
Computational Modeling of Arterial Walls: Evaluating Model
Complexity and the Influence of Model Parameters on Deformation
Outcomes
Seda Aslan
1
, Xiaolong Liu
2
, Enze Chen
3
, Miya Mese-Jones
4
, Bryan Gonzalez
5
, Ryan O’Hara
5
,
Yue-Hin Loke
5,6
, Narutoshi Hibino
7
, Laura Olivieri
8
, Axel Krieger
1
and Thao D. Nguyen
1
1
Department of Mechanical Engineering, Johns Hopkins University, Baltimore, MD, U.S.A.
2
Department of Mechanical Engineering, Texas Tech University, Lubbock, TX, U.S.A.
3
Department of Civil and Systems Engineering, Johns University, Baltimore, MD, U.S.A.
4
Baltimore Polytechnic Institute, Baltimore, MD, U.S.A.
5
Sheikh Zayed Institute of Pediatric Surgical Innovation, Children’s National Hospital, Washington DC, U.S.A.
6
Division of Cardiology, Children’s National Hospital, Washington DC, U.S.A.
7
Section of Cardiac Surgery, Department of Surgery, The University of Chicago Medicine, Chicago, IL, U.S.A.
8
Division of Pediatric Cardiology, University of Pittsburgh Medical Center, Pittsburgh, PA, U.S.A.
{bgonzalez, rohara, yloke}@childrensnational.org, nhibino@bsd.uchicago.edu, olivierilj@upmc.edu,
{axel, vicky.nguyen}@jhu.edu
Keywords:
Biomechanical Modeling, Arterial Wall Modeling, Patient-Specific FE Models.
Abstract:
Computational models have been instrumental in advancing cardiovascular applications, particularly in simu-
lating arterial behaviors for pre-surgical treatment strategies. Nonetheless, uncertainties arising from patient-
specific parameters, such as arterial wall thickness and material properties, pose challenges to their precision.
This study utilized finite element analysis to simulate the deformation response of the porcine pulmonary
artery to a pressure change and performed a sensitivity analysis of the effects of material properties and vessel
wall thickness on the deformation. The widely recognized Holzapfel-Gasser-Ogden (HGO) model was used
to describe the stress-strain behavior of the arterial wall. Initially, the arterial walls were modeled as a single
layer, then as separate adventitia and intima-media layers with constant thickness. The model complexity
was increased by varying thickness and specific material properties of different regions in pulmonary arteries,
based on ex vivo data from existing literature. For the sensitivity analysis, the HGO model parameters were
adjusted within their measured variance to study their impact on deformation. The results showed that a sin-
gle layer, regionally varying wall thickness is needed to reproduce the in vivo measure strain response of the
cardiac cycle. The strain response was also most sensitive to variations in the thickness and isotropic shear
modulus of the vessel wall. Using this knowledge, we tuned the model parameters for three porcine models
until the deformation results were within 10% of the MRI-measured deformations. This study offers valuable
insights to identify key model features for specimen-specific computational modeling of the pulmonary artery,
thus providing a foundation for enhancing the realism of soft tissue deformation simulations.
1 INTRODUCTION
The importance of computational models cannot be
overstated in the rapidly evolving field of cardiovas-
cular medicine. Computational models serve as crit-
ical tools, aiding clinicians in visualizing and under-
standing arterial behaviors, especially when creating
surgical plans (Lashkarinia et al., 2018; Aslan et al.,
2022; Liu et al., 2022), predicting growth remodel-
ing (Lashkarinia et al., 2021), and making patient-
specific clinical decisions regarding treatment strate-
gies. Patient-specific computational models offer a
comprehensive insight into the intricacies of the car-
diovascular system, paving the way for more effective
and safer surgical interventions.
Among the various modeling techniques, finite el-
ement (FE) analysis is notable for the ability to sim-
ulate complex mechanical behaviors. FE modeling is
454
Aslan, S., Liu, X., Chen, E., Mese-Jones, M., Gonzalez, B., O’Hara, R., Loke, Y., Hibino, N., Olivieri, L., Krieger, A. and Nguyen, T.
Computational Modeling of Arterial Walls: Evaluating Model Complexity and the Influence of Model Parameters on Deformation Outcomes.
DOI: 10.5220/0012391700003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 454-461
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.

widely used for various cardiovascular applications,
including simulating an artery pre- and post-surgery,
creating patient-specific designs of stents (Caimi
et al., 2018; He et al., 2019; Razaghi et al., 2018),
grafts (Fegan et al., 2022), and patches (Lashkarinia
et al., 2018; Lashkarinia et al., 2021) for virtual surgi-
cal planning, visualizing surgical outcomes, and pre-
dicting the mechanical behavior of the repaired or re-
constructed artery (Lashkarinia et al., 2018). A va-
riety of constitutive models have been developed to
describe the stress-strain response of the arterial wall,
such as the Ogden (Ogden, 1972), Fung (Fung, 1967),
and Holzapfel-Gasser-Ogden (HGO) (Gasser et al.,
2006) models. Experimental studies, including infla-
tion (Sanders et al., 2020), uniaxial, and biaxial tests
(Azadani et al., 2012), have been conducted to un-
derstand the behavior of arteries and to develop these
constitutive models. Arterial walls exhibit a highly
nonlinear behavior (Hoffman et al., 2017) and are
composed of 3 layers: intima, media, and adventi-
tia. The mechanical properties of arterial walls sig-
nificantly vary based on the species, age, health, and
anatomy (Hayashi, 2003). Therefore, the FE models
must account for these variances to accurately sim-
ulate the behavior of arterial walls in both unloaded
and loaded states (Humphrey, 1995).
