ReLu 128×128×1
Max pool 2×2 32×124×124
Conv 5×5/32 ReLu 32×123×123
Max pool 2×2 32×119×119
Conv 5×5/32 ReLu 32×118×118
Max pool 2×2 32×115×115
4 EXPERIMENTS
We implement our model by using one of the deep
learning library tensors flows with Keras backend,
which supports a graphical processing unit (GPU).
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
60
This tensor flow backend Keras with GPU speeds up
the process of a deep learning algorithm. We describe
the parameters of our model, such as kernel size,
convolution layer, pooling layer, hidden layer, stride
and others, in Table 2. The parameter in the table is
the one in which our model achieves the best
performance on the validation set. The training
hyperparameters, including initial momentum, end
momentum, learning rate, and weight decay, were
configured as 0.5, 0.8, 0.001, and 0.001, respectively.
Stride 1 was applied to convolution and max-pool
layers to maintain per-pixel precision. The filters for
all layers, except the softmax layer's parameter
initialized to the label's log, were randomly initialized
from uniform distributions (-0.005, 0.005). Finally,
the network's bias was set to zero.We have shown our
results in Table 3.
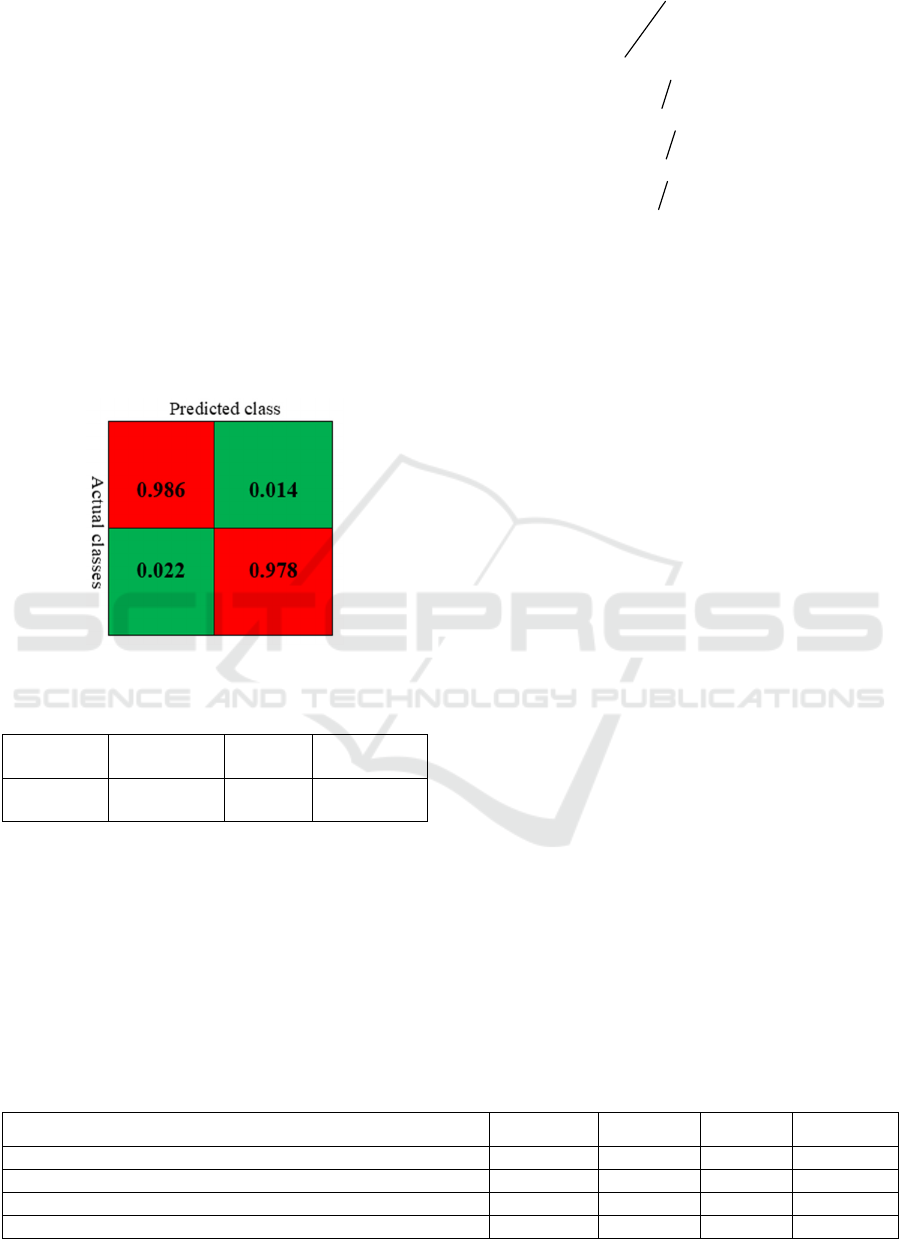
Figure 6: Confusion matrix of our model.
