
Evaluating the Viability of Neural Networks for Analysing
Electromyography Data in Home Rehabilitation: Estimating Foot
Progression Angle
Finn Siegel
1a
, Christian Buj
1b
, Ricarda Merfort
2
, Andreas Hein
1
and Frerk Müller-Von Aschwege
1
1
OFFIS e.V.- Institute for Information Technology, Escherweg 2, Oldenburg, Germany
2
Universitätsklinikum Aachen, Aachen, Germany
Keywords: Electromyography, Neural Network, Deep Learning, Rehabilitation, Intramedullary Nailing, Femur Shaft
Fracture, Foot Progression Angle.
Abstract: Intramedullary (IM) nailing is a widely accepted treatment for femoral shaft fractures due to its good healing
rate and rapid return to full weight bearing. However, a significant number of patients experience impairments
years after treatment. One possible cause is a malrotation of the femur, resulting in altered foot progression
angles (FPAs), which can lead to changes in gait or persistent pain. To gain a better understanding of
compensation mechanisms and improve rehabilitation strategies, a continuous surface electromyography
(EMG) measurement system worn on vastus lateralis (VL) and vastus medialis (VM) is proposed. To test the
feasibility of this approach, a study is conducted with healthy participants (N=10) simulating different FPA.
The EMG signal was recorded and analysed using a convolutional neural network (CNN). The feasibility
study showed promising results, as the CNN could on average achieve a validation accuracy of 74% in
classifying FPAs as normal, inward (-15°), or outward (+15°). These results show the potential of using EMG
measurements from VL and VM to monitor changes in FPA during rehabilitation. This approach offers the
opportunity to increase our understanding of compensatory mechanisms and improve rehabilitation outcomes
following malrotation caused by IM nailing.
1 INTRODUCTION
The established gold standard for the treatment of
femoral shaft fractures is the use of an intramedullary
(IM) nail. The widespread adoption of this method is
attributed to its compelling properties, including a
high likelihood of fracture union (99%) (Mavrogenis
et al., 2016), a low risk of infection (El Moumni et al.,
2012) and a rapid weight bearing (Rommens &
Hessmann, 2015). However, potential risks include
implant failure (Mavrogenis et al., 2016) or non-
union of the fractured femur (El Moumni et al., 2012).
Despite the relatively low risk associated with IM
nailing, approximately 20% of patients subjected to
this procedure suffer from long-term residual
impairments. These complications can include pain,
hip ossification, altered gait patterns or restricted
a
https://orcid.org/0000-0002-9319-4304
b
https://orcid.org/0000-0002-5357-5516
mobility in hip and knee (El Moumni et al., 2012;
Hamahashi et al., 2019). The cause of these
complications remains a topic of ongoing debate.
Surgical factors, including the risk of injuring
surrounding muscle tissue, nerve supply or articular
cartilage may contribute (El Moumni et al., 2012).
Another potential factor could be, that there is no
direct visibility of the femur during surgery making it
difficult to precisely restore rotation and length of the
fractured femur and thus increasing the risk of
malrotation or malpositioning (Jaarsma & van
Kampen, 2004). Such misalignments are defined as
deviations greater than 5° in the frontal or sagittal
plane, 15° in the axial plane, and 2 cm in length (Ricci
et al., 2008). The incidence of such deviations varies
between 22.7% to 28% across studies (Jaarsma & van
Kampen, 2004; Papachristos, 2019; Rommens &
Hessmann, 2015).
132
Siegel, F., Buj, C., Merfort, R., Hein, A. and Aschwege, F.
Evaluating the Viability of Neural Networks for Analysing Electromyography Data in Home Rehabilitation: Estimating Foot Progression Angle.
DOI: 10.5220/0012385100003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 2, pages 132-141
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.

Irrespective of an identified cause, long-term
residual impairments pose a substantial burden to the
affected patient. Effective rehabilitation, essential for
moderating or even resolving these consequences,
depends on accurate identification of limitations.
After hospitalization, as patients transition to a home-
based care, monitoring is mostly based on subjective
self-assessments, which tend to be inaccurate and can
reduce the quality of rehabilitation (Toogood et al.,
2016).
Previous research by Siegel et al. (2023) suggests
the use of wearable home devices as a strategy to
improve the accuracy of rehabilitation monitoring,
allowing the identification of long-term residual
impairments, thereby providing a basis for the
treating specialist to take adapted countermeasures. In
addition, a continuous monitoring system could
detect possible malrotation and monitor any changes
during rehabilitation (Siegel et al., 2023). Research
by Jaarsma et al. (2004) showed that, on average,
patients are capable of compensating for
approximately 71% of a given malrotation. However,
an enhanced understanding of malrotation
mechanisms could provide further insight into the
compensation strategies and enable clinicians to help
patients cope by training targeted supporting muscles.
This is relevant, as some studies show a high
likelihood for malrotation to be a major source of pain
(Dagneaux et al., 2018).
To gain insight into malrotation, compensation
mechanisms and coping strategies, it is necessary to
continuously measure foot progression angle (FPA).
In a previous paper, the concept of employing a
wearable device, positioned above the knee, equipped
with electromyography (EMG) sensors to measure
the FPA, was introduced (Siegel et al., 2023).
An EMG measurement records biopotentials
when an electrochemical stimulus triggers muscle
fibre (Al-Ayyad et al., 2023). The possibility of using
EMG measurements to draw conclusions about FPA
is based on the premise that alterations in movement
are accompanied by a corresponding change in the
measurable EMG signal (Akuzawa et al., 2017). In
addition, Benedetti el al., 2003 found that altered
muscle contractions, quantifiable through EMG
measurements, may account for a compensation
mechanism (Benedetti et al., 2003).
In order to detect these alterations in muscle
activity, a surface EMG measurement should ideally
record the electrical activity of uniformly active
motor units within one muscle. However, the
resulting EMG signal is subject to many influences,
including fatigue, quantity of active motor units,
firing rates, firing amplitudes, superposition from
surrounding muscles, low-pass characteristics of
surrounding tissue, sensor properties and extraneous
signals such as ambient noise. This complexity makes
it difficult to reliably classify EMG recordings using
basic filter algorithms or feature extraction
methodologies. As a possible answer to this
challenge, deep learning has proven to be a successful
tool (Faust et al., 2018). Especially the usage of
convolutional neural networks (CNNs) has been
proven reliable (Olsson et al., 2019; Yang et al., 2019;
Zia Ur Rehman et al., 2018). CNNs are particularly
suitable for detecting patterns in one-dimensional or
multi-dimensional data due to a high degree of
invariance to translation, scaling, skewing or
distortion. This is possible because each neuron
receives its input from a local receptive field from the
previous layer. Thus, the position of features becomes
less important as long as they maintain their relative
position to each other (Al-Jabery,Khalid et al., 2020),
enabling a classification of variant time series (Zhao
et al., 2017). I.e. Bakircioğlu and Öskurt (2020) used
a CNN to classify EMG recordings of movements
made while gripping six different objects and
achieved 95.9% accuracy. Olsson et al. (2019) used a
CNN, classifying 16 independent states of the hand
recorded using an EMG system and achieved 78.7%
accuracy.