In previous research, patient-specific FE models
for pulmonary artery (PA) walls were developed to
understand the mechanical and structural changes in
PAs using invasive measurements and Magnetic Res-
onance Imaging (MRI) in vivo (Lashkarinia et al.,
2018; Pourmodheji et al., 2021). However, these stud-
ies had limitations due to the assumption of constant
thickness or uniform material properties along the ar-
teries. The main challenges faced by FE modeling in-
clude uncertainty in the artery wall thickness and ma-
terial properties. These properties exhibit significant
intra- and inter-subject variability and are challenging
to measure in vivo. The effects of these uncertain-
ties, along with various modeling assumptions, on the
mechanical behavior of specimen-specific PA models
remain unclear.
In this paper, we developed an FE model to sim-
ulate the behavior of PA in a 14-week-old pig model
using in vivo MRI data. Our aim is to identify the key
features of the computational model that accurately
capture the spatially-varying deformation response of
the vessel walls during the cardiac cycle. We used a
well-known HGO model to describe the stress-strain
response of the PA walls. In our study, we undertook a
stepwise approach to modeling the behavior of the PA
walls using FE methods. We began with a simple rep-
resentation, treating the PA walls as a single-layered
structure. This was then expanded to a more intricate
two-layered model with different material properties,
yet kept the thickness constant across the PA. We in-
troduced further refinement by adjusting the thickness
and material properties in distinct PA regions based
on ex vivo measurements from porcine PAs (Pillala-
marri et al., 2021). We aimed to simulate the deforma-
tion response of the PA walls under a pressure change,
specifically a 13 mmHg increase from diastole to sys-
tole (Mueller-Graf et al., 2021). To understand the ro-
bustness of the model, we varied its parameters within
their standard deviation and analyzed the subsequent
impact on deformation.
Our findings demonstrated the necessity of wall
thickness gradients, especially from the ventricle to-
wards the lungs, to capture observed arterial wall de-
formation in-vivo. We also noted a pronounced sensi-
tivity in the pressure-strain response to wall thickness
and the isotropic shear modulus. Based on these find-
ings, we adjusted the shear modulus in our porcine
models (n=3), striving for a match within 10% of
MRI-measured deformations during the cardiac cy-
cle. Our study offers valuable insights into the com-
plexities of modeling and parameter adjustments re-
quired for individualized computational artery simu-
lations, setting a foundation for advanced soft tissue
deformation simulations. It aims to improve surgical
planning, enhance predictions of disease progression,
and inform clinical decisions related to treatment ap-
proaches.
2 METHODS
Our methodology is outlined in Fig. 1. We first
obtained three-dimensional (3D) geometries of a
porcine PAs in vivo at both peak systole and end di-
astole, then generated circumferential curves on its
surface along the centerline (Fig. 1a). Using the
PA geometry at the diastolic state, a computational
model was constructed by imposing boundary condi-
tions simulating the increase to peak systolic pressure
(Fig. 1b). Once the geometry underwent deforma-
tion, we analyzed the deformation pattern throughout
the PAs (Fig. 1c) and plotted circumferential strain
patterns along the centerline of the PAs. We tuned
the model parameters to mimic the strain pattern ob-
served in-vivo MRI measurements (Fig. 1d). We in-
cluded three porcine models to demonstrate the re-
sults of specimen-specific tuning. Further specifics of
these procedures are detailed in the subsequent sec-
tions.
