
Investigation of Artifact Contamination Impact on EEG Oscillations
Towards Enhanced Motor Function Characterization
Mojisola Grace Asogbon
1,2 a
, Oluwarotimi Williams Samuel
1,2 b
, Farid Meziane
1,2,
*
c
,
Guanglin Li
3
and Yongcheng Li
3,
*
1
School of Computing, University of Derby, Derby, DE22 3AW, U.K.
2
Data Science Research Centre, University of Derby, Derby DE22 3AW, U.K.
3
CAS Key Laboratory of Human-Machine Intelligence-Synergy Systems, Shenzhen Institutes of Advanced Technology
(SIAT), Chinese Academy of Sciences (CAS), Shenzhen, Guangdong, China
Keywords: Electroencephalogram (EEG), Signal Processing, Artifact Removal Methods, Motor Recovery.
Abstract: The significant advancements in electroencephalography (EEG)-driven technology have led to its widespread
use in assessing stroke-related conditions. Over the years, various studies have explored the potential of EEG
oscillatory patterns in neurological research, with several of them giving limited attention to the signal
processing techniques employed, precluding a proper understanding of EEG oscillatory patterns under various
conditions. To resolve this issue, we systematically investigated how artifacts impact EEG oscillatory rhythms
associated with upper limb movement-related tasks. Thus, the EEG signals of motor tasks were acquired non-
invasively from healthy subjects and processed using automated artifact-attenuation methods. Subsequently,
the Mu and Beta bands in the brain's motor cortex region were extracted through time-frequency analysis and
analyzed using relevant metrics. Experimental results revealed that artifacts in EEG would substantially
influence the brain activation strength and response during motor tasks. Notably, signals preprocessed with
Reduction of Electroencephalographic Artifacts based on Multi Wiener Filter and Enhanced Wavelet
Independent Component Analysis (RELAX_MWF_wICA) showed better brain responses and high task
classification performance compared to other methods and the raw signal across motor tasks. This study's
findings revealed that the choice of signal processing technique is crucial, as it would influence its analysis
and interpretation, thus highlighting the need for careful consideration and usage.
1 INTRODUCTION
The study of neural oscillations, driven by the
coordinated activity of numerous neurons and
assessed through techniques such as functional
magnetic resonance imaging (fMRI),
electroencephalography (EEG), and magneto-
encephalography (MEG), among others, has been a
prominent and extensively explored area in
neurological research (Ward, 2015; Gui et al., 2010;
Jee, 2021).
Notable advances in EEG technology have led to
its wide usage in assessing stroke-related brain
function. EEG is a non-invasive and safe method
with an excellent temporal resolution that offers
valuable insights into brain activity through direct
a
https://orcid.org/ 0009-0006-1503-9356
b
https://orcid.org/ 0000-0003-1945-1402
c
https://orcid.org/ 0000-0001-9811-6914
measurement of electrical potentials from the
underlying neural tissue (Wu et al., 2016; Lin et al.,
2017; Asogbon et al., 2021; Anapama et al., 2012).
EEG signals represent recurring patterns resulting
from the coordinated activity of neurons firing in
synchrony, and they can be observed across a range
of frequencies (including delta, theta, alpha, beta, and
gamma bands). Brain activities during upper limb
movements in these bands are affected by stroke
(Maura et al., 2023; Bartur et al., 2019). Therefore,
they are considered promising predictors that can
offer valuable insights into stroke patients’ status,
helping clinicians identify distinct biological
subgroups and determine which treatment approach
might be more appropriate and effective (Cassidy et
al., 2019).
Asogbon, M., Samuel, O., Meziane, F., Li, G. and Li, Y.
Investigation of Artifact Contamination Impact on EEG Oscillations Towards Enhanced Motor Function Characterization.
DOI: 10.5220/0012373400003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 755-762
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
755

For instance, in contemporary stroke therapeutic
treatment, EEG oscillations are utilized as predictive
indicators, incorporated with clinical intervention
techniques. This integration further enhances
diagnosis, treatment, and recovery in stroke patients
with motor impairments (Keser et al., 2022). In 2019,
Cassidy et al. investigated EEG oscillations as a
potential predictor of injury and motor function
recovery in stroke survivors. By experimenting with
EEG recordings from both healthy controls and stroke
patients, the study examined the connection between
EEG oscillations and injury and motor condition,
utilizing delta and high-beta frequency bands. The
study's outcome revealed that delta-frequency
oscillations reflect both injury and motor function
recovery after a stroke.