1.1 Aim of this Study
The aim of this study is to evaluate the potential utility
of EMG sensors in improving the reliability of
monitoring FPAs during home-based rehabilitation, a
crucial aspect considering the FPA contributes
significantly in long-term outcome following femoral
shaft fracture treatment.
As of now, EMG measurements have been
successfully used in numerous rehabilitation
applications, such as:
• In neuromuscular rehabilitation, EMG
measurements can be used to quantitatively
assess spasticity as well as monitor treatment
progress (Campanini et al., 2020).
• In post-stroke rehabilitation, EMG
measurements can be used to monitor the
healing process (Simpson et al., 2011) or to
control an exoskeleton aimed to reactivate
paralysed limbs (Nam et al., 2022).
• In orthopaedic rehabilitation, EMG
measurements can be used to evaluate muscle
function, to detect abnormalities or to manage
pain-inducing syndromes during sessions with
specialists (Barton et al., 2013; Benedetti et al.,
2003).
Evaluating the Viability of Neural Networks for Analysing Electromyography Data in Home Rehabilitation: Estimating Foot Progression
Angle
133
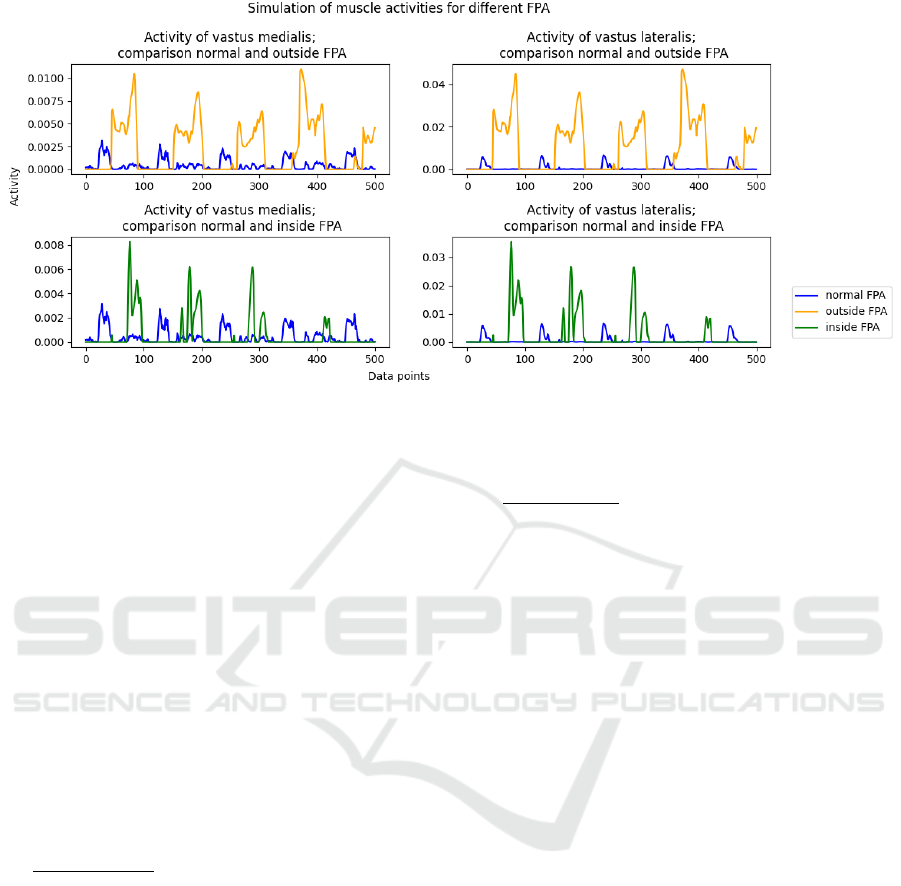
Figure 1: Presentation of simulation results, generated with Anybody software. Simulated muscle activity during normal gait
and gait with an intentional alteration (approximately 15° inside or outside) of foot progression angle is displayed. The
overview is shown for vastus medialis and vastus lateralis.
However, until now, EMG measurements have
not been used to monitor a patient’s activity or
malrotation in a home environment.
There are already sensor systems measuring
FPAs, such as inertial measurement units (IMUs) or
pressure sensors, but these have limitations such as
inaccurate results indoors or a dependency on the
footwear (Siegel et al., 2023). An EMG system, worn
on the leg, could potentially overcome these
limitations while increasing data availability, and is
therefore being tested in this study.
Since this study is intended to provide a first
overview of the usability of EMG measurements for
FPA monitoring, it was decided to conduct the tests
on a healthy cohort rather than on patients. The study
will evaluate the following hypotheses:
Hypothesis A: A CNN can classify FPAs of
unknown steps for a single proband, after training on
EMG data obtained from the same proband.
If a CNN is capable do discriminate EMG signals
from different FPAs within a single proband, this
knowledge holds potential to monitor changes in
FPAs during rehabilitation. However, this is limited,
since the proband would need to simulate different
FPAs in order to train such neural network. In
practical clinical scenarios, this data aggregation may
not be feasible, due to the recently treated femur shaft
fracture. Therefore, it is important to investigate,
whether a neural network can be trained using data
from diverse patients and enabling it to classify EMG
signal for different FPAs without prior subject
specific training. To test this the following
hypotheses is formulated:
Hypothesis B: A CNN can classify FPAs for an
unknown proband, after training on EMG data
obtained only different probands.
To test hypotheses A and B, EMG signals of
several probands simulating different FPAs are
recorded and analysed using a CNN.
2 MATERIAL AND METHODS
For the acquisition of EMG data across different
FPAs, ten volunteers were recruited. Exclusion
criteria were adhesive tape and silver allergy,
implanted electrical devices and known deformities
of the lower limb. The gender distribution was 50%
male to 50% female with a mean age of 36.5 years
(±14.3 years).
2.1 Sensor Placement
To ensure optimal sensor placement, aligned to answer
the hypotheses, literature was reviewed to find poten-
tial correlations between lower limb muscle activity
and FPA. A simulation was used to verify the results.
Mohammad and Elsais (2020) investigated the
correlation between EMG signal amplitude and hip
rotation in male runners. They found such a
relationship for Vastus Lateralis (VL), Vastus Medius
(VM) and Gluteus Maximus. As the overall goal,
outlined in earlier work (Siegel et al., 2023), is to
wear a sensor array positioned above the knee, the
electrical activity of VL and VM are promising
muscles for conclusions to be drawn about FPAs.