Computational Modeling of Arterial Walls: Evaluating Model Complexity and the Influence of Model Parameters on Deformation Outcomes
455
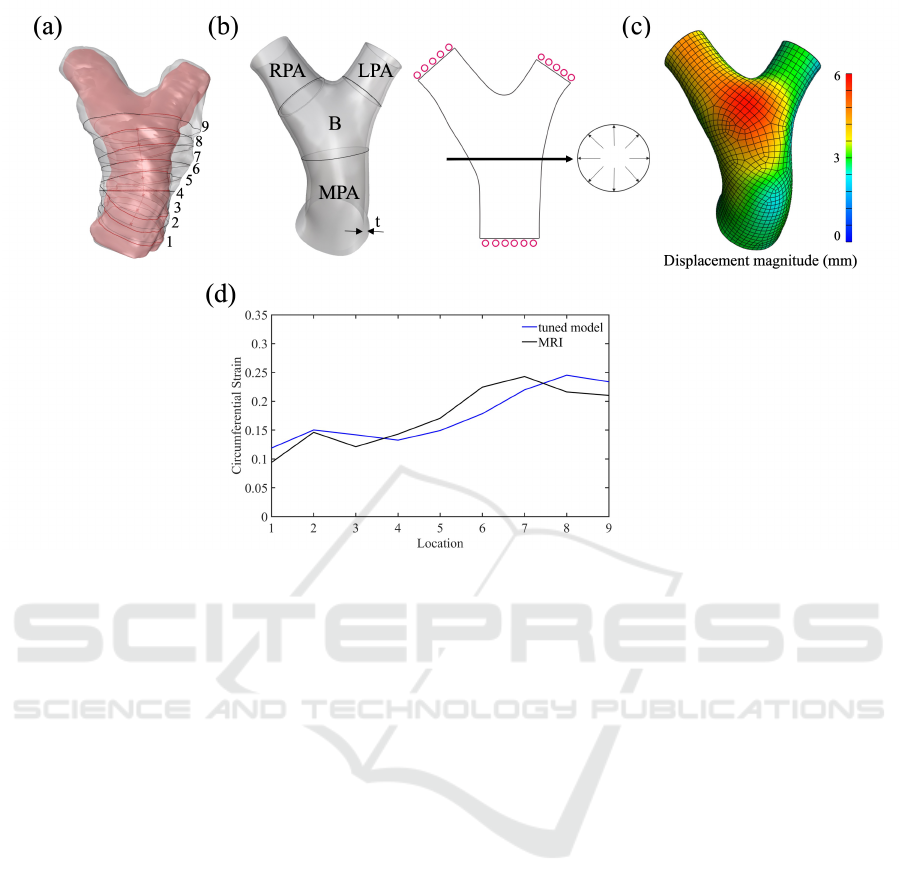
Figure 1: (a) Acquisition of in vivo PA geometries (b) Computational model of PA. The solid model was created as thin wall
with a thickness, t. Fixed normal displacements were applied at the distal surfaces of MPA, LPA, and RPA, and pressure was
applied at the luminal surface along PAs. (c) Resulting displacement magnitude along the geometry. (d) The comparison
between strain obtained from tuned model and MRI data.
2.1 Acquisition of In-Vivo PA Geometry
The data were acquired as part of an Institutional Ani-
mal Care & Use Committee (IACUC) approved study.
Magnetic resonance imaging (MRI) was used to ob-
tain the images of PA from three 14-week-old porcine.
The segmentation of images was performed using
Mimics software (Materialise, Leuven, Belgium) to
create 3D anatomies at end-diastole and at peak sys-
tole as shown in Fig. 1a in pink and grey, respectively.
Nine circumferential curves in the normal directions
(Fig. 1a) to the centerline were created on the luminal
surface of the PAs. The lengths of the curves were
measured from the geometries (at peak systole and
end diastole) to estimate the circumferential strain as
the difference in circumference normalized by the cir-
cumference at diastole.
2.2 Computational Model of the PA
A solid model of the luminal surface at the diastolic
pressure was reconstructed from magnetic resonance
(MRI) using Rhino3D (McNeel and Associates). The
PA wall model included main PA (MPA), bifurcation
region B, left PA branch (LPA), and right PA branch
(RPA), as shown in Fig. 1b. The walls were created by
extruding the luminal surface in the normal direction.
In reality, PA walls consist of three layers: the in-
nermost layer (intima), the middle layer (media), and
the outermost layer (adventitia) (Gasser et al., 2006).
Previous studies have measured the thickness of these
layers in various regions of PAs using ex-vivo samples
(Pillalamarri et al., 2021). They also conducted uni-
axial and biaxial tension tests (Lally et al., 2004), as
well as inflation tests (Boekhoven et al., 2016), to de-
termine the material properties of the walls. Because
separating the layers can be challenging, the intima
and media (IM) are often tested together after adven-
titia (ADV) is separated (Tian and Chester, 2012).
In our modeling approach shown in Fig. 2, we in-
troduced varying degrees of complexity to represent
the walls. Our geometries include 1 layer (IM) of
constant thickness (Fig.2a), 2 layers (IM and ADV)
of constant thicknesses (Fig.2b), 1 layer of varying
thickness (Fig.2c), and 1 layer of varying thickness
with varying material properties (Fig.2d) along the
PAs. The material properties used in models in Fig.2
a, b, and c were adapted from (Pillalamarri et al.,
2021) from the region designated as MPA-M in their
study. In the dual-layer model (Fig2.b), ADV and
IM had specific properties. In the model shown in
Fig.2d, the region-specific parameters (i.e. MPA, B,
BIOINFORMATICS 2024 - 15th International Conference on Bioinformatics Models, Methods and Algorithms
456

LPA, RPA) were adapted from the same study.
The solid model was discretized using trilinear
hexahedral elements using Cubit (v.2023.4 Core-
form). The well-established (HGO) model (Gasser
et al., 2006), given in equations (1), (2) and (3) for
the strain energy density was used to describe the
anisotropic elastic stress-strain response of the walls.