Table 3: Result achieved from our model.
Accuracy Precision Recall Specificity
98.2% 98.6% 97.8% 98.5%
4.1 Performance Evaluation
Researchers have put up a number of performance
evaluations for medical image identification.
Accuracy, recall, and specificity are among the
metrics that are frequently used. We frequently use
performance metrics like accuracy (A), recall (R),
precision (P), and specificity (S) to gauge how well
our model performs.
()
()
tp tn
A
tp fp fn tn
+
=
+++
(7)
nn p
Stt f=+
(8)
pp n
Rtt f=+
(9)
pp P
Ptt f=+
(10)
5 ABLATION STUDY
Our suggested lung cancer detection model, which
uses a dual-kernel technique with the fusion of two
different feature types, Histogram of Oriented
Gradients (HOG) and Local Binary Patterns (LBP),
conducts an ablation study to examine the influence
of individual components. Understanding how each
feature type contributes to the performance of the
entire model is the primary goal of this study.
For the purpose of detecting lung cancer, our
foundational model, known as the dual-kernel with
dual-feature fusion, combines both HOG and LBP
features. After that, while maintaining the values of
all other model elements and hyper parameters, we
systematically assess how well the model performs
when one of these feature types is removed.
Considered are three main experimental conditions:
The combination of dual kernels and multiple
features represents our entire model architecture.
In this configuration, we use a dual-kernel with
LBP (without HOG) and only LBP features for
classification, excluding HOG features from the
model.
HOG and dual kernels without LBP Here, we use
HOG features for classification and omit LBP
features from the model.
With both HOG and LBP features included, the
dual-kernel with dual-feature fusion achieves the
most remarkable accuracy of 98.2%. This
demonstrates how the dual-kernel technique with
feature fusion effectively improves the model's
performance for detecting lung cancer. The accuracy
of the model falls to 96.7% when HOG features are
Table 4: Contestation of our method with other methods.
Methods Accuracy Precision Recall Specificity
DCLCCST (Y. Chen et al., 2022) 94.7% 95.6% 93.9% 95.5%
MLBLCDMIF (Nazir, AlQahtani, Jadoon, & Dahshan, 2023) 97.1% 97.8% 96.4% 97.7%
GLCDGM (Salama, Shokr
y
, & Al
y
, 2022) 97.6% 98.4% 96.8% 98.3%
DKCDF 98.2% 98.6% 97.8% 98.5%
DKCDF: Dual-Kernel CNN with Dual Feature Fusion for Lung Cancer Detection
61
removed, and only LBP features are used. This shows
that HOG characteristics highly influence the model's
capacity to identify lung cancer. The accuracy decline
(-1.5%) highlights the significance of HOG elements
in our model.
Conversely, the accuracy stays high at 97.1%
when we do not include LBP characteristics and
solely use HOG features. Even while this setup
outperforms employing only LBP characteristics, it
still falls short of the dual-kernel with dual-feature
fusion model. This shows that, although to a lesser
extent than HOG, LBP features help offer additional
information. Our lung cancer detection model's
overall accuracy is improved by both the HOG and
LBP features, according to our ablation study. As
their removal causes a more significant accuracy loss
than the omission of LBP features, HOG features, in
particular, are more crucial to improving model
performance. The significance of feature fusion and
the dual-kernel technique in enhancing the
performance of deep learning models for lung cancer
detection is therefore highlighted by our research.