HEALTHINF 2024 - 17th International Conference on Health Informatics
134
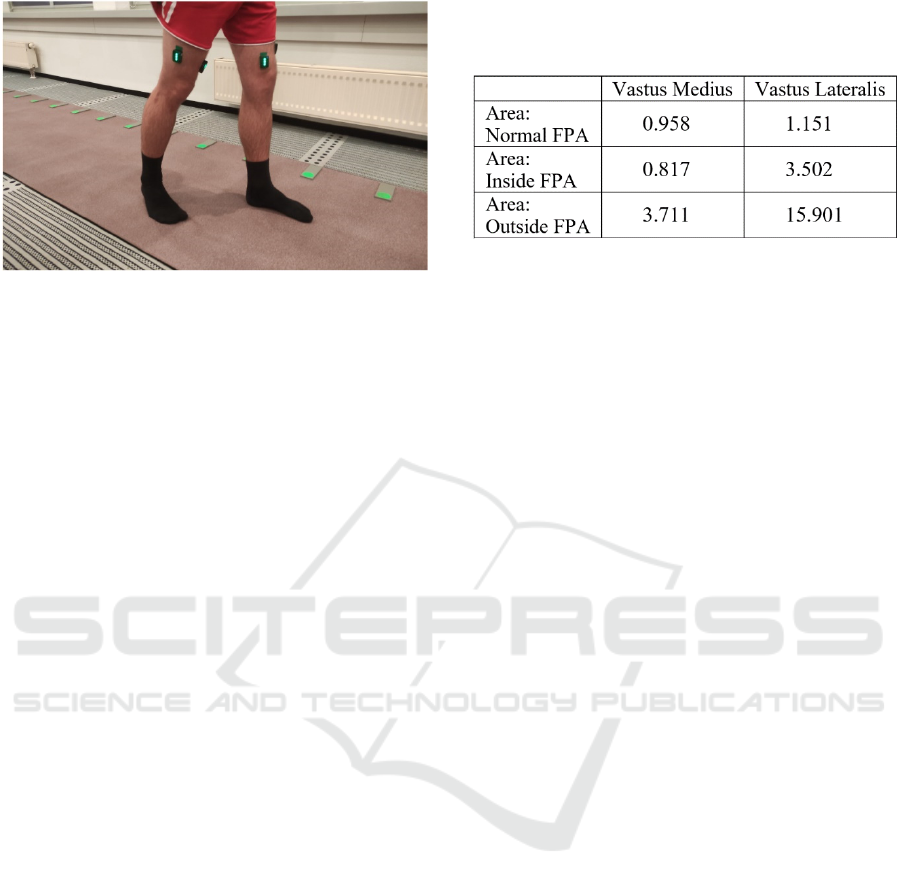
Figure 2: Exemplary image from the study displaying
placement of EMG sensors on VL and VM. The GaitRite
mat, used for FPA verification, is also visible.
To further confirm that change in FPA induces
change in muscle activity for VL and VM, a
simulation was performed in collaboration with
university hospital in Aachen, Germany. A volunteer
was fitted with a MTw Awinda motion tracker system
(Paulich et al., 2018) and walks with different FPAs
were conducted. The FPA was varied between 15°
inward, normal and 15° outward. To derive muscle
activity from the collected data, the AnyBody
Modelling System (Paul & Doweidar, 2023) was used
in combination with the AnyBody Managed Model
Repository. This approach allows an inverse
dynamics analysis to be performed, based on a third-
order polynomial muscle recruitment criterion, which
produces a simulation of the electrical activity in the
lower limb muscles during walks. The results are
shown in figure 1. This figure displays muscle
activity during gait with normal FPA compared to
gait with an inward or outward FPA. The simulated
activity is shown for VM and VL. It is immediately
noticeable, that the shape of the curves for normal and
modified FPA are distinctly different. To quantify
these observations, the integral of the curves was
calculated (python library: numpy.trapz (Harris et al.,
2020)), displayed in table 1. The results show
variation in the area under the curve for normal FPAs
compared to modified FPAs. The differences are
particularly significant for outside FPAs in
comparison to inside FPAs. The simulation supports
the choice of using VL and VM as EMG
measurement points to detect differences in FPA.
In conclusion it was decided to place the EMG
sensors on VL and VM. The European
recommendations for sensors and sensor placement
for EMG (Hermens et al., n.d.) was used as a guide to
ensure optimal placement of the sensors on VL and
VM, minimising superposing of signals by
surrounding muscles. To further improve signal
Table 1: To quantify figure 1, the area under the plotted
curves is calculated using the trapezoid method (python
library: numpy.trapz) and the results are shown in this table.
quality, the skin was shaved and cleaned prior to
sensor placement. An example of placed sensors is
given in figure 2.
2.2 Signal Acquisition
The EMG signal was recorded using the Delsys
Trigno-Wireless-Biofeedback System (Delsys, n.d.).
This system consists of a base station that wirelessly
collects data from individual sensors. Each sensor is
capable of collecting data at a frequency of 4 kHz
with a bandwidth of 20-450 Hz and an input range of
11 mV.
For the purpose of supervised learning, it is
necessary to label EMG data recordings.
Consequently, a GaitRite mat was used to record the
FPAs. The mat is manufactured by CIR Systems
represents a gold standard in gait analysis. 36 864
pressure sensors evenly distributed of over a length of
914 cm allows steps to be recorded and a gait profile
to be created. This profile includes the FPA for each
step executed. A section of the mat can be seen in the
figure 2.
In order to conduct this study, each proband had
to perform a total of 45 walks along the entire length
of the GaitRite. 15 normal walks, 15 walks with
outward FPAs and 15 walks with inward FPAs. For
each simulated malrotation, the participants were
asked to change their FPA by -15° inward or +15°
outward. Prior to the study, foot positions were
trained using the GaitRite mat. During the study any
steps deviating by more than ±8° from proposed FPA
were removed from the dataset. Only one foot was
varied during the different walks. The side to be
varied was freely chosen by the proband, the choice
remained consistent throughout the study. A
metronome was used to ensure uniform walking
speed during different walks. In order to merge EMG
data with GaitRite data, software was developed to
record both systems simultaneously. Both data
streams were synchronised by an output signal
generated by the GaitRite system.
Evaluating the Viability of Neural Networks for Analysing Electromyography Data in Home Rehabilitation: Estimating Foot Progression
Angle
135
2.2.1 Data Preprocessing
Data preprocessing is performed according to a
general data preparation paradigm (Al-Jabery et al.,
2020). During the study, walks across the GaitRite
mat were recorded alongside the corresponding EMG
signals, resulting in 15 datasets per class (inward,
outward and normal FPA). However, this quantity
proved insufficient to train a supervised deep learning
algorithm (Alwosheel et al., 2018). To increase the
size of each class, the walks are divided into
individual steps. For this purpose, software was
developed that extracts individual steps based on
EMG peak detection and assigns them to the
appropriate FPA class. This results in a dataset for
each proband containing the EMG signal for VL and
VM and the corresponding FPA for each step.
