
OCT Image Analysis of Internal Changes in Leaves due to Ozone
Stresses
Hayate Goto
1a
, Nofel Lagrosas
2b
and Tatsuo Shiina
1c
1
Chiba University, Yayoi-cho, Inage-ku, Chiba-shi, Chiba, Japan
2
School of Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka, Japan
Keywords: OCT, Indicator Plant, Environmental Assessment, Ozone, GLCM.
Abstract: Changes in environmental conditions can be evaluated by detecting the conditions in indicator plants.
Indicator plants are sensitive to specific environmental stresses. This research focused on white clover as an
indicator plant for ozone. To analyze the effects of weaker stresses, compact OCT (Optical coherence
tomography) for plants was developed, which allows for non-invasive and non-contact cross-sectional
imaging of white clover (Trifolium repens) leaves exposed to ozone gas. OCT image changes on each level
of ozone damage were evaluated using parameters such as the OCT signal level of the leaf palisade layer, the
thickness of the leaf palisade layer, and texture analysis using GLCM (Gray-Level Co-occurrence Matrix).
Measurements of leaves grown in our laboratory showed increased palisade tissue signal, thicker palisade
tissue, a smaller distribution of palisade layer thickness, increased OCT image contrast, and decreased OCT
image homogeneity.
1 INTRODUCTION
In Japan, due to the pollution problems caused by
high economic growth around the 1950s, it became
clear that plants were affected by air pollution
(Takeshi, 2020). Using plants to help evaluate the
atmospheric environment is attracting attention.
Indicator plants are sensitive to specific
environmental stresses which can provide vital
information on the local environmental conditions.
For example, white clover is an indicator plant for
ozone, and white spots of visible damage occur near
the leaf’s main veins when exposed to high
concentrations of ozone gas. Ozone concentrations
can be high in urban areas due to the influence of
traffic. There are significant differences in ozone
concentrations between rural and urban areas.
The white clover in the field is greatly affected by
ozone, and it is possible to quickly estimate the
environment by observing its leaves. Indicator plants
are generally evaluated by visual inspection, satellite
observation, and spectroscopic observation.
Spectroscopic observations can observe a decrease in
a
https://orcid.org/0000-0001-5387-9109
b
https://orcid.org/0000-0002-8672-4717
c
https://orcid.org/0000-0001-9292-4523
the chlorophyll of the plants. However, this
phenomenon is caused by dryness, insect damage,
and nutritional deficiencies, and it is challenging to
elucidate the cause of the change in the experimental
results(Takeshi, 2020).
Optical coherence tomography (OCT) can detect
morphological and intracellular tomographic images
using near-infrared light (Huang et al., 1991). The
internal change in plants is related to specific
environmental stresses or diseases. Additionally,
since OCT is an in-situ and non-invasive technique, it
can be used to observe indicator plants in long-term
changes over time (Wijesinghe et al., 2017; Lee et al.,
2019). Recent studies showed that OCT is used in
ophthalmology (Drexler et al., 2001; Wojtkowski et
al., 2005; Chopra, 2022), dentistry (Sinescu et al.,
2008; Colston et al., 1998), and dermatology (Liu et
al., 2020; Gambichler et al., 2005). OCT is also used
for observation of seed germination process (De Silva
et al., 2021; Saleah et al., 2022) and diagnosis of
vegetables and fruits (Zhou et al., 2022; Saleah et al.,
2022; Gocławski et al., 2017). Compared to
technologies that visualize internal structures, such as
Goto, H., Lagrosas, N. and Shiina, T.
OCT Image Analysis of Internal Changes in Leaves due to Ozone Stresses.
DOI: 10.5220/0012392200003651
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 12th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2024), pages 65-71
ISBN: 978-989-758-686-6; ISSN: 2184-4364
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
65

MRI and X-rays, OCT is high resolution, and capa
ble of non-invasive quantitative analysis. Since the
developed OCT is compact to bring outside, plant
internal structures can be measured at growing area.
Environmental conditions can assess to measuring the
indicator plant in the growth area.
Ozone is purposely absorbed by the plant through
its stomata which destroys the palisade tissue near the
adaxial epidermis. In our previous study, white clover
was exposed to high concentrations of ozone gas, and
it was confirmed that the OCT signal decreased
measured above the palisade tissue (Goto et al., 2023).
To evaluate the internal changes caused by ozone, this
research extracted features from the OCT images of
white clover leaves.