We used the HGO model parameters determined by
ex-vivo tests of porcine PAs by (Pillalamarri et al.,
2021). In the equations, Ψ represents strain energy
with an isotropic, Ψ
iso
, and an anisotropic, Ψ
aniso
,
contribution. C is the isochoric Cauchy-Green defor-
mation tensor, a
01
and a
02
vectors represent the ori-
entations of the collagen fiber families, c is the shear
modulus of the isotropic matrix composed of the non-
fibrillar components of the PA wall, and k
1
and k
2
characterize the exponential behavior of the collagen
fibers.
The average pressure difference between peak
systole and end diastole was measured as 13 mmHg in
previous porcine studies (Mueller-Graf et al., 2021),
therefore, a 13 mmHg pressure was applied to the lu-
minal surface to simulate the increase in blood pres-
sure from the diastolic to the systolic phase. The dis-
placement of the proximal surface of the MPA and
the distal surface of the LPA and RPA were fixed in
the normal direction. FeBio software was utilized to
solve the equations and perform FE simulations using
the time step size of 0.1 seconds for 10 time steps.
Ψ = Ψ
iso
(C) + Ψ
aniso
(C, a
01
, a
02
) (1)
Ψ
iso
=
c
2
(I
1
− 3) (2)
Ψ
aniso
=
∑
layer=M,A
∑
i=4,6
k
1
2k
2
exp
k
2
I
layer
i
− 1
2
− 1
(3)
2.3 Postprocessing and Comparison of
Deformations
The pressurized geometry was exported in stere-
olithography (STL) format. The curves on the inner
luminal surface along the centerline of PAs were cre-
ated and their lengths were measured. The percent
difference in curve lengths between original model
and deformed model was measured as circumferen-
tial strain using:
curve length
de f ormed
− curve length
original
2(curve length
de f ormed
+ curve length
original
)
(4)
The results were compared against measured
strains from MRI data at the same locations to deter-
mine the level of complexity required to capture the
strain trend along PAs.
3 RESULTS
3.1 Comparison of Different
Computational Models
The circumferential strains along the centerlines of
MPA and B regions obtained using different models
(Fig.2) are compared in Fig.3.
The difference in circumferential strains between
the single-layer and two-layer models, with constant
material properties, was negligible. The deforma-
tion decreased along the centerline, transitioning from
MPA to B when a uniform thickness was applied
throughout the PAs. Contrarily, when comparing with
MRI-derived strain results, the trend was reversed.
Consequently, models with a constant thickness fail
to accurately represent the in vivo deformation of the
PA walls.
Varying the wall thickness along the center-
line, decreasing from MPA to the branches, pro-
duced the increasing strain variation congruently with
MRI measurements, as depicted by the pink line in
Fig.3. Moreover, when material properties were var-
ied along with wall thickness, the strain outcomes in-
creasingly resemble the MRI-measured strain trend,
represented by the red line in Fig.3. While the overall
trend remains consistent, they were still consistently
lower than those measured by MRI across all regions.
The aforementioned results indicated that the ex-
perimentally measured regional variation in thickness
and material properties are essential for capturing the
spatial variation in the pressure-strain response of the
PA. Adjusting and fine-tuning the material properties
can bring us closer to strains measured in vivo, allow-
ing for a more accurate depiction of the mechanical
behavior of PA walls across different regions.
The HGO model contains 4 parameters, and it is
essential to understand the sensitivity of the pressure-
strain response to the model parameters. In the sub-
sequent section, we highlight the sensitivity of strain
results to the thickness t, and the HGO model param-
eters: shear modulus of the isotropic matrix (non-
fibrous components of the arterial wall) c, tensile
modulus of the collagen fibers k
1
, the strain-stiffening
parameter of the collagen fiber k
2
, and fiber orienta-
tion angle β, prior to fine-tuning these to more closely
match MRI data.