To extract non-stationary properties from the
EMG signal, time windowing is performed (Zha et
al., 2021). Initially, the EMG signal of one step spans
over a duration of one second. This can be contracted,
since VL and VM are only active for approximately
20% to 25% of the time during one gait cycle (Róisín
Howard, 2017). The average duration of a gait cycle
is around one second (Murray et al., 1964), enabling
the EMG signal be to contracted to a duration of 250
ms. As data was collected at 4 kHz, the EMG signal
is truncated to a time window of 1000 data points
(250ms). The next step is a high and low pass filtering
(Morbidoni et al., 2019), already conducted by the
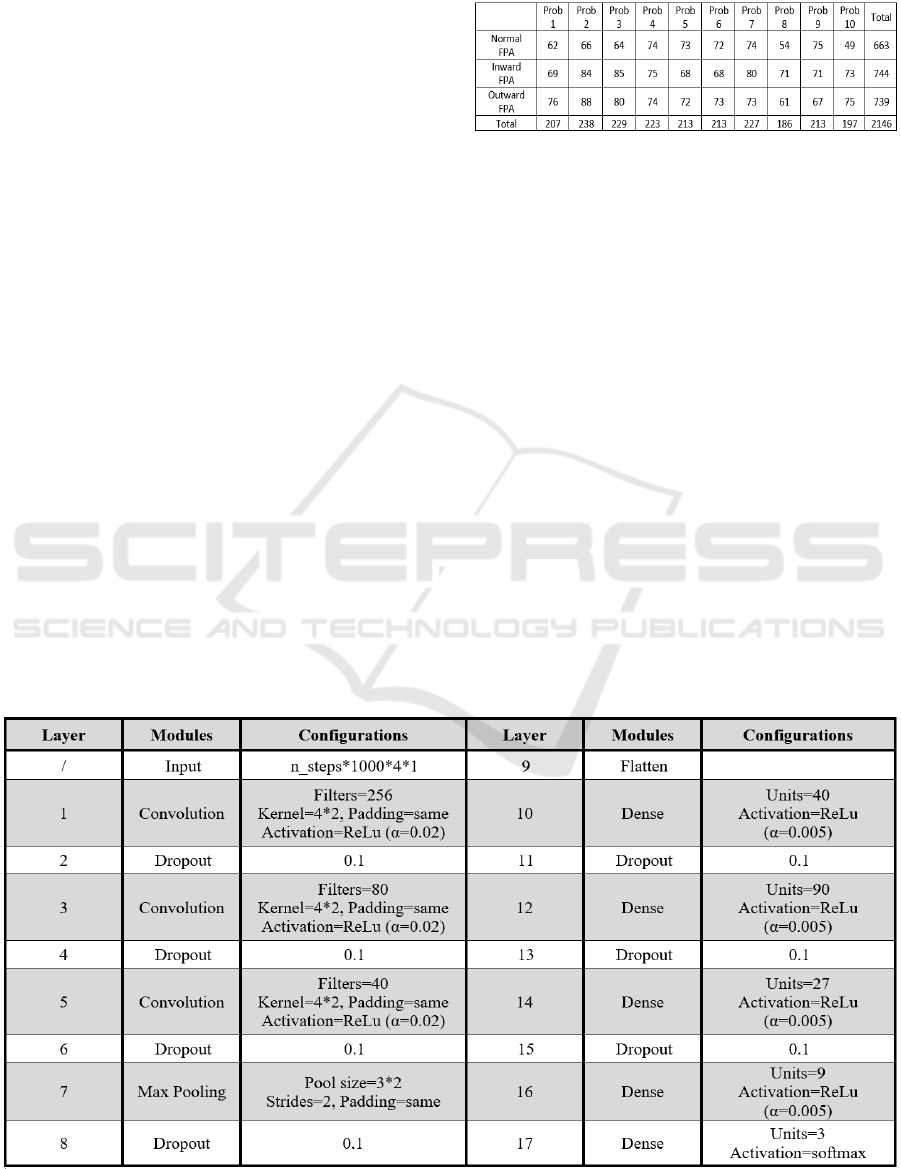
Table 2: This table displays the distribution of steps
generated in this study across different subjects and FPAs,
showing the class sizes used to train the neural network.
sensor. To filter motion artifacts, a low-pass filter
with a cutoff frequency of 20 Hz is used. A high-pass
filter with a cutoff frequency of 450 Hz is applied, as
not much additional information is available above
this frequency (Bakircioğlu & Özkurt, 2020). This is
followed by a rectification of the data, enhancing the
chances of successful training of deep learning
algorithms (Li et al., 2011). Next, a Fast Fourier
Transformation (FFT) is performed creating
additional input features and enhancing information
density (Yang et al., 2019). Finally, data is
normalised using the peak-dynamic method,
requiring each data point to be divided by the
maximum value. While this method results in a loss
of information regarding the degree of muscle
activation, it enhances the comparability between
probands.
In Conclusion, a matrix is generated containing
both a time series and a frequency series, for each
labelled step and each sensor. Combining the
measurements for VL and VM results in a matrix of
Table 3: This table shows the structure of the CNN used. The optimizer adam and sparse categorical crossentropy were used
for training.
HEALTHINF 2024 - 17th International Conference on Health Informatics
136
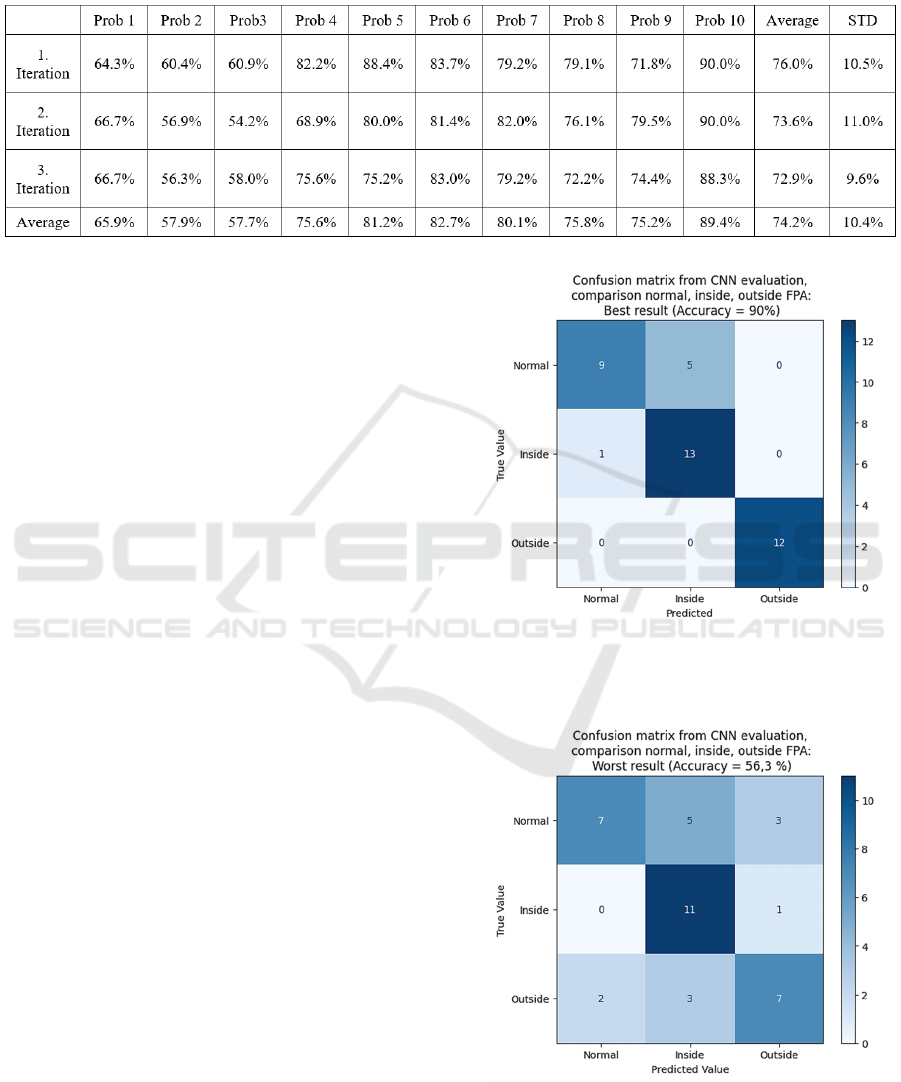
Table 4: Representation of the accuracy achieved for individual subjects using a CNN for classifying the classes normal,
inside and outside FPAs. The network was trained and evaluated three times. The average accuracy and the corresponding
standard deviation are also presented.