This study aimed to explore OCT signal
processing and image analysis methods for
classifying the level of environmental damage to
white clover leaves using machine learning. Since the
effect of ozone gas is likely to appear in the thickness
and OCT image texture of the palisade tissue, we
performed thickness evaluation by layer detection
using peak detection and OCT image texture analysis
and developed the optimum feature extraction for
OCT signal analysis.
2 METHOD
2.1 Developed Plant OCT System
The developed plant time domain (TD)-OCT system
is based on a Michelson interferometer (Fig.1). The
light irradiated from the super luminescent diode
(SLD (ANRITSU, AS3E113HJ10M)) light source is
split into a reference optical path and a sample optical
path by a fiber coupler. The light returning from the
sample optical path has different optical path lengths
due to the light backscattered by different layers
within the sample leaf. The reference optical path
length changes at a constant speed, performing the
rotation mechanism (Fig.2). The light path in the
reference optical path (red arrow) is shown in Figure
2. The light comes from the light upper position,
returns to the same position, and goes back to the fiber
coupler. To rotate the mirror on the stage, the
reference optical path length is changed. Since low-
coherence light is used, the intensity of the
interference light can be observed only when the
optical path lengths match within the coherence
length. Since the reference optical path length
changes linearly with time, linear analysis can be
conducted directly (Shiina et al., 2003; Saeki et al.,
2021).
Figure 1: The configuration of the plant OCT system.
Figure 2: Reference optical path system.
Table 1: Specification of OCT system.
Parameters Values
Center wavelength 1310 nm
FWHM 53 nm
SLD output 15μW
Axial resolution 14.2 μm
Lateral resolution 10 μm
A-scan rate 25 Hz
OCT size 198×168×98 mm
Probe size φ6 mm×9 mm
Table 1 shows the characteristics of the developed
OCT (Goto et al., 2023). The central wavelength is
1310 nm, which has low absorption by chlorophyll
and the local minimum value of the absorption by
water. The axial resolution is 14.2 μm related to SLD
coherent length. At 1310 nm, the resolution is lower
than that of light wavelength around 800 nm, which
is commonly used in medical OCT, but the measuring
depth is more extended because it is less affected by
scattering and absorption. The signal acquisition rate
is 25 Hz, and 16 accumulated signals were averaged
to collect one A-line data which reduces noise. The
probe moves every 10 μm to produce an image from
400 signals. The size of OCT is small enough to be
carried outside and measured in the field.
PHOTOPTICS 2024 - 12th International Conference on Photonics, Optics and Laser Technology
66
2.2 Exposure to Ozone Gas
White clover was grown in an incubator at 20℃ with
an ozone generator (KENWOOD, CAX-DM01) at a
concentration of 0.17-0.21 ppm. The red and blue
light of the cold cathode lamp that is easy to absorb
by plant’s leaves is irradiated for 15 hours a day inside
the incubator. Measurements were done before
placing plants in the incubator and after placing plants
in the incubator every 3, 6, 10, and 14 days. The
plants were measured in growing conditions to
analyze effects within the same leaf.
2.3 Parameter Extraction
To confirm the influence of ozone, multiple analysis
parameters were utilized by measuring the intensity
of the OCT signal, the thickness of the palisade
structure using peak detection, and texture analysis
using the Gray-Level Co-occurrence Matrix (GLCM)
within the palisade structure.
2.3.1 The Intensity of Palisade Tissue
To obtain the OCT interference intensity of the
palisade tissue, the acquired data was processed by
background subtraction, focal length correction, and
distance squared correction. To make one A-line, 400
A-lines were aligned and averaged. The averaged A-
line was performed by using normalization with the
maximum value, and logarithmic transformation.