In addition, Thibaut et al. (2017) found, in their
work, that brain activity in both lesioned and
unlesioned hemispheres of stroke patients, as
measured by EEG, provides new insights into the
relationship between high-frequency rhythms and
motor impairment. Their findings highlight the role
of an excess of beta activity in the affected central
cortical region, contributing to poor motor function
during stroke recovery.
A research study conducted by López-Larraz et al.
(2018) emphasized the significance of employing
suitable techniques to eliminate artifacts in EEG
recordings of stroke patients. The study aimed to
uncover the true neural activity by eliminating
unwanted interference. The findings revealed that
during motor tasks, EEG-cortical activation is
heightened, and the presence of artifacts can
introduce an overly optimistic bias in the performance
evaluation of brain-machine interfaces (BMIs).
Unarguably, several works have conducted
exploratory investigations using EEG oscillatory
rhythms to predict motor function recovery in stroke
patients. Considering that the EEG signal is
susceptible to contamination from various artifacts,
these disturbances can significantly influence the
resulting signal, potentially leading to
misinterpretation if a robust cleaning method is not
implemented at the signal processing stage.
Unfortunately, relatively little attention has been
directed towards the methodologies employed for
processing EEG oscillations in relation to upper limb
motor tasks, representing a fundamental drawback in
the field.
In addressing this concern, we systematically
investigated the influence of artifacts on cortical
activation and the recognition of motor tasks using
EEG-based neural oscillations, with a specific focus
on the Mu (μ) and Beta (β) bands. The study involved
the analysis of non-invasively collected EEG signals
from healthy individuals participating in four distinct
movement execution (ME) tasks.
These signals were individually processed using
five automated data-driven methods capable of
removing either single or multiple artifacts. These
methods were selected from widely used EEG artifact
attenuation techniques based on performance criteria
evident in existing works.
The ICA decomposition method is applied to the
processed signal, and an automated independent
component (IC) classification method was used to
detect or flag artefactual ICs based on specific
thresholding parameters. After that, the signal
segment that is time-locked to a specific event was
epoched and analysed using time-frequency analysis.
2 MATERIALS AND METHODS
2.1 Participants Information
In this study, 20 healthy subjects volunteered to
participate in the experiment. Specifically, right hand
dominated individuals including male and female
aged between 20 and 35 years were recruited. Prior to
the experiment, all volunteers were briefed on the
study objective and the experimental procedure.
Subsequently, they all agreed and gave written
consent for the publication of their data. The
Institutional Review Board of Shenzhen Institutes of
Advanced Technology, Chinese Academy of
Sciences, approved the recruitment and experimental
process.
2.2 Equipment Setup and Data
Collection
The experiment was conducted at the Shenzhen
Institute of Advanced Technology, Chinese Academy
of Sciences. The EEG signals were acquired from the
subjects using a 64-channel gel-based AgCl electrode
cap combined with a Neuroscan acquisition system.
The EEG cap was positioned on each of the
volunteer’s head following international 10–20
electrode placement configuration. The ground
electrode was positioned at AFz and referenced to
CPz during signal recording. All electrode channels
were sampled at 1000Hz and based on the volunteer’s
tolerance level, the impedance varies between 5-8kΩ.
Before commencing the experiment, the subjects
were trained on the experimental procedure and
instructed on how to perform the ME tasks. The ME
tasks, including key grip (KGME), power grip
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
756
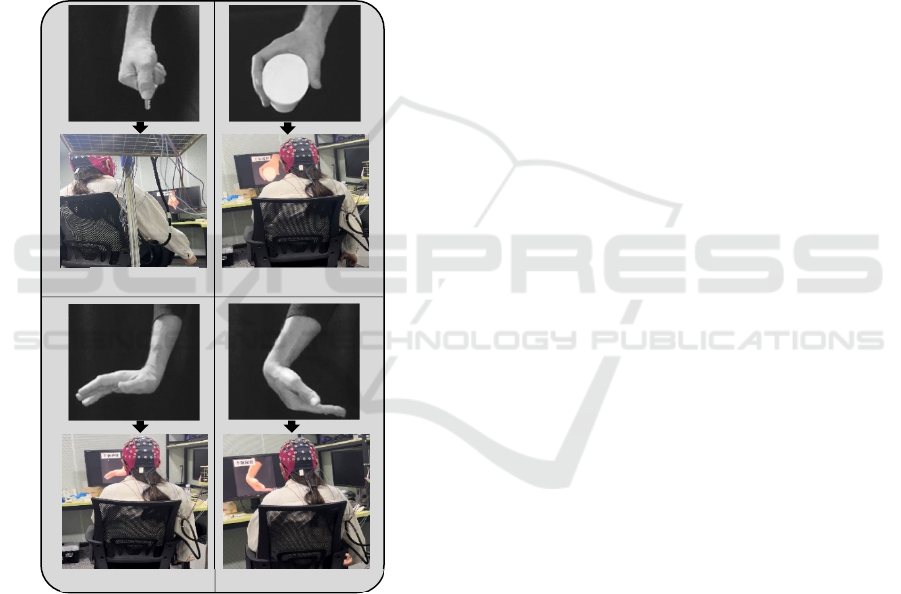
(PGME), wrist extension (WEME), and wrist flexion
(WFME), shown in Figure 1, were performed by each
subject. The subjects were instructed to sit on a
comfortable high-back chair and watch a visual
display unit (VDU) placed 1m in front of them. To
ensure the tasks were performed correctly, pre-
recorded video containing an active (say wrist
extension) and non-active task (rest) was developed.