3.2 Effect of Input Parameters on
Deformation
The ranges for PA wall thickness and parameters
within the HGO model, specified as mean ± stan-
Computational Modeling of Arterial Walls: Evaluating Model Complexity and the Influence of Model Parameters on Deformation Outcomes
457
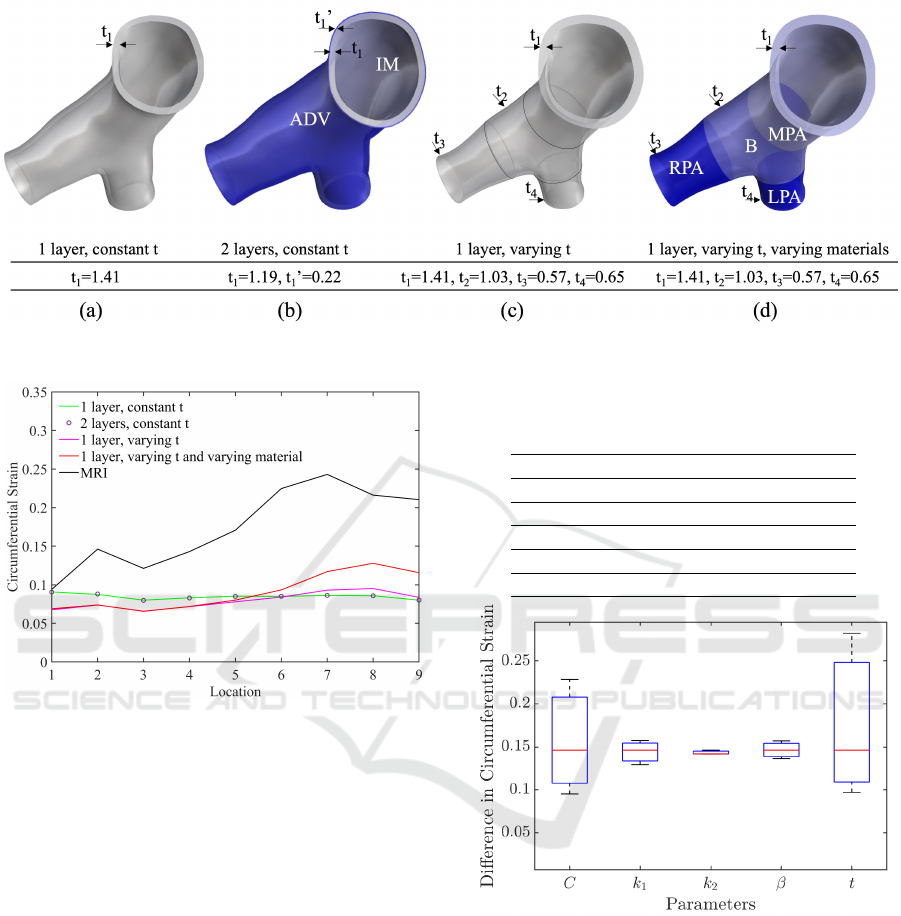
Figure 2: Computational models with different wall layers and thicknesses. The unit of thickness is mm.
Figure 3: Comparison of circumferential strain results in
MPA and B regions using different models. The x-axis in-
dicates the locations where the circumferential lengths were
measured on the wall surface along the centerline.
dard deviation (STD), were obtained from (Pillala-
marri et al., 2021). We used the HGO model param-
eters for region B (as detailed in Table 1) to perform
the simulations for investigating the impact of input
parameters on deformations. The wall thicknesses for
regions B, LPA, and RPA were varied to maintain
a consistent standard deviation percentage from the
mean, mirroring that of the MPA region. The thick-
ness value in Table 1 corresponds to the MPA, and
a proportional adjustment was made for B, LPA, and
RPA regions. We performed simulations by applying
the same pressure across all the models, and subse-
quently analyzed the resulting circumferential strains.
Input parameters were varied between their high
(mean+STD) and low (mean-STD) values one by one
and the resulting differences in circumferential strains
were plotted in Fig.4. As seen in the plot, the circum-
ferential strain is highly sensitive to the changes in the
thickness t and the isotropic shear matrix C, and less
Table 1: The HGO model parameters adapted from (Pil-
lalamarri et al., 2021). The values for each parameter were
varied within their standard deviation.
mean standard deviation
t (mm) 1.19 0.52
C (kPa) 30.03 11.52
k
1
(kPa) 80.73 58.53
k
2
0.67 0.77
β (degree) 55.24 28.71
Figure 4: The difference in circumferential strain resulting
from changes in parameters between their high and low val-
ues.
sensitive to the changes in k
1
, k
2
, and β. The parame-
ter k
2
has the least effect on the deformation.
The circumferential strains along the centerline of
MPA, B, LPA, and RPA regions were plotted to show
the effect of varying input parameters individually in
Fig.5. The MRI strains were also included in the
figure (bottom right) for comparison of strain trends.
The strains obtained with mean values of all param-
eters were able to capture a similar trend as MRI in
MPA and B regions. However, in LPA and RPA re-