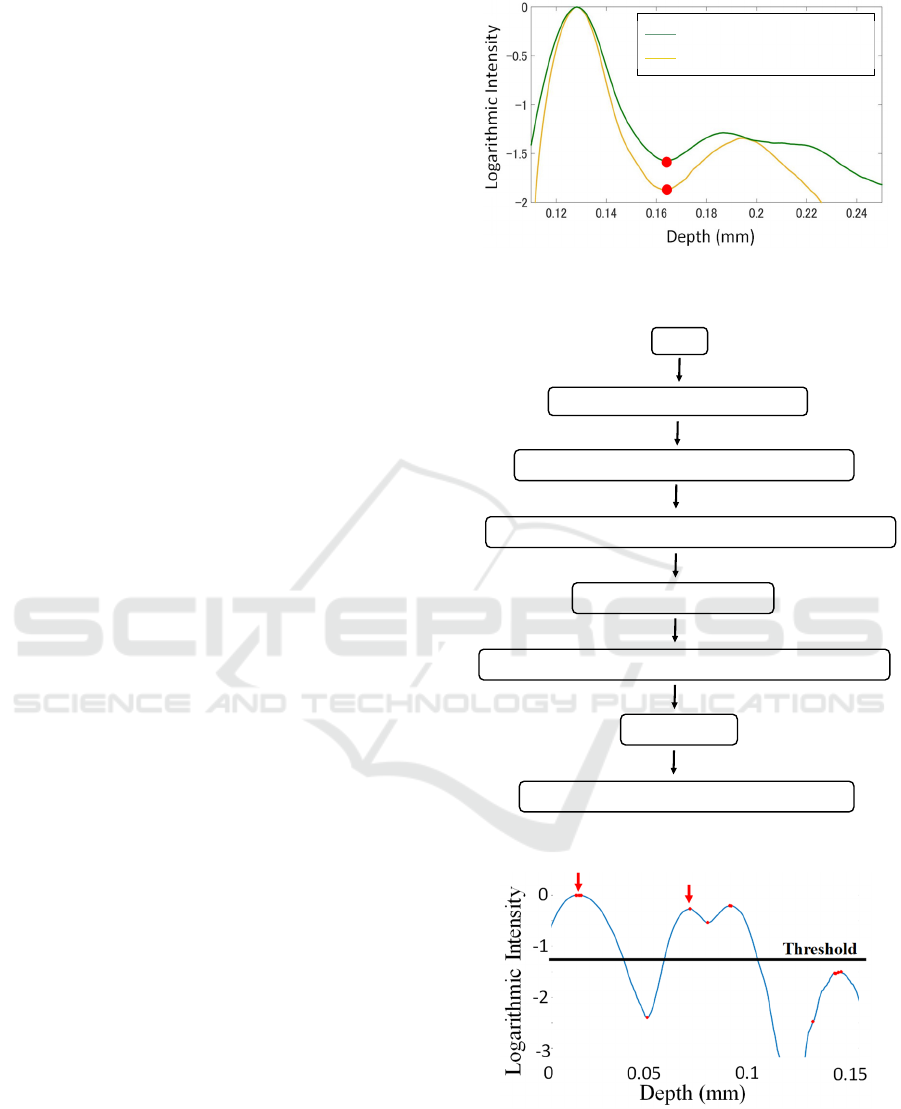
Figure 3 shows the OCT signal change in the A-line
before and after 10 days of exposure to ozone gas.
The x-axis is the depth of the sample leaves, and the
y-axis is the logarithmic intensity of the OCT signal.
The green line in Fig.3 is before exposure to ozone,
the yellow line is after ten days of exposure to ozone.
This graph shows smooth lines because averaging to
evaluate the change in a whole image of a leaf itself.
The first minimum value after the maximum position
(red dots points in Fig. 3) was defined as the intensity
of the middle position of the palisade layer.
2.3.2 Thickness Calculations
Figure 4 shows the flowchart of the thickness
calculation program. This program selects the
positions of the first and second peaks. Since the first
peak is the surface layer, and the second peak is the
palisade and spongy layer boundary, the difference
between these peaks is considered as the palisade
layer thickness.
Peak detection was performed using Matlab’s
PeakFinder command (Fig.5). The x-axis shows the
depth information of the leaf, and the y-axis shows
Figure 3: The signal comparison in the palisade layer before
and after exposed to ozone gas.
Figure 4: Flowchart of thickness calculations.
Figure 5: Peak detection of A-line.