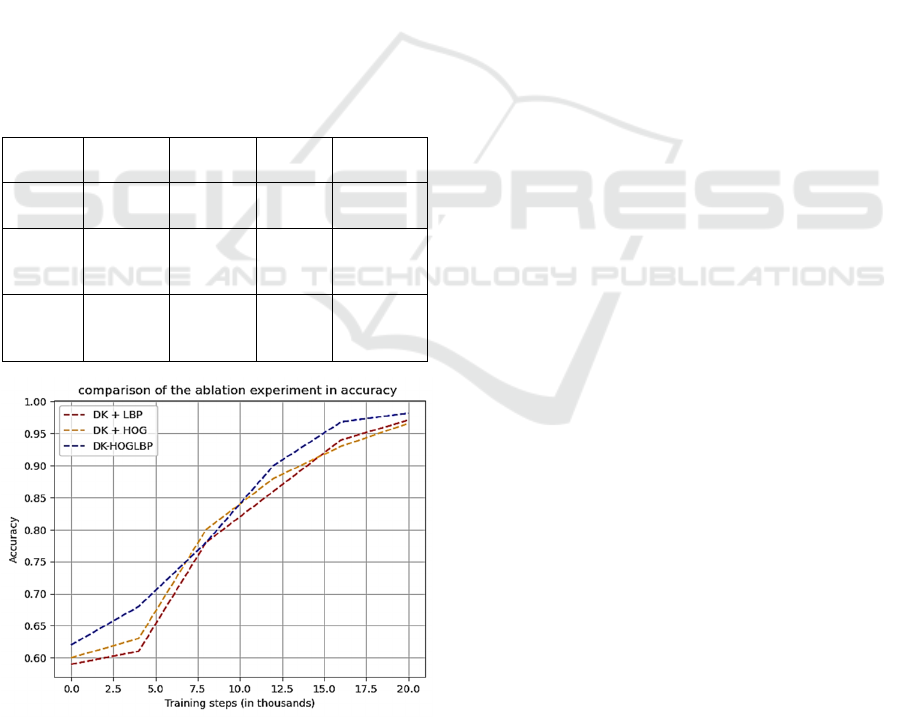
Table 5: Result analysis from ablation experiment.
Methods Accuracy Precision Recall Specificity
DKCDF 98.2% 98.6% 97.8% 98.5%
DKC-
HOG
97.1% 97.8% 96.4% 97.7%
DKC-
LBP
96.6% 97.0% 96.4% 96.9%
Figure 7: Accuracy graph from the ablation experiment.
6 CONCLUSIONS
The article presents an innovative approach to lung
cancer detection by employing a dual-kernel with
dual-feature fusion method, incorporating Histogram
of Oriented Gradient and Local Binary Pattern fusion
techniques. Our assessment using the Kaggle Data
Science Bowl 2017 (KDSB, 2017) dataset reveals
superior outcomes when compared to recent
methodologies, highlighting advancements in
accuracy, recall, precision, and specificity. To be
specific, our model demonstrates an enhancement of
98.2%, 98.6%, 97.8% and 98.5% of accuracy,
precision, recall and specificity respectively
achieved.
These improved findings emphasize the potential
impact of our approach on enhancing lung cancer
detection, with implications for early diagnosis and
treatment strategies. In our forthcoming research, we
aim to investigate transfer learning methods to further
refine the accuracy of our proposed model. This
strategic approach seeks to leverage the insights
gained from our current model and apply them to new
data, fostering ongoing enhancements in lung cancer
detection.
ACKNOWLEDGEMENTS
This work is supported by The National Natural
Science Foundation of China under Grant Numbers
61671185 and 62071153.
REFERENCES
Ahonen, T., Hadid, A., & Pietikainen, M. (2006). Face
description with local binary patterns: Application to
face recognition. IEEE transactions on pattern analysis
and machine intelligence, 28(12), 2037-2041.
Al-Absi, H.R., Belhaouari, S.B., & Sulaiman, S. (2014). A
computer aided diagnosis system for lung cancer based
on statistical and machine learning techniques. J.
Comput., 9(2), 425-431.
Alakwaa, W., Nassef, M., & Badr, A. (2017). Lung cancer
detection and classification with 3d convolutional
neural network (3d-cnn). International Journal of
Advanced Computer Science and Applications, 8(8).
Ani Brown Mary, N., & Dejey, D. (2018). Classification of
coral reef submarine images and videos using a novel z
with tilted z local binary pattern (z⊕ tzlbp). Wireless
Personal Communications, 98, 2427-2459.
Bade, B.C., & Cruz, C.S.D. (2020). Lung cancer 2020:
Epidemiology, etiology, and prevention. Clinics in
chest medicine, 41(1), 1-24.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
62

Cai, Z., Xu, D., Zhang, Q., Zhang, J., Ngai, S.-M., & Shao,
J. (2015). Classification of lung cancer using ensemble-
based feature selection and machine learning methods.
Molecular BioSystems, 11(3), 791-800.
Chen, S., Han, Y., Lin, J., Zhao, X., & Kong, P. (2020).