four features with 1000 data points times the number
of steps. In this study, a total of 186-238 steps were
recorded, per participant. Resulting in 2146 steps
available to train the CNN, see table 2. This is a
relatively modest dataset size for the application of
deep learning (Alwosheel et al., 2018), but the
purpose of this study is to provide an initial insight in
the possibility of determining FPAs using EMG
measurements in conjunction with deep learning
evaluations and therefore declared acceptable for this
feasibility study.
2.2.2 Used Network
The structure of the CNN used is shown in the table
3. The network is built using TensorFlow (Martín
Abadi et al., 2015) and Keras (Chollet & others,
2015) libraries in Python. To obtain reliable results,
each run was performed three times and the average
validation accuracy is taken as the result.
3 RESULTS
In the following the results gained from the analysis
of the study data are presented in relation to the tested
hypothesis.
3.1 Results for Testing Hypothesis A
H: A CNN can classify FPAs of unknown steps for a
single proband, after training on EMG data obtained
from the same proband.
To test this hypothesis, data from the gait study
obtained by each proband individually was used to
train the CNN. The labelled data was combined,
randomly mixed and 85% was used for training and
the remaining 15% served as validation data. To
Figure 3: Confusion matrix, showing the result for the best
prediction. The predicted value is shown against the true
value.
Figure 4: Confusion matrix, showing the result for the least
successful prediction. The predicted value is shown against
the true value.
Evaluating the Viability of Neural Networks for Analysing Electromyography Data in Home Rehabilitation: Estimating Foot Progression
Angle
137
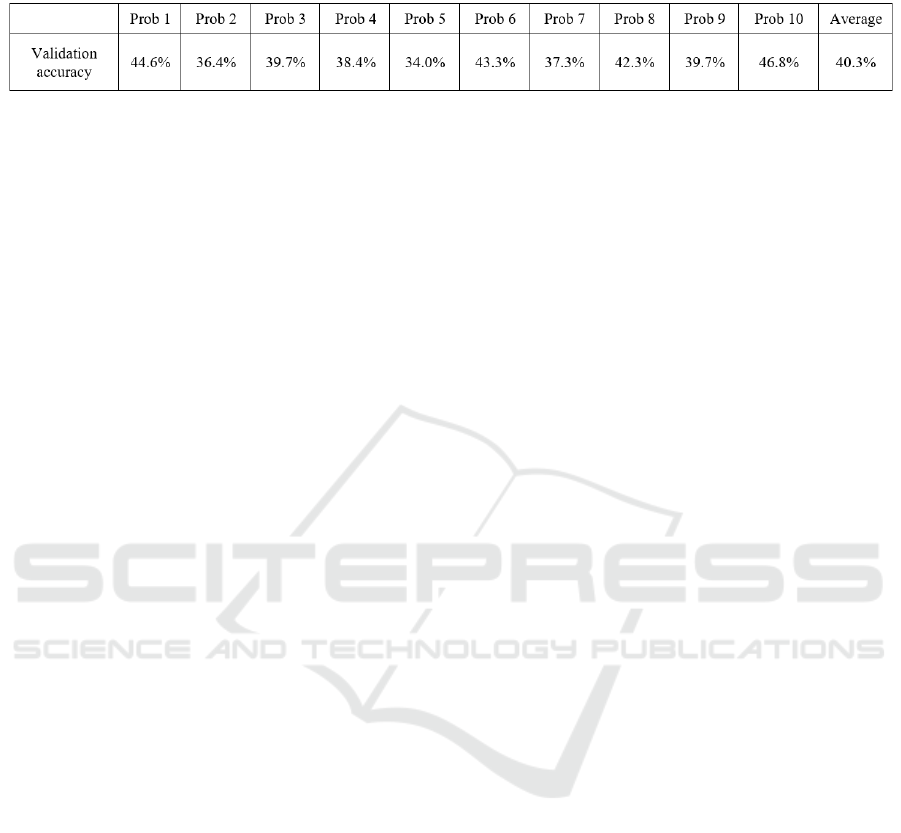
Table 5: Presentation of the interproband validation accuracy achieved using a CNN to classify the classes normal, inside and
outside FPAs. Shown is the average validation accuracy.
ensure a reliable conclusion, each training iteration
was performed three times. The results are presented
in table 4.
Steps are classified into three classes (normal,
internal and external rotated FPAs) with an average
classification accuracy of 74.2% (±10.4%). This
performance exceeds chance level of 33. 3
% .
Additionally, a confusion matrix is displayed, for the
most successful and the least successful
classification, see figure 3.
The result suggests that a CNN can learn and
discriminate features within the EMG signal allowing
conclusions to be drawn about FPAs. However, the
high variance of 10.4% indicates inconsistency in the
quality of features identified by the CNN between
probands.
3.2 Results for Testing Hypothesis B
H: A CNN can classify FPAs for an unknown
proband, after training on EMG data obtained from
different probands.
To test this hypothesis, datasets from all probands
excluding one for validation were combined and used
to train a CNN. This process was repeated, ensuring
each proband’s data was tested against the combined
majority. The results are shown in table 5. It can be
seen that a CNN, trained on a whole population, can
distinguish validation steps an average accuracy of
40% across inward, outward and normal FPAs. The
results indicate a limited reliability for a classification
of unknown EMG data recorded from different FPAs.
4 DISCUSSION
In this study, each class (normal, inside and outside
FPA) contains 1326-1488 trails (for VL and VM
combined), which, in the context of deep learning,
accounts for a relatively small dataset (Alwosheel et
al., 2018). However, when working with EMG
measurements, the availability of data is limited by
the number of times a person can repeat a specific
movement. This limitation restricts the size of
available datasets, which needs to be considered when
working with deep neural networks. Nevertheless,
researchers have shown that small datasets can be used
successfully, i.e. Grag et al. (2021) used three classes
of EMG recordings and a total of 1575 trails while
achieving an accuracy of 85.44%.