the logarithmic intensity of the OCT signal. At this
time, a threshold value was used to prevent peaks
below a particular value. After that, all of the A-line
peaks in the OCT images were detected. The outliers
START
Find the peaks positon and intensity
Group peaks with peak location difference < 100
Select the maximum of signals at first group as the 1
st
peak
Select next peak as 2
nd
peak
Interpolate the average of the adjacement value to outliers
Moving average
Calculate the distance between 1
st
and 2
nd
peak
Before exposure to ozone
After 10 days exposure to ozone
OCT Image Analysis of Internal Changes in Leaves due to Ozone Stresses
67
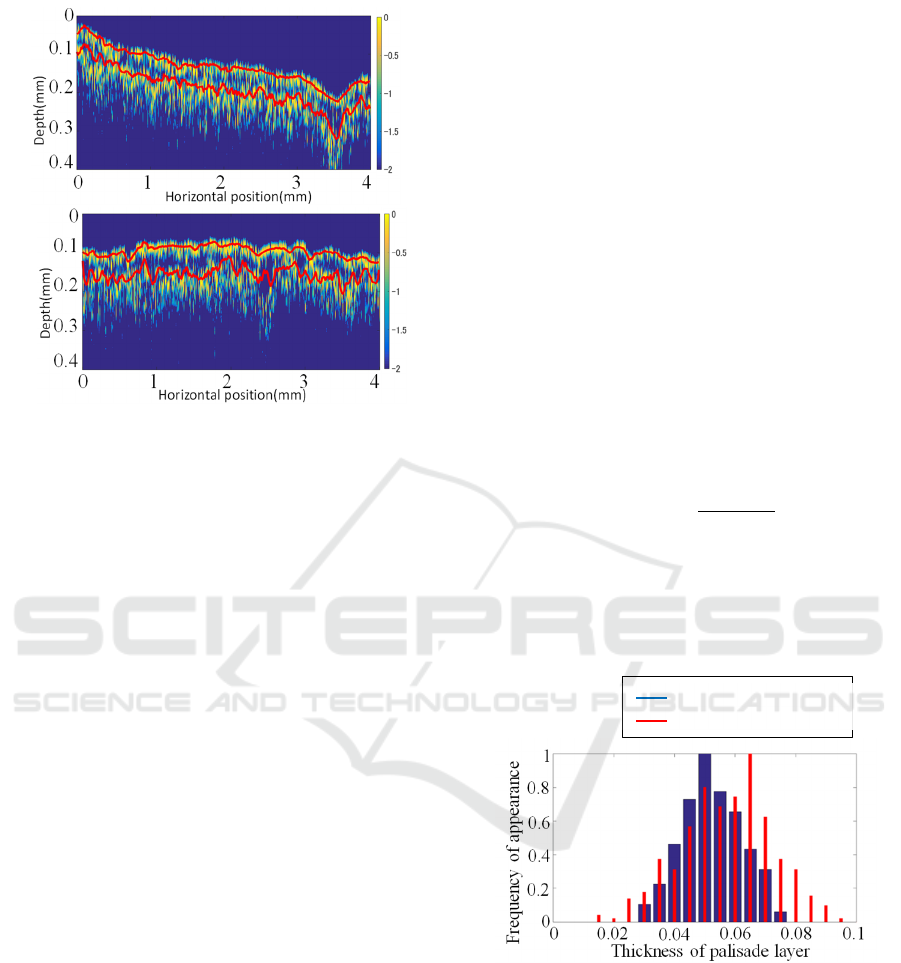
Figure 6: Peak detection of B-scan image (a) Before
exposure to ozone gas and (b) After 10 days exposure to
ozone gas.
of peak positions in the images were removed and
interpolated. The moving average was taken so the
peak detection results fit smoothly with the
OCTimage. Finally, we obtained the distance
between the two peaks and made a histogram. The red
arrow indicates the peak point that is defined by this
method.
Figure 6(a) and 6(b) show the results of peak
detection before and after exposure to ozone gas,
respectively. The image's upper part is the leaf's
adaxial surface, and measurements are taken with
light incident from this direction. The x-axis is the
scanning direction, and the y-axis is the depth
direction of the white clover leaf. The image's aspect
ratio has been changed to make it easier to see peak
detection results. The red line in the image is the
result of peak detection, and it can be confirmed that
the two layers can be detected correctly. Since the
ozone gas destroys the palisade tissue, the two peaks
in Figure 6(b) have a larger distance than the two
peaks in Figure 6(a).
From each histogram, the kurtosis, which
indicates the degree of concentration of the
distribution, and the average value were obtained and
compared with the measurement results of white
clover leaves grown under each condition.
Figure 7 is an example of the histogram. The blue
bar graph is the result before exposure to ozone gas,
and the red bar graph is the result after 10 days of
exposure to ozone gas. A detailed discussion of
Figure 7 is shown in subsection 3.2 Thickness
calculation.
2.3.3 GLCM (Gray-Level Co-Occurrence
Matrix)
GLCM is a method that creates a matrix from the
frequency of appearance of specific pixel value pairs
and evaluates the image texture of an object. In the
previous OCT research, OCT images could evaluate
the moisture change because of the storage using
contrast, correlation, energy, and homogeneity from
the GLCM matrix and classify them using machine
learning of support vector machine (SVM)
(Srivastava et al., 2018).
Figure 8 shows the method for making GLCM.
One pixel intensity compares with the intensities of
adjacent pixels. GLCM creates a matrix that counts
the number of identical intensity pairs. Contrast and
homogeneity were calculated using the following
equations (1) and (2) using the created matrix.