gions, trends look significantly different. Specifically,
BIOINFORMATICS 2024 - 15th International Conference on Bioinformatics Models, Methods and Algorithms
458
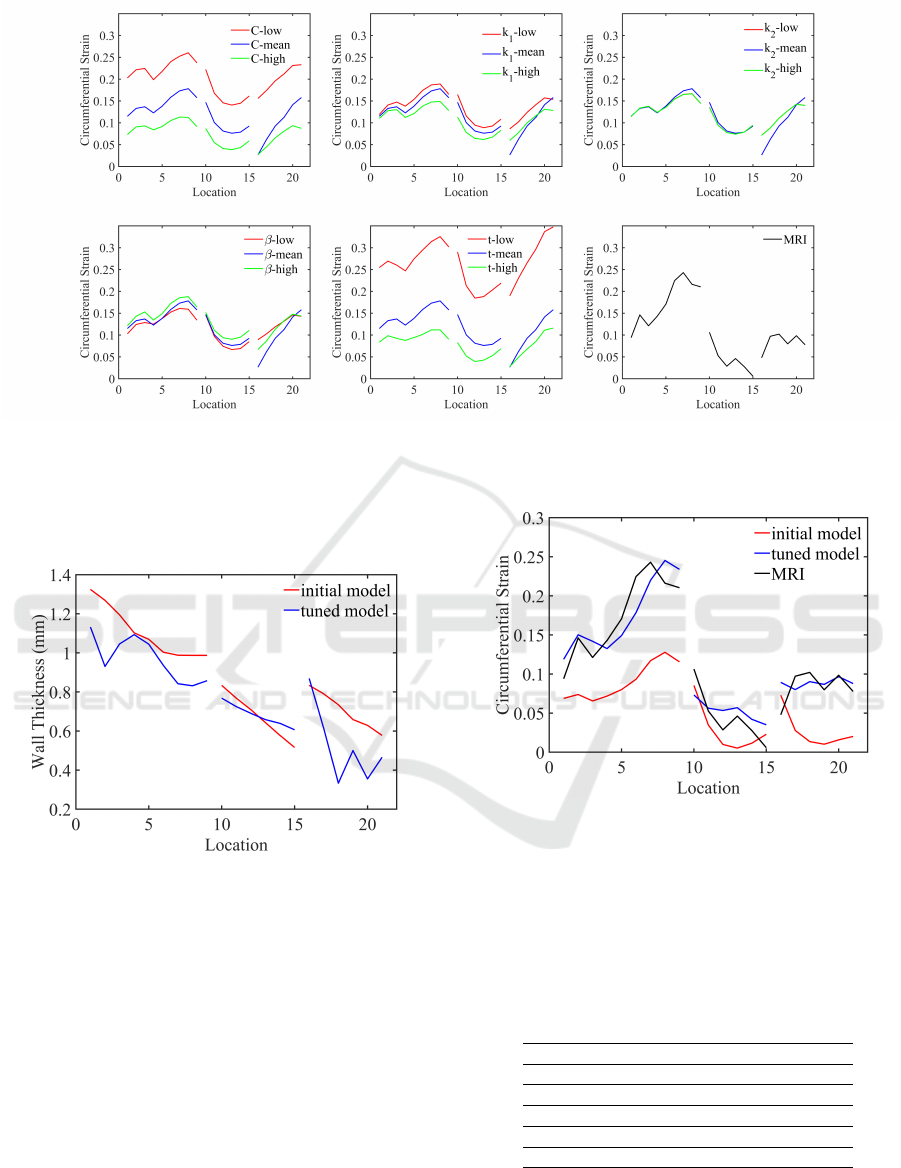
Figure 5: The effect of varying HGO model parameters and wall thicknesses on circumferential strain along the PAs and the
comparison of strain trends against MRI measurements. On x-axis, locations 1 through 9 correspond to MPA and B regions,
10 through 15 correspond to LPA, and 16 through 21 correspond to RPA regions.
the trend in RPA region suggests an adjustment of
thickness to mimic the behavior of PA walls.
Figure 6: The comparison of wall thicknesses along the cen-
terlines between the initial and tuned model.
3.3 Model Parameter Tuning for
Porcine Pulmonary Arteries
We identified that the circumferential strain is most
sensitive to the changes in the isotropic shear modulus
C and thickness from our analysis in the previous sec-
tion. We also found that the thickness should be var-
ied to change the strain trend along the PAs from com-
paring the models with different complexities. Based
on these findings, we first tuned the thickness to ob-
tain a more accurate strain trend. We then varied C,
which had a scaling effect on the strain trend. The it-
erative process continued until the difference between
Figure 7: The circumferential strains along the centerline
of PAs obtained from initial and tuned model compared
against MRI-measured strains.
predicted and MRI-measured circumferential strains
was less than 10 percent. The initial and tuned thick-
nesses along the PAs are shown in Fig.6. The initial
and tuned C values are listed in Table 2 in each region.
Table 1.
C
initial tuned range
MPA 40 34 40.77±11.02
B 30 21 30.03±11.52
LPA 43 60 43.24±71.72
RPA 82 80 82.61±90.54
A closer match to MRI strains was obtained as
a result of the iterative tuning process and demon-
strated in Fig.7. We repeated the same process for two
Computational Modeling of Arterial Walls: Evaluating Model Complexity and the Influence of Model Parameters on Deformation Outcomes
459
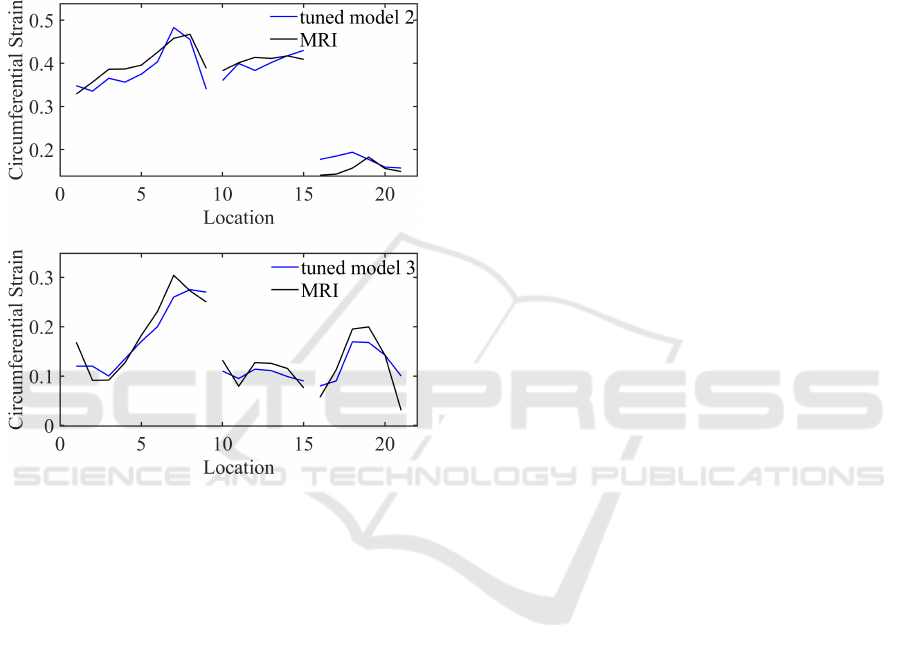
additional porcine models. The results are demon-
strated in Fig.8. All tuned models show good agree-
ment with the MRI results by reproducing similar
strain spatial variation and magnitude. For the three
porcine models, the mean discrepancies between the
tuned and MRI-measured circumferential strains were
0.035±0.043 in the MPA and B region, 0.009±0.016
in the LPA region, and 0.011±0.045 in the RPA re-
gion.