The inclusion of 10 probands, as in this study, is
in line with the approach of other researchers, when
experimenting with EMG data. I.e. Rehman et al.
(2018) collected data from seven healthy probands
and Bakircioğlu and Öskurt (2020) had five probands
enrolled in their study.
4.1 Discussion of Hypothesis A
H: A CNN can classify FPAs of unknown steps for a
single proband, after training on EMG data obtained
from the same proband.
This study has shown a CNN can learn features from
EMG recordings of VL and VM to distinguish
between outward, inward and normal FPAs with an
average success rate of 74.2%. The standard deviation
of 10.4% reflects the high variance of the EMG
signal, which has also been reported by other
researchers (Rane et al., 2019). The variability of the
EMG signal can be attributed to its inherent nature,
which is non-stationary, non-linear, stochastic, and
unpredictable (Geng et al., 2016). At the same time,
the characteristics of the sensor play a role, as the
signal varies depending on the position relative to the
muscle and the quality of the contact with the skin. In
addition, the signal is prone to noise, including
instrument noise, ambient noise, motion artefacts, and
signal instability (Reaz et al., 2006).
The result of this part of the study is in line with
results of other studies, i.e. Tryon et al. (2021)
achieved an accuracy of 74.7% in discriminating
EMG signals into three classes related to elbow
flexion while holding different weights.
It is important to note, when dealing with hand
gestures using a CNN, results tend to be significantly
better. For instance, Lee at al. (2020) achieved an
accuracy of 94% when discriminating between ten
gesture classes. The differences in performance may
be due to the availability of distinct movements,
whereas this study focuses on detecting small changes
HEALTHINF 2024 - 17th International Conference on Health Informatics
138

in movement sequences, which are easily masked by
the noise of the EMG signal.
An improvement in results could be achieved by
using CNNs in combination with other deep learning
algorithms. For example, by connecting CNNs to
bidirectional LSTM networks. Karnam et al. (2022)
were able to improve the accuracy of classifying
EMG recordings of hand gestures by up to 18.7%
compared to state-of-the-art models.
Another way to improve the accuracy of the CNN
is using transfer learning. This involves pre-training
the network on subjects with comparable data
recorded from other subjects followed by training on
target data. Soroushmojdehi et al. (2022) showed that
this methodology can improve the accuracy of a
CNN, when predicting hand movements based on
EMG data, up to 10%.
4.2 Discussion of Hypothesis B
H: A CNN can classify FPAs for an unknown
proband, after training on EMG data obtained from
different probands.
The result of this hypothesis testing shows an average
accuracy of 40.3%, barely surpassing chance level
(33.33%). A major contributing factor is the high
interpatient variability. This high variability has
already been reported by Anders et al. (2019), who
demonstrated substantial interindividual variability
and Guidetti et al. (1996) found significant variation
between subjects.
Furthermore, the interpatient comparison results
are consistent with findings in existing literature. In
this study, three classes of FPAs were classified with
up to 46.8% validation accuracy, see table 4. This
performance is comparable to that of Castellini’s team,
who achieved an accuracy of 51.7% for three classes in
an interpatient evaluation (Castellini et al., 2009).
One way to improve the results could be to use the
normal gait pattern of a subject under investigation as
calibration followed by detecting changes in FPAs
with the help of a trained CNN. Cano et al. (2022)
showed, that the accuracy of predicting high blood
pressure in unknown subjects could be increased by
up to 30% this way.
5 CONCLUSIONS
The aim of this study was to provide initial insights
into the potential utility of EMG sensors in improving
the reliability of FPA monitoring during home
rehabilitation. It has been demonstrated that EMG
measurements, evaluated by a CNN trained on an
individual proband, can be used to classify between
inward, outward and normal FPAs with an average
validation accuracy of 70.4%. In conclusion, while
the results show that such a system is not yet ready
for use as a medical device, they highlight the
potential and need for further research into this
approach.
The major goal for the future is to develop a user-
friendly measuring device capable of precisely
detecting changes in FPA, providing essential data for
the recovery process. The next steps on this path
include minimising the variance between different
patients. The use of an EMG sensor array is one
possible solution for this, as it allows the
determination of the sensor with the optimal signal
quality, thus reducing the need for precise sensor
placement. In addition, increasing the size of the data
set is a critical factor. The possibility to integrate
more steps could significantly increase the accuracy
of a neural network. other optimisation approaches
include combining different deep learning algorithms
and testing the usability of transfer learning.
To the best of our knowledge, this study
represents the first instance of utilizing EMG
measurements in combination with CNNs to provide
insight into FPA.
ACKNOWLEDGEMENTS
This work is founded by the German Federal Ministry
of Education and Research (BMBF) (FKZ:
01IS21085) and is part of the ITEA Secure-e-Health
project.
REFERENCES
Akuzawa, H., Imai, A., Iizuka, S., Matsunaga, N., &
Kaneoka, K. (2017). The influence of foot position on
lower leg muscle activity during a heel raise exercise
measured with fine-wire and surface EMG. Physical
Therapy in Sport, 28, 23–28. https://doi.org/10.1016/
j.ptsp.2017.08.077
Al-Ayyad, M., Owida, H. A., De Fazio, R., Al-Naami, B., &
Visconti, P. (2023). Electromyography Monitoring
Systems in Rehabilitation: A Review of Clinical
Applications, Wearable Devices and Signal Acquisition
Methodologies. Electronics, 12(7), 1520. https://doi.org/
10.3390/electronics12071520
Al-Jabery,Khalid, Obafemi-Ajayi, Tayo, Olbricht, Gayla, &
Wunsch, Donald. (2020). Computational learning
approaches to data analytics in biomedical applications.
Academic press.
Evaluating the Viability of Neural Networks for Analysing Electromyography Data in Home Rehabilitation: Estimating Foot Progression
Angle
139

Alwosheel, A., Van Cranenburgh, S., & Chorus, C. G.
(2018). Is your dataset big enough? Sample size
requirements when using artificial neural networks for
discrete choice analysis. Journal of Choice Modelling,
28, 167–182. https://doi.org/10.1016/j.jocm.2018.07.002
Anders, J. P. V., Smith, C. M., Keller, J. L., Hill, E. C.,
Housh, T. J., Schmidt, R. J., & Johnson, G. O. (2019).
Inter- and Intra-Individual Differences in EMG and
MMG during Maximal, Bilateral, Dynamic Leg
Extensions. Sports, 7(7), 175. https://doi.org/10.3390/
sports7070175
Bakircioğlu, K., & Özkurt, N. (2020). Classification of Emg
Signals Using Convolution Neural Network.
International Journal of Applied Mathematics
Electronics and Computers, 8(4), 115–119.
https://doi.org/10.18100/ijamec.795227
Barton, C. J., Lack, S., Malliaras, P., & Morrissey, D. (2013).