Contrast
|
𝑖𝑗
|
𝑝
𝑖,𝑗
,
(1)
Homogeneity
𝑝𝑖,𝑗
1|𝑖𝑗|
(2)
The i,j indicates the positions within the GLCM
matrix, and p(i,j) indicates the frequency of
appearance of specific pixel value pairs. μ and σ are
each row and column's average and standard
deviation, respectively.
Figure 7: Histgram of the thickness.
3 RESULTS AND DISCUSSION
3.1 Intensity of Palisade Layer
Figure 9 shows the result of the intensity change
inside the palisade tissues. The vertical axis indicates
the differences between the epidermis surface and the
middle position of palisade tissue intensity, and the
horizontal axis is the day of growing in the incubator.
Before exposure to ozone
After 10 days exposure to ozone
(a)
(b)
PHOTOPTICS 2024 - 12th International Conference on Photonics, Optics and Laser Technology
68
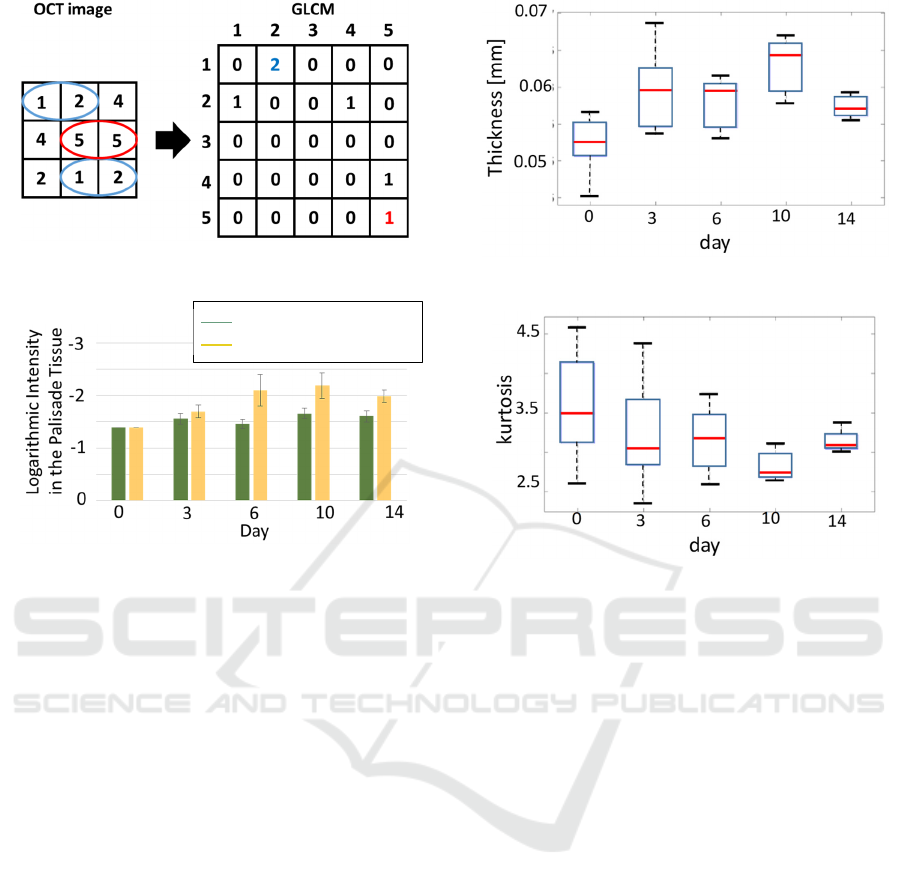
Figure 8: GLCM.
Figure 9: Average of thickness.
The yellow bar indicates the result of growth in
the high concentration of ozone gas, and the green bar
indicates the result of growth in the normal air
condition. Higher intensities are observed in the
palisade tissues under high ozone concentration as
compared to tissues under normal air conditions.
In the case of normal conditions, the intensity of
palisade tissue did not have significant changes.
However, in the case of a high ozone concentration,
the palisade tissue intensity became higher from Day
0 to Day 10. Ozone gas enters the inside of leaves
from the stoma and generates the reactive oxygen
species (Takeshi, 2020). Since the oxidation stress
occurs due to the reactive oxygen species, the
palisade tissue is highly affected by ozone as
compared to spongy tissue. The less scattering effect
observed inside the destroyed leaf palisade tissue
leads to a lower signal.