Figure 8: The circumferential strains along the centerline of
PAs obtained from tuned models of two additional porcine
compared against MRI-measured strains.
4 DISCUSSION
In this paper, we explored the complexity of computa-
tional models necessary for accurate modeling of ar-
terial walls. We also investigated the effects of model
parameters on deformations by simulating the pres-
sure increase from end-diastole to peak systole in a
porcine PA. Our findings indicate that wall thickness
and shear matrix, C, have a notably greater impact on
deformation outcomes than other HGO model param-
eters. We showed that wall thickness variation along
the PAs is necessary to accurately mimic the mechan-
ical response of arterial walls. By tuning the input
parameters to which the model is highly sensitive, we
replicated the in-vivo deformations.
The tuning of input parameters was performed in
an iterative manner. The final values we settled on
might not yield the smallest discrepancy against MRI,
implying that additional tuning might further enhance
deformation predictions. In subsequent research, we
intend to incorporate an optimization component into
our framework for improved predictions.
In our simulations, we applied a 13 mmHg pres-
sure difference between peak systole and end diastole
to the inner luminal surface of the walls. This value
was sourced from the mean measurement across 10
porcine in a prior study (Mueller-Graf et al., 2021),
due to the absence of invasive measurements on our
part. Despite this limitation, our findings retain their
importance. With access to pressure measurements
alongside patient-specific images, prediction accu-
racy could be further enhanced.
Our computational framework for arterial model-
ing can enhance surgical planning and inform clinical
decisions. In instances like artery reconstruction, sub-
optimal outcomes or complications may arise from
post-surgical shape deformations. The model has the
potential to predict these shape changes pre-surgery.
We aim to validate our model using post-surgical re-
sults of a reconstructed artery in a future study. In the
future, as we gain access to detailed material prop-
erties of the human arteries through advanced me-
chanical testing, it will become feasible to translate
these specimen-specific porcine models into patient-
specific models. This transition will enable a more ac-
curate approach to the personalized treatment of vas-
cular conditions in patients.
5 CONCLUSIONS
This study investigated the complexity required for
computational models to predict specimen-specific
in-vivo deformations of arterial walls and explored
the effects of thickness and HGO parameters on de-
formations. Our findings underscored the importance
of varying wall thickness regionally to accurately re-
produce MRI-measured strain response in vivo and
highlight the significant influence of wall thickness
and isotropic shear modulus on strain response re-
sults.
Our findings offer valuable insights to identify key
model features for specimen-specific computational
modeling of the arteries, thus providing a foundation
for enhancing the realism of soft tissue deformation
simulations. This enhancement could further improve
the outcomes of surgical planning, predictions of dis-
ease progression, and clinical decision-making.
BIOINFORMATICS 2024 - 15th International Conference on Bioinformatics Models, Methods and Algorithms
460

ACKNOWLEDGEMENTS
This work was supported by the National Sci-
ence Foundation under Award NSF FRR CAREER
2144348.
REFERENCES
Aslan, S., Liu, X., Wu, Q., Mass, P., Loke, Y.-H., Hib-
ino, N., Olivieri, L., and Krieger, A. (2022). Virtual
planning and simulation of coarctation repair in hy-
poplastic aortic arches: is fixing the coarctation alone
enough? In BIOINFORMATICS, pages 138–143.
Azadani, A. N., Chitsaz, S., Matthews, P. B., Jaussaud, N.,
Leung, J., Wisneski, A., Ge, L., and Tseng, E. E.
(2012). Biomechanical comparison of human pul-
monary and aortic roots. European journal of cardio-
thoracic surgery, 41(5):1111–1116.
Boekhoven, R. W., Peters, M. F., Rutten, M. C., van Sam-
beek, M. R., van de Vosse, F. N., and Lopata, R. G.
(2016). Inflation and bi-axial tensile testing of healthy
porcine
´
acarotid arteries. Ultrasound in Medicine &
Biology, 42(2):574–585.