The VDU was used to display the video of each task
to guide them throughout the experiment. Each active
task (ME) was performed for a duration of 5s,
followed by 5s of non-active task (rest) to mitigate
fatigue. In total, participants completed two
consecutive sessions, each comprising 10 active tasks
and 10 non-active tasks.
Figure 1: A representation of a participant during the motor
execution tasks which includes key grip , power grip ,wrist
extension and wrist flexion .
2.3 Data Processing
The signal recorded for each participant underwent
offline processing and analysis utilizing the EEGLAB
(Delorme and Makeig 2004) and MATLAB (The
MathWorks Inc. 2019) toolkits. Towards
understanding the relevance of artifacts on EEG
oscillatory patterns five popularly used automatic
data-driven EEG artifact attenuation methods were
applied to the recorded signals. The procedure for the
signal processing is described as follows:
1. Utilizing EEGLAB, each of the ME tasks of the
recorded signals trials/session were merged.
Subsequently, the signals were filtered using a
passband edge frequency of 1Hz and 30Hz. The
1Hz signifies the upper limit of the lower
frequency range, and 30Hz represents the lower
limit of the higher frequency range that can pass
through the filter.
2. Afterward, the following automated EEG
artifacts elimination methods were individually
applied to the filtered signal:
(a) Independent Component Analysis (ICA)
based Extended Information-maximization
(INFOMAX) (Jutten & Herault, 1991;
Comon, 1994).
(b) Artifact Subspace Reconstruction (ASR)
(Bloniasz, 2022; Chang et al., 2019, blum et
al., 2019).
(c) ICA based Automated Artifact Removal
(CCACCA) (Gómez-Herrero et al., 2006;
De Clercq et al., 2006).
(d) Reduction of Electroencephalographic
Artifacts based on Multi Wiener Filter and
Enhanced Wavelet ICA
(RELAX_MWF_wICA) (Bailey et al.,
2022; Somers et al., 2018; Castellanos et al.,
2006).
(e) Reduction of Electroencephalographic
Artifacts based on Multi Wiener Filter
(RELAX_MWF) (Bailey et al., 2022;
Somers et al., 2018).
Importantly, the single and multiple automated
artifact reduction methods were chosen from
commonly used EEG artifact removal techniques
based on their superior performance. The detail
description of each method can found in the provided
references above.
3. Next, ICs of the individually processed signals
were computed using the ICA decomposition
method.
4. The IC_Label, an accurate and computationally
efficient classifier compared to other commonly
used automated IC component classification
method (Pion-Tonachini et al., 2019), was
applied to detect or flag artefactual ICs based on
thresholding parameters. Thereafter, the flagged
artefactual ICs were subtracted from the
processed signals.
Ke
y
Gri
p
Power Gri
p
Wrist Extension Wrist Flexion
Investigation of Artifact Contamination Impact on EEG Oscillations Towards Enhanced Motor Function Characterization
757

5. The resulting continuous EEG signals are
epoched by extracting data epochs that are time-
locked to a specified ME task.
6. In examining the task-related EEG dynamics of
the signals, each task was epoched, choosing a
window from -1s to 5s.
7. The epoched datasets are saved for time-
frequency analysis and other analyses in
MATLAB using a custom-built script.
It is worth stating that the signal processing steps
presented above were performed on each subject’s
EEG recording.