Comparing the ozone exposure result of Day 0
with other days except for Day 10 in Figure 9 using a
t-test, a significant change didn’t appear in the normal
air conditions (Day 3 : p = 0.140, Day 6 : p = 0.460,
Day 10 : p = 0.021, Day 14 : p = 0.059). Day 10 result
shows a significant change compared to Day 0
because of the growth or senescence. On the other
hand, a significant change was observed from Day 0
to later days (Day 3: p = 0.037, Day 6 : p = 0.047, Day
10 : p = 0.012, Day 14 : p = 0.000064) after exposure
Figure 10:
Average thickness.
Figure 11: Kurtosis of thickness distribution.
to ozone gas. In this research, visible inspection
where white spots appear near the leaf's main vein can
be observed after 10 days of exposure to ozone gas.
Thus, the indicator plants measurement using OCT
can detect the leaf change after 3 days of exposure to
ozone gas, and it is earlier than visible inspection.
OCT can detect the early stage of the inspection and
small inspection due to environmental stresses or
diseases.
3.2 Thickness Calculations
Figure 7 shows the histogram of leaf thickness after 0
and 10 days of exposure to ozone gas. This thickness
value was calculated as described in subsection ‘2.3.2
Thickness calculations’ in the section ‘2 METHOD’.
The median value of the thickness in 10 days became
thicker than the median value in 0 days. The 10-day
histogram has a wider distribution than the 0-day
histogram due to the ozone gas effect.
Figures 10 and 11 show the average and kurtosis
of the thickness measured from the OCT image,
respectively. The horizontal axis is the day of
growing in the incubator, and the vertical axis is the
average and kurtosis of the thickness. As observed,
the average thickness increases, and the kurtosis
decreases during the sampling period. Since the part
of destroyed tissue by ozone was filled with water, the
Growing at normal atmosphere
Growing at ozone atmosphere
OCT Image Analysis of Internal Changes in Leaves due to Ozone Stresses
69
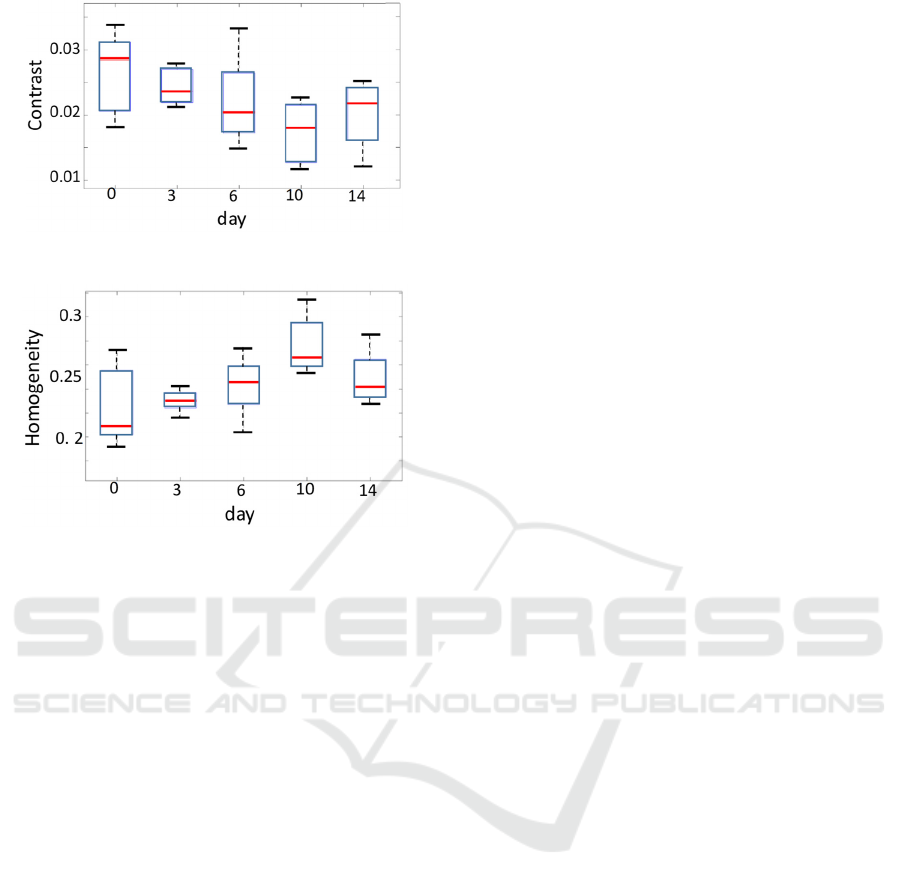
Figure 12: Contrast of palisade layer.