Gluteal muscle activity and patellofemoral pain
syndrome: A systematic review. British Journal of Sports
Medicine, 47(4), 207–214. https://doi.org/10.1136/
bjsports-2012-090953
Benedetti, M. G., Catani, F., Bilotta, T. W., Marcacci, M.,
Mariani, E., & Giannini, S. (2003). Muscle activation
pattern and gait biomechanics after total knee
replacement. Clinical Biomechanics, 18(9), 871–876.
https://doi.org/10.1016/S0268-0033(03)00146-3
Campanini, I., Disselhorst-Klug, C., Rymer, W. Z., &
Merletti, R. (2020). Surface EMG in Clinical Assessment
and Neurorehabilitation: Barriers Limiting Its Use.
Frontiers in Neurology, 11, 934. https://doi.org/
10.3389/fneur.2020.00934
Cano, J., Fácila, L., Gracia-Baena, J. M., Zangróniz, R.,
Alcaraz, R., & Rieta, J. J. (2022). The Relevance of
Calibration in Machine Learning-Based Hypertension
Risk Assessment Combining Photoplethysmography and
Electrocardiography. Biosensors, 12(5), 289.
https://doi.org/10.3390/bios12050289
Castellini, C., Fiorilla, A. E., & Sandini, G. (2009). Multi-
subject/daily-life activity EMG-based control of
mechanical hands. Journal of NeuroEngineering and
Rehabilitation, 6(1), 41. https://doi.org/10.1186/1743-
0003-6-41
Chollet, F. & others. (2015). Keras. https://keras.io
Dagneaux, L., Allal, R., Pithioux, M., Chabrand, P., Ollivier,
M., & Argenson, J.-N. (2018). Femoral malrotation from
diaphyseal fractures results in changes in patellofemoral
alignment and higher patellofemoral stress from a finite
element model study. The Knee, 25(5), 807–813.
https://doi.org/10.1016/j.knee.2018.06.008
Delsys. (n.d.). Trigno Wireless Biofeedback System.
https://www.delsys.com/downloads/USERSGUIDE/trig
no/wireless-biofeedback-system.pdf
El Moumni, M., Voogd, E. H., Ten Duis, H. J., & Wendt, K.
W. (2012). Long-term functional outcome following
intramedullary nailing of femoral shaft fractures. Injury,
43(7), 1154–1158. https://doi.org/10.1016/j.injury.20
12.03.011
Faust, O., Hagiwara, Y., Hong, T. J., Lih, O. S., & Acharya,
U. R. (2018). Deep learning for healthcare applications
based on physiological signals: A review. Computer
Methods and Programs in Biomedicine, 161, 1–13.
https://doi.org/10.1016/j.cmpb.2018.04.005
Garg, N., Balafrej, I., Beilliard, Y., Drouin, D., Alibart, F., &
Rouat, J. (2021). Signals to Spikes for Neuromorphic
Regulated Reservoir Computing and EMG Hand Gesture
Recognition. International Conference on Neuromorphic
Systems 2021, 1–8. https://doi.org/10.1145/3477145.
3477267
Geng, W., Du, Y., Jin, W., Wei, W., Hu, Y., & Li, J. (2016).
Gesture recognition by instantaneous surface EMG
images. Scientific Reports, 6(1), 36571. https://doi.org/
10.1038/srep36571
Guidetti, L., Rivellini, G., & Figura, F. (1996). EMG patterns
during running: Intra- and inter-individual variability.
Journal of Electromyography and Kinesiology, 6(1), 37–
48. https://doi.org/10.1016/1050-6411(95)00015-1
Hamahashi, K., Uchiyama, Y., Kobayashi, Y., Ebihara, G.,
Ukai, T., & Watanabe, M. (2019). Clinical outcomes of
intramedullary nailing of femoral shaft fractures with
third fragments: A retrospective analysis of risk factors
for delayed union. Trauma Surgery & Acute Care Open,
4(1), e000203. https://doi.org/10.1136/tsaco-2018-
000203
Harris, C. R., Millman, K. J., Walt, S. J. van der, Gommers,
R., Virtanen, P., Cournapeau, D., Wieser, E., Taylor, J.,
Berg, S., Smith, N. J., Kern, R., Picus, M., Hoyer, S.,
Kerkwijk, M. H. van, Brett, M., Haldane, A., Río, J. F.
del, Wiebe, M., Peterson, P., … Oliphant, T. E. (2020).
Array programming with NumPy. Nature, 585(7825),
357–362. https://doi.org/10.1038/s41586-020-2649-2
Hermens, Freriks, Merletti, Rau, Disselhorst-Klug, &
Stegeman. (n.d.). SENIAM (Surface ElectroMyoGraphy
for the Non-Invasive Assessment of Muscles) project.
http://www.seniam.org/
Jaarsma, R. L., Ongkiehong, B. F., Grüneberg, C.,
Verdonschot, N., Duysens, J., & van Kampen, A. (2004).
Compensation for rotational malalignment after
intramedullary nailing for femoral shaft fractures. Injury,
35(12), 1270–1278. https://doi.org/10.1016/j.injury.20
04.01.016
Jaarsma, R. L., & van Kampen, A. (2004). Rotational
malalignment after fractures of the femur. The Journal of
Bone and Joint Surgery. British Volume, 86-B(8), 1100–
1104. https://doi.org/10.1302/0301-620X.86B8.15663
Karnam, N. K., Dubey, S. R., Turlapaty, A. C., & Gokaraju,
B. (2022). EMGHandNet: A hybrid CNN and Bi-LSTM
architecture for hand activity classification using surface
EMG signals. Biocybernetics and Biomedical
Engineering, 42(1), 325–340. https://doi.org/10.1016/
j.bbe.2022.02.005
Lee, S., Sung, M., & Choi, Y. (2020). Wearable fabric sensor
for controlling myoelectric hand prosthesis via
classification of foot postures. Smart Materials and
Structures, 29(3), 035004. https://doi.org/10.1088/1361-
665X/ab6690
Li, G., Li, Y., Yu, L., & Geng, Y. (2011). Conditioning and
Sampling Issues of EMG Signals in Motion Recognition
of Multifunctional Myoelectric Prostheses. Annals of
Biomedical Engineering, 39(6), 1779–1787.
https://doi.org/10.1007/s10439-011-0265-x
Martín Abadi, Ashish Agarwal, Paul Barham, Eugene
Brevdo, Zhifeng Chen, Craig Citro, Greg S. Corrado,
Andy Davis, Jeffrey Dean, Matthieu Devin, Sanjay
Ghemawat, Ian Goodfellow, Andrew Harp, Geoffrey
HEALTHINF 2024 - 17th International Conference on Health Informatics
140

Irving, Michael Isard, Jia, Y., Rafal Jozefowicz, Lukasz
Kaiser, Manjunath Kudlur, … Xiaoqiang Zheng. (2015).