2.4 Feature Extraction and Task
Decoding
In gaining insights into how the automated methods
for reducing artifacts affect brain activation strength,
the z- The 𝜇 and 𝛽 oscillatory pattern in the brain's
motor cortex region during ME tasks were
considered. As demonstrated in existing works, these
bands were selected based on their modulation
characteristics during movement. In addition,
alterations in these bands during ME tasks have been
found to correspond with motor impairment in stroke
patients. (Bartur et al., 2019; Leonardi et al., 2022).
Apply a time-frequency analysis-based approach,
the 𝜇 and 𝛽 bands in the range of 10-14Hz and 16-
26Hz were extracted from the cleaned/processed
signal from the 18 electrodes at the motor cortex
region (excluding the midline electrodes).
The short-time Fourier transform analysis was
performed on each processed signal within the
designated frequency bands (μ and β). Following the
time-frequency decomposition, the data were z-
scored (eqn. 1). A statistical comparison of the z-
scored power in both frequency bands during rest and
movement task was conducted across all EEG
channels using the Wilcoxon rank-sum test, and the
results were subsequently topographically mapped. In
cases where no significant difference was observed
between rest and movement for a channel, the value
was set to 0.
𝑋
^
=
𝑋−𝜇
𝜎
(1)
where X ̂ is the Z-scored signal, X denotes the
processed signal, μ and σ is the mean and standard
deviation of the signal during rest time for each trial.
For the motor task recognition, the preprocessed
EEG signals were divided into smaller windows using
a sliding segmentation approach. Subsequently, a
feature extraction method based on wavelet analysis
was employed to extract pertinent features from each
segment. Each resulting feature matrix was used to
construct individual machine-learning models,
including Linear Discriminant Analysis (LDA), k-
nearest Neighbors (kNN), and Random Forest (RF).
A five-fold cross-validation technique was
applied to partition the extracted feature matrices into
training and testing datasets to ensure optimal data
utilization. The five-fold cross-validation involves
randomly dividing the entire dataset into five subsets,
and this process is repeated five times. During each
iteration, the model is trained on four of the folds,
while the remaining one is used for testing the model.
The performances of the models were assessed
using classification accuracy (CA; eqn. 2), positive
predictive value (PPV; eqn. 3), negative predictive
value (NPV; eqn. 4), and false positive rate (FPR;
eqn. 5). PPV is the percentage chance that a positive
result is a true positive. NPV is the percentage chance
that a negative result is a true negative. The FPR
measures the proportion of negative instances that are
inaccurately identified as positive instances.
𝐶𝐴
=
∑
𝑇𝑃
+𝑇𝑁
𝑇𝑃
+𝐹𝑁
+𝐹𝑃
+𝑇𝑁
𝑁
(
2)
𝑃𝑃𝑉
=
𝑇𝑃
𝑇𝑃
+𝐹𝑃
(
3)
𝑁𝑃𝑉
=
𝑇𝑁
𝑇𝑁
+𝐹𝑁
(
4)
𝐹𝑃𝑅
=
𝐹𝑃
𝐹𝑃
+𝑇𝑁
(
5)
where N denotes the number of ME classes, 𝑇𝑃
: true
positive, 𝐹𝑃
: false positive, 𝐹𝑁
: false positive, and
𝑇𝑁
: true negative.
The Friedman test was employed to check the
statistically significant effect between the
preprocessed signals with the artifact attenuation
methods and the original
EEG signal recordings.