Figure 13: Homogeneity of palisade layer.
intercellular space expanded, and the thickness of
palisade tissue increased as shown in Figure 10. The
decrease of kurtosis is due to the morphological
changes in the leaf. As the influence of the ozone
effect increased, the damaged region in the leaf
expanded and made a uniform condition. This caused
a decrease in the distribution of thickness, and the
kurtosis was decreased. The leaf at the initial state has
a large variation of the kurtosis. The initial state of
cells that are not damaged and have several
conditions due to senescence. Additionally, the 14th-
day result of an average thickness is increased, and
the kurtosis of thickness distribution is decreased. It
did not fit the same trend in earlier days.
3.3 GLCM
Figures 12 and 13 show the GLCM results of contrast
and homogeneity, respectively. The horizontal axis is
the day of growing in the incubator, and the vertical
axis is the contrast and homogeneity. The contrast
value is decreased and the homogeneity value is
increased with the day except for 14 days result.
These results showed a similar trend to the thickness
results (Fig. 10 and 11). This confirms that the
palisade tissues were destroyed and filled with water
upon exposure to ozone gas. As observed in the OCT
images, the water part showed less contrast and high
homogeneity compared with the cell part.
Day 14 has the other trend same as Figures 10 and
11 such as high contrast and less homogeneity. The
plants may become partially senescence by the ozone
effect. Since this causes the dryness inside the leaf
and the high scattering of the light, the thickness
became thicker (Fig.10), bigger kurtosis of thickness
(Fig.11), high contrast (Fig.12), and low homogeneity
(Fig.13).
4 CONCLUSIONS
In this study, we examined the possibility of the white
clover leaves as an indicator plant for ozone gas using
TD-OCT. This study shows the use of OCT as an
early detection device for ozone gas. If the plant
leaves are exposed to ozone gas, ozone gas enters
inside the leaves from the stoma and destroys the
palisade tissue. This phenomenon causes the OCT
intensity to decrease in the palisade tissue. This study
performed peak detection and texture analysis to
evaluate the effects of ozone gas on white clover
leaves. Additionally, the thickness of palisade tissue
became thicker and has smaller distribution. The
GLCM result of palisade tissue has low contrast and
high homogeneity. After 14 days of exposure to
ozone gas, due to necrosis, they showed other trends
compared to before 14 days.
To classify the degree of damage caused by ozone
gas, this research will increase the number of samples
and use machine learning for classification. To
distinguish the ozone effect from other stresses, the
plants will be measured and evaluated under other
conditions such as dryness, lack of nutrients, lack of
light, and varying temperatures. The parameters such
as interfered intensity of palisade tissue, thickness
distribution of the palisade tissue, and image texture
that only appeared in the ozone effect will be selected
for evaluation of the condition of the plant growing
area.
Using OCT in the field, the relationship between
the field conditions and indicator plant parameters
can be properly assessed. This method can be helpful
in the field of agriculture research because the
selection of crops to be planted, and proper
application of pesticides and other growth parameters
can be studied.
ACKNOWLEDGMENTS
This work was supported by JST, the establishment
of university fellowships towards the creation of
PHOTOPTICS 2024 - 12th International Conference on Photonics, Optics and Laser Technology
70

science and technology innovation, Grant Number
JPMJFS2107.
REFERENCES
Takeshi, I. (2020). Atmospheric environment and Plant
(Taiki kankyo to syokubutu. Asakura Shoten, ISBN
987-4-254-42045-6 C 3061 ,Japan.
Huang, D., Swanson, E. A., Lin, C. P., Schuman, J. S.,
Stinson, W. G., Chang, W., Hee, M. R., Flotte, T.,
Gregory, K., & Puliafito, C. A. (1991). Optical
coherence tomography. Science (New York, N.Y.),
254(5035), 1178–1181.
Drexler, W., Morgner, U., Ghanta, R. K., Kärtner, F. X.,
Schuman, J. S., & Fujimoto, J. G. (2001). Ultrahigh-
resolution ophthalmic optical coherence tomography.
Nature medicine, 7(4), 502–507.
Wojtkowski, M., Srinivasan, V., Fujimoto, J. G., Ko, T.,
Schuman, J. S., Kowalczyk, A., & Duker, J. S. (2005).
Three-dimensional retinal imaging with high-speed
ultrahigh-resolution optical coherence tomography.
Ophthalmology, 112(10), 1734–1746.
Chopra V. (2022). Association of Rates of Optical
Coherence Tomography Angiography Vessel Density
Loss on Initial Visits With Future Visual Field
Progression. JAMA ophthalmology, 140(4), 326–327.