Caimi, A., Sturla, F., Pluchinotta, F. R., Giugno, L., Secchi,
F., Votta, E., Carminati, M., and Redaelli, A. (2018).
Prediction of stenting related adverse events through
patient-specific finite element modelling. Journal of
Biomechanics, 79:135–146.
Fegan, K. L., Green, N. C., Britton, M. M., Iqbal, A. J.,
and Thomas-Seale, L. E. (2022). Design and simula-
tion of the biomechanics of multi-layered composite
poly (vinyl alcohol) coronary artery grafts. Frontiers
in cardiovascular medicine, 9:883179.
Fung, Y. (1967). Elasticity of soft tissues in simple elon-
gation. American Journal of Physiology-Legacy Con-
tent, 213(6):1532–1544.
Gasser, T. C., Ogden, R. W., and Holzapfel, G. A. (2006).
Hyperelastic modelling of arterial layers with dis-
tributed collagen fibre orientations. Journal of the
royal society interface, 3(6):15–35.
Hayashi, K. (2003). Mechanical properties of soft tissues
and arterial walls. In Biomechanics of soft tissue in
cardiovascular systems, pages 15–64. Springer.
He, R., Zhao, L., Silberschmidt, V. V., Liu, Y., and Vogt,
F. (2019). Finite element modelling of stent deploy-
ment in a patient-specific coronary artery. Procedia
Structural Integrity, 15:28–32.
Hoffman, A. H., Teng, Z., Zheng, J., Wu, Z., Woodard,
P. K., Billiar, K. L., Wang, L., and Tang, D. (2017).
Stiffness properties of adventitia, media, and full
thickness human atherosclerotic carotid arteries in
the axial and circumferential directions. Journal of
biomechanical engineering, 139(12):124501.
Humphrey, J. D. (1995). Mechanics of the arterial wall: re-
view and directions. Critical Reviews™ in Biomedical
Engineering, 23(1-2).
Lally, C., Reid, A., and Prendergast, P. J. (2004). Elastic
behavior of porcine coronary artery tissue under uni-
axial and equibiaxial tension. Annals of biomedical
engineering, 32:1355–1364.
Lashkarinia, S. S., Coban, G., Kose, B., Salihoglu, E.,
and Pekkan, K. (2021). Computational modeling of
vascular growth in patient-specific pulmonary arte-
rial patch reconstructions. Journal of Biomechanics,
117:110274.
Lashkarinia, S. S., Piskin, S., Bozkaya, T. A., Salihoglu, E.,
Yerebakan, C., and Pekkan, K. (2018). Computational
pre-surgical planning of arterial patch reconstruction:
parametric limits and in vitro validation. Annals of
biomedical engineering, 46:1292–1308.
Liu, X., Hibino, N., Loke, Y.-H., Kim, B., Mass, P., Fuge,
M. D., Olivieri, L., and Krieger, A. (2022). Surgical
planning and optimization of patient-specific fontan
grafts with uncertain post-operative boundary condi-
tions and anastomosis displacement. IEEE Transac-
tions on Biomedical Engineering, 69(11):3472–3483.
Mueller-Graf, F., Merz, J., Bandorf, T., Albus, C. F.,
Henkel, M., Krukewitt, L., Kuehn, V., Reuter, S., Voll-
mar, B., Pulletz, S., et al. (2021). Correlation of
pulse wave transit time with pulmonary artery pres-
sure in a porcine model of pulmonary hypertension.
Biomedicines, 9(9):1212.
Ogden, R. W. (1972). Large deformation isotropic
elasticity–on the correlation of theory and experiment
for incompressible rubberlike solids. Proceedings of
the Royal Society of London. A. Mathematical and
Physical Sciences, 326(1567):565–584.
Pillalamarri, N. R., Patnaik, S. S., Piskin, S., Gueldner, P.,
and Finol, E. A. (2021). Ex vivo regional mechanical
characterization of porcine pulmonary arteries. Exper-
imental mechanics, 61:285–303.
Pourmodheji, R., Jiang, Z., Tossas-Betancourt, C.,
Figueroa, C. A., Baek, S., and Lee, L.-C. (2021). In-
verse modeling framework for characterizing patient-
specific microstructural changes in the pulmonary ar-
teries. journal of the mechanical behavior of biomed-
ical materials, 119:104448.
Razaghi, R., Karimi, A., and Taheri, R. A. (2018). Patient-
specific finite element model of coronary artery stent-
ing. Current pharmaceutical design, 24(37):4492–
4502.
Sanders, S. N., Lopata, R. G., van Breemen, L. C., van de
Vosse, F. N., and Rutten, M. C. (2020). A novel
technique for the assessment of mechanical proper-
ties of vascular tissue. Biomechanics and Modeling
in Mechanobiology, 19:1585–1594.
Tian, L. and Chester, N. C. (2012). In vivo and in vitro
measurements of pulmonary arterial stiffness: a brief
review. Pulmonary circulation, 2(4):505–517.
Computational Modeling of Arterial Walls: Evaluating Model Complexity and the Influence of Model Parameters on Deformation Outcomes
461