Pulmonary nodule detection on chest radiographs using
balanced convolutional neural network and classic
candidate detection. Artificial Intelligence in Medicine,
107, 101881.
Chen, Y., Feng, J., Liu, J., Pang, B., Cao, D., & Li, C.
(2022). Detection and classification of lung cancer cells
using swin transformer. Journal of Cancer Therapy,
13(7), 464-475.
Dalal, N., & Triggs, B. (2005). Histograms of oriented
gradients for human detection. Paper presented at the
2005 IEEE computer society conference on computer
vision and pattern recognition (CVPR'05).
DENG, Z., & CHEN, X. (2019). Pulmonary nodule
detection algorithm based on deep convolutional neural
network. Journal of Computer Applications, 39(7),
2109.
El-Baz, A., Elnakib, A., El-Ghar, A., Gimel'farb, G., Falk,
R., & Farag, A. (2013). Automatic detection of 2d and
3d lung nodules in chest spiral ct scans. International
journal of biomedical imaging, 2013.
Fakoor, R., Ladhak, F., Nazi, A., & Huber, M. (2013).
Using deep learning to enhance cancer diagnosis and
classification. Paper presented at the Proceedings of the
international conference on machine learning.
Fang Lei, B. (2019). Barriers to lung cancer screening with
low-dose computed tomography. Paper presented at the
Oncology nursing forum.
Fedewa, S.A., Kazerooni, E.A., Studts, J.L., Smith, R.A.,
Bandi, P., Sauer, A.G., . . . Silvestri, G.A. (2021). State
variation in low-dose computed tomography scanning
for lung cancer screening in the united states. JNCI:
Journal of the National Cancer Institute, 113(8), 1044-
1052.
Greenspan, H., Van Ginneken, B., & Summers, R.M.
(2016). Guest editorial deep learning in medical
imaging: Overview and future promise of an exciting
new technique. IEEE transactions on medical imaging,
35(5), 1153-1159.
Guo, Y., Feng, Y., Sun, J., Zhang, N., Lin, W., Sa, Y., &
Wang, P. (2014). Automatic lung tumor segmentation
on pet/ct images using fuzzy markov random field
model. Computational and mathematical methods in
medicine, 2014.
Gupta, B., & Tiwari, S. (2014). Lung cancer detection using
curvelet transform and neural network. International
Journal of Computer Applications, 86(1).
Gurcan, M.N., Sahiner, B., Petrick, N., Chan, H.P.,
Kazerooni, E.A., Cascade, P.N., & Hadjiiski, L. (2002).
Lung nodule detection on thoracic computed
tomography images: Preliminary evaluation of a
computer‐aided diagnosis system. Medical Physics,
29(11), 2552-2558.
Hamedianfar, A., Mohamedou, C., Kangas, A., &
Vauhkonen, J. (2022). Deep learning for forest
inventory and planning: A critical review on the remote
sensing approaches so far and prospects for further
applications. Forestry, 95(4), 451-465.
Han, G., Liu, X., Zhang, H., Zheng, G., Soomro, N.Q.,
Wang, M., & Liu, W. (2019). Hybrid resampling and
multi-feature fusion for automatic recognition of cavity
imaging sign in lung ct. Future Generation Computer
Systems, 99, 558-570.
Highamcatherine, F., & Highamdesmond, J. (2019). Deep
learning. SIAM Rev, 32, 860-891.
Jonas, D.E., Reuland, D.S., Reddy, S.M., Nagle, M., Clark,
S.D., Weber, R.P., . . . Armstrong, C. (2021). Screening
for lung cancer with low-dose computed tomography:
Updated evidence report and systematic review for the
us preventive services task force. Jama, 325(10), 971-
987.
Kaggle. KDSB (2017). Data Science Bowl 2017 lung
Cancer Detection (dsb3).
Kuruvilla, J., & Gunavathi, K. (2014). Lung cancer
classification using neural networks for ct images.
Computer methods and programs in biomedicine,
113(1), 202-209.
Liang, H., Hu, M., Ma, Y., Yang, L., Chen, J., Lou, L., . . .
Xiao, Y. (2023). Performance of deep-learning
solutions on lung nodule malignancy classification: A
systematic review. Life, 13(9), 1911.
Ma, L., Wan, C., Hao, K., Cai, A., & Liu, L. (2023). A novel
fusion algorithm for benign-malignant lung nodule
classification on ct images. BMC Pulmonary Medicine,
23(1), 474.