TensorFlow: Large-Scale Machine Learning on
Heterogeneous Systems. https://www.tensorflow.org/
Mavrogenis, A. F., Panagopoulos, G. N., Megaloikonomos,
P. D., Igoumenou, V. G., Galanopoulos, I., Vottis, C. T.,
Karabinas, P., Koulouvaris, P., Kontogeorgakos, V. A.,
Vlamis, J., & Papagelopoulos, P. J. (2016).
Complications After Hip Nailing for Fractures.
Orthopedics, 39(1). https://doi.org/10.3928/01477447-
20151222-11
Mohammad, W. S., & Elsais, W. M. (2020). Association
Between Hip Rotation and Activation of the Quadriceps
and Gluteus Maximus in Male Runners. Orthopaedic
Journal of Sports Medicine, 8(11), 232596712096280.
https://doi.org/10.1177/2325967120962802
Morbidoni, C., Cucchiarelli, A., Fioretti, S., & Di Nardo, F.
(2019). A Deep Learning Approach to EMG-Based
Classification of Gait Phases during Level Ground
Walking. Electronics, 8(8), 894. https://doi.org/
10.3390/electronics8080894
Murray, M. P., Drought, A. B., & Kory, R. C. (1964).
Walking Patterns Of Normal Men. The Journal of Bone
and Joint Surgery. American Volume, 46, 335–360.
Nam, C., Rong, W., Li, W., Cheung, C., Ngai, W., Cheung,
T., Pang, M., Li, L., Hu, J., Wai, H., & Hu, X. (2022). An
Exoneuromusculoskeleton for Self-Help Upper Limb
Rehabilitation After Stroke. Soft Robotics, 9(1), 14–35.
https://doi.org/10.1089/soro.2020.0090
Olsson, A. E., Sager, P., Andersson, E., Björkman, A.,
Malešević, N., & Antfolk, C. (2019). Extraction of Multi-
Labelled Movement Information from the Raw HD-
sEMG Image with Time-Domain Depth. Scientific
Reports, 9(1), 7244. https://doi.org/10.1038/s41598-019-
43676-8
Papachristos, I. V. (2019). Complications of Femoral
Intramedullary Nailing: What should the Surgeon
Remember? . . EC, 7.
Paul, G., & Doweidar, M. H. (Eds.). (2023). Digital human
modeling and medicine: The digital twin. Academic
Press, an imprint of Elsevier.
Paulich, M., Schepers, M., Rudigkeit, N., & Bellusci, G.
(2018). Xsens MTw Awinda: Miniature Wireless Inertial-
Magnetic Motion Tracker for Highly Accurate 3D
Kinematic Applications. https://doi.org/10.13140/
RG.2.2.23576.49929
Rane, L., Ding, Z., McGregor, A. H., & Bull, A. M. J. (2019).
Deep Learning for Musculoskeletal Force Prediction.
Annals of Biomedical Engineering, 47(3), 778–789.
https://doi.org/10.1007/s10439-018-02190-0
Reaz, M. B. I., Hussain, M. S., & Mohd-Yasin, F. (2006).
Techniques of EMG signal analysis: Detection,
processing, classification and applications. Biological
Procedures Online, 8(1), 11–35. https://doi.org/10.1251/
bpo115
Ricci, W. M., Schwappach, J., Tucker, M., Coupe, K.,
Brandt, A., Sanders, R., & Leighton, R. (2008).
Trochanteric versus Piriformis Entry Portal for the
Treatment of Femoral Shaft Fractures. Journal of
Orthopaedic Trauma, 22, S9–S13. https://doi.org/
10.1097/01.bot.0000248472.53154.14
Róisín Howard. (2017). The application of data analysis
methods for surface electromyography in shot putting
and sprinting. https://doi.org/10.13140/RG.2.2.1590
7.04640
Rommens, P. M., & Hessmann, M. H. (Eds.). (2015).
Intramedullary Nailing. Springer London.
https://doi.org/10.1007/978-1-4471-6612-2
Siegel, F., Buj, C., Schwanbeck, R., Petersik, A., Hoffmann,
U., Kemper, J., Hildebrand, F., Kobbe, P., Eschweiler, J.,
Greven, J., Merfort, R., Freimann, C., Schwaiger, A., &
Aschwege, F. (2023). Concept for General
Improvements in the Treatment of Femoral Shaft
Fractures with an Intramedullary Nail: Proceedings of
the 16th International Joint Conference on Biomedical
Engineering Systems and Technologies, 360–367.
https://doi.org/10.5220/0011679100003414
Simpson, K. M., Munro, B. J., & Steele, J. R. (2011).
Backpack load affects lower limb muscle activity
patterns of female hikers during prolonged load carriage.
Journal of Electromyography and Kinesiology, 21(5),
782–788. https://doi.org/10.1016/j.jelekin.2011.05.012
Soroushmojdehi, R., Javadzadeh, S., Pedrocchi, A., &
Gandolla, M. (2022). Transfer learning in hand
movement intention detection based on surface
electromyography signals. Frontiers in Neuroscience,
16, 977328. https://doi.org/10.3389/fnins.2022.977328
Toogood, P. A., Abdel, M. P., Spear, J. A., Cook, S. M.,
Cook, D. J., & Taunton, M. J. (2016). The monitoring of
activity at home after total hip arthroplasty. The Bone &
Joint Journal, 98-B(11), 1450–1454. https://doi.org/
10.1302/0301-620X.98B11.BJJ-2016-0194.R1
Tryon, J., & Trejos, A. L. (2021). Evaluating Convolutional
Neural Networks as a Method of EEG–EMG Fusion.
Frontiers in Neurorobotics, 15, 692183. https://doi.org/
10.3389/fnbot.2021.692183
Yang, W., Yang, D., Liu, Y., & Liu, H. (2019). EMG Pattern
Recognition Using Convolutional Neural Network with
Different Scale Signal/Spectra Input. International
Journal of Humanoid Robotics, 16(04), 1950013.
https://doi.org/10.1142/S0219843619500130
Zha, X., Wehbe, L., Sclabassi, R. J., Mace, Z., Liang, Y. V.,
Yu, A., Leonardo, J., Cheng, B. C., Hillman, T. A., Chen,
D. A., & Riviere, C. N. (2021). A Deep Learning Model
for Automated Classification of Intraoperative
Continuous EMG. IEEE Transactions on Medical
Robotics and Bionics, 3(1), 44–52.
https://doi.org/10.1109/TMRB.2020.3048255
Zhao, B., Lu, H., Chen, S., Liu, J., & Wu, D. (2017).
Convolutional neural networks for time series
classification. Journal of Systems Engineering and
Electronics, 28(1), 162–169. https://doi.org/10.21629/
JSEE.2017.01.18
Zia Ur Rehman, M., Waris, A., Gilani, S., Jochumsen, M.,
Niazi, I., Jamil, M., Farina, D., & Kamavuako, E. (2018).
Multiday EMG-Based Classification of Hand Motions
with Deep Learning Techniques. Sensors, 18(8), 2497.
https://doi.org/10.3390/s18082497
Evaluating the Viability of Neural Networks for Analysing Electromyography Data in Home Rehabilitation: Estimating Foot Progression
Angle
141