Sinescu, C., Negrutiu, M. L., Todea, C., Balabuc, C., Filip,
L., Rominu, R., Bradu, A., Hughes, M., & Podoleanu,
A. G. (2008). Quality assessment of dental treatments
using en-face optical coherence tomography. Journal of
biomedical optics, 13(5), 054065.
Colston, B., Sathyam, U., Dasilva, L., Everett, M., Stroeve,
P., & Otis, L. (1998). Dental OCT. Optics express, 3(6),
230–238.
Liu, Y., Zhu, D., Xu, J., Wang, Y., Feng, W., Chen, D., Li,
Y., Liu, H., Guo, X., Qiu, H., & Gu, Y. (2020).
Penetration-enhanced optical coherence tomography
angiography with optical clearing agent for clinical
evaluation of human skin. Photodiagnosis and
photodynamic therapy, 30, 101734.
Gambichler, T., Moussa, G., Sand, M., Sand, D., Altmeyer,
P., & Hoffmann, K. (2005). Applications of optical
coherence tomography in dermatology. Journal of
dermatological science, 40(2), 85–94.
De Silva, Y. S. K., Rajagopalan, U. M., Kadono, H., & Li,
D. (2021). Positive and negative phenotyping of
increasing Zn concentrations by Biospeckle Optical
Coherence Tomography in speedy monitoring on lentil
(Lens culinaris) seed germination and seedling growth.
Plant Stress, 2, 100041.
Saleah, S. A., Lee, S. Y., Wijesinghe, R. E., Lee, J., Seong,
D., Ravichandran, N. K., Jung, H. Y., Jeon, M., & Kim,
J. (2022). Optical signal intensity incorporated rice seed
cultivar classification using optical coherence
tomography. Computers and Electronics in Agriculture,
198, 107014.
Zhou, Y., Wu, Y., & Chen, Z. (2022). Detection of Mold-
Contaminated Maize Kernels Based on Optical
Coherence Tomography. Food Anal. Methods, 15,
1619-1625.
Saleah, S. A., Wijesinghe, R. E., Lee, S. Y., Ravichandran,
N. K., Seong, D., Jung, H. Y., Jeon, M., & Kim, J.
(2022). On-field optical imaging data for the pre-
identification and estimation of leaf deformities.
Scientific data, 9(1), 698.
Gocławski, J., Nalewajko, J. S., Korzeniewsk, E.,&
Piekarsk, A. (2017). The use of optical coherence
tomography for the evaluation of textural changes of
grapes exposed to pulsed electric field. Computers and
Electronics in Agriculture, 142, 29-40.
Wijesinghe, R. E., Lee, S. Y., Ravichandran, N. K., Han, S.,
Jeong, H., Han, Y., Jung, H. Y., Kim, P., Jeon, M., &
Kim, J. (2017). Optical coherence tomography-
integrated, wearable (backpack-type), compact
diagnostic imaging modality for in situ leaf quality
assessment. Applied optics, 56(9), D108–D114.
Lee, J., Lee, S. Y., Wijesinghe, R. E., Ravichandran, N. K.,
Han, S., Kim, P., Jeon, M., Jung, H. Y., & Kim, J.
(2019). On-Field In situ Inspection for Marssonina
Coronaria Infected Apple Blotch Based on Non-
Invasive Bio-Photonic Imaging Module. IEEE Access,
7, 148684-148691.
Goto, H., & Shiina, T. (2023). Environmental Pollution
Assessment with Indicator Plant Under Ozone Gas
Atmosphere by Using OCT. Proceedings of the 11th
International Conference on Photonics, Optics and
Laser Technology, 1, PHOTOPTICS, 34-39.
Shiina, T., Moritani, Y., Ito, M., & Okamura, Y. (2003).
Long-optical-path scanning mechanism for optical
coherence tomography. Applied optics, 42(19), 3795–
3799.
Saeki, K., Huyan, D., Sawada, M., Nakamura, A., Kubota,
S., Uno, K., Ohnuma, K., & Shiina, T. (2021). Three-
dimensional measurement for spherical and
nonspherical shapes of contact lenses. Applied optics,
60(13), 3689–3698.
Srivastava, V., Dalal, D., Kumar, A., Prakash, S., & Dalal,
K. (2018). In vivo automated quantification of quality
of apples during storage using optical coherence
tomography images. Laser Physics, 28, 066207
OCT Image Analysis of Internal Changes in Leaves due to Ozone Stresses
71