Mandal, M., & Vipparthi, S.K. (2021). An empirical review
of deep learning frameworks for change detection:
Model design, experimental frameworks, challenges
and research needs. IEEE Transactions on Intelligent
Transportation Systems, 23(7), 6101-6122.
Masud, M., Muhammad, G., Hossain, M.S., Alhumyani, H.,
Alshamrani, S.S., Cheikhrouhou, O., & Ibrahim, S.
(2020). Light deep model for pulmonary nodule
detection from ct scan images for mobile devices.
Wireless Communications and Mobile Computing,
2020, 1-8.
Nazir, I., AlQahtani, S.A., Jadoon, M.M., & Dahshan, M.
(2023). Machine learning-based lung cancer detection
using multiview image registration and fusion. Journal
of Sensors, 2023.
Ojala, T., Pietikäinen, M., & Harwood, D. (1996). A
comparative study of texture measures with
classification based on featured distributions. Pattern
recognition, 29(1), 51-59.
Penedo, M.G., Carreira, M.J., Mosquera, A., & Cabello, D.
(1998). Computer-aided diagnosis: A neural-network-
based approach to lung nodule detection. IEEE
Transactions on Medical Imaging, 17(6), 872-880.
Rao, P., Pereira, N.A., & Srinivasan, R. (2016).
Convolutional neural networks for lung cancer
screening in computed tomography (ct) scans. Paper
presented at the 2016 2nd international conference on
contemporary computing and informatics (IC3I).
Ronneberger, O., Fischer, P., & Brox, T. (2015). U-net:
Convolutional networks for biomedical image
segmentation. Paper presented at the Medical Image
DKCDF: Dual-Kernel CNN with Dual Feature Fusion for Lung Cancer Detection
63

Computing and Computer-Assisted Intervention–
MICCAI 2015: 18th International Conference, Munich,
Germany, October 5-9, 2015, Proceedings, Part III 18.
Salama, W.M., Shokry, A., & Aly, M.H. (2022). A
generalized framework for lung cancer classification
based on deep generative models. Multimedia Tools
and Applications, 81(23), 32705-32722.
Shah, A.A., Malik, H.A.M., Muhammad, A., Alourani, A.,
& Butt, Z.A. (2023). Deep learning ensemble 2d cnn
approach towards the detection of lung cancer.
Scientific Reports, 13(1), 2987.
Shen, D., Wu, G., & Suk, H.-I. (2017). Deep learning in
medical image analysis. Annual review of biomedical
engineering, 19, 221-248.
Shen, S., Han, S.X., Aberle, D.R., Bui, A.A., & Hsu, W.
(2019). An interpretable deep hierarchical semantic
convolutional neural network for lung nodule
malignancy classification. Expert systems with
applications, 128, 84-95.
Shimizu, R., Yanagawa, S., Monde, Y., Yamagishi, H.,
Hamada, M., Shimizu, T., & Kuroda, T. (2016). Deep
learning application trial to lung cancer diagnosis for
medical sensor systems. Paper presented at the 2016
International SoC Design Conference (ISOCC).
Siegel, R.L., Miller, K.D., Wagle, N.S., & Jemal, A. (2023).
Cancer statistics, 2023. CA: a cancer journal for
clinicians, 73(1), 17-48.
Soerjomataram, I., Cabasag, C., Bardot, A., Fidler-
Benaoudia, M.M., Miranda-Filho, A., Ferlay, J., . . .
Znaor, A. (2023). Cancer survival in africa, central and
south america, and asia (survcan-3): A population-
based benchmarking study in 32 countries. The Lancet
Oncology, 24(1), 22-32.
Taher, F., & Sammouda, R. (2011). Lung cancer detection
by using artificial neural network and fuzzy clustering
methods. Paper presented at the 2011 IEEE GCC
conference and exhibition (GCC).
Tian, G., Fu, H., & Feng, D.D. (2008). Automatic medical
image categorization and annotation using lbp and
mpeg-7 edge histograms. Paper presented at the 2008
international conference on information technology and
applications in biomedicine.
Tong, G., Li, Y., Chen, H., Zhang, Q., & Jiang, H. (2018).
Improved u-net network for pulmonary nodules
segmentation. Optik, 174, 460-469.
Wang, S., Chen, A., Yang, L., Cai, L., Xie, Y., Fujimoto, J.,
. . . Xiao, G. (2018). Comprehensive analysis of lung
cancer pathology images to discover tumor shape and
boundary features that predict survival outcome.
Scientific reports, 8(1), 10393.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
64