3 RESULTS
3.1 Analysis of Cortical Activation via
Z-Score Power
Figure 2a-b depicts the average z-score results for the
μ and β bands during motor execution (ME) across all
participants. In the figures, each color bar represents
the raw signal and different artifact reduction
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
758
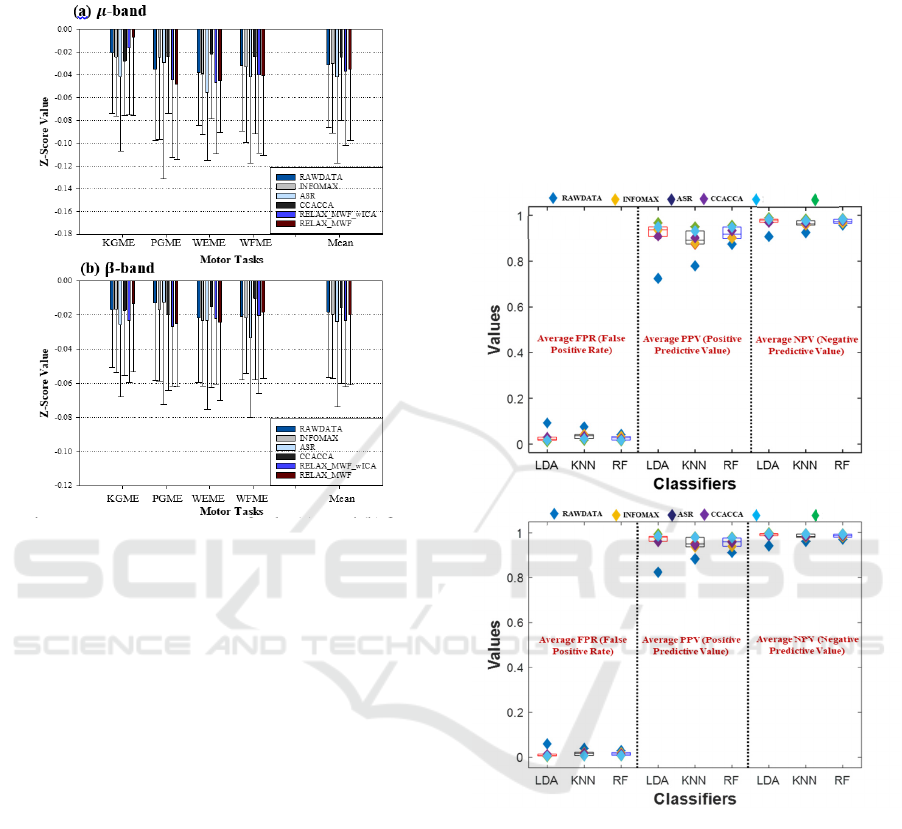
methods, with the z-score power varying for all
methods task by task.
Figure 2: Average z-score power for the (a) μ and (b) β
frequencies band across all participants.
Generally, the z-score value is expected to increase
(more negative) after artifacts are eliminated from the
signals. A high negative z-score value indicates a
stronger brain signal or activation and vice-versa.
Through careful examination, the ASR-based signal
obtained increased z-score value for all tasks
compared to the raw data in both bands.
Similarly, RELAX_MWF_wICA and
RELAX_MWF methods achieved better z-scores
than others for all tasks except for KGME. However,
the CCACCA method recorded the lowest z-score
value, followed by INFOMAX. The performance of
INFOMAX and CCACCA methods may be due to
removing more brain signals during pre-processing. It
could also be because of their inability to remove
other artifacts unrelated to ocular or muscular
artifacts. Across all tasks and bands, the
RELAX_MWF_wICA and ASR methods recorded
consistently higher average z-score values. At the
same time, some artifact removal methods showed an
increment in the z-score values; there is no statistical
significance between the raw data and the artifact
attenuation methods (
𝜇
: p = 0.1815 and β: p =
0.6126).
3.2 Performance Estimation Using
FPR, PPV and NPV
The effectiveness of the classifier's performances
with respect to the attenuation methods was validated
using false positive rate (FPR), positive predictive
value (PPV), and negative predictive value (NPV)
metrics. The average FPR, PPV, and NPV results for
the bands across tasks are presented in Figure 3a-b
using a scatter plot by group graph.
Figure 3: Performance evaluation of the methods for FPR,
PPV and NPV (a) 𝜇 and (b) 𝛽 bands.
Each plot consists of average (i) FPR, (ii) PPV, and
(iii) NPV (partitioned with a black dotted line) for the
classifiers (including LDA, KNN, and RF).
Observing the plots for the
𝜇
and
𝛽 bands, the
effectiveness of the raw data, and the attenuation
methods based on the RF classifier for the metrics are
also relatively the same compared to LDA and KNN
classifiers.
However, an obvious difference is noticeable
between the artifact attenuation methods and the raw
signal for all the metrics, especially PPV. Overall,
RELAX_MWF_wICA based on the LDA classifier
achieved the lowest FPR value (μ: 0.0108, β: 0.0026),
(i)
(ii) (iii)
RELAX_
MWF_wICA
RELAX_
MWF
(a) 𝝁-band
(i)
(ii)
(iii)
RELAX_
MWF_wICA
RELAX_
MWF
(b) 𝜷-band
Investigation of Artifact Contamination Impact on EEG Oscillations Towards Enhanced Motor Function Characterization
759
highest PPV (μ: 0.9679, β: 0.9923), and NPV (μ:
0.9892, β: 0.9974) values compared to other methods.
3.3 Evaluation of Individual Task
Decoding Performance
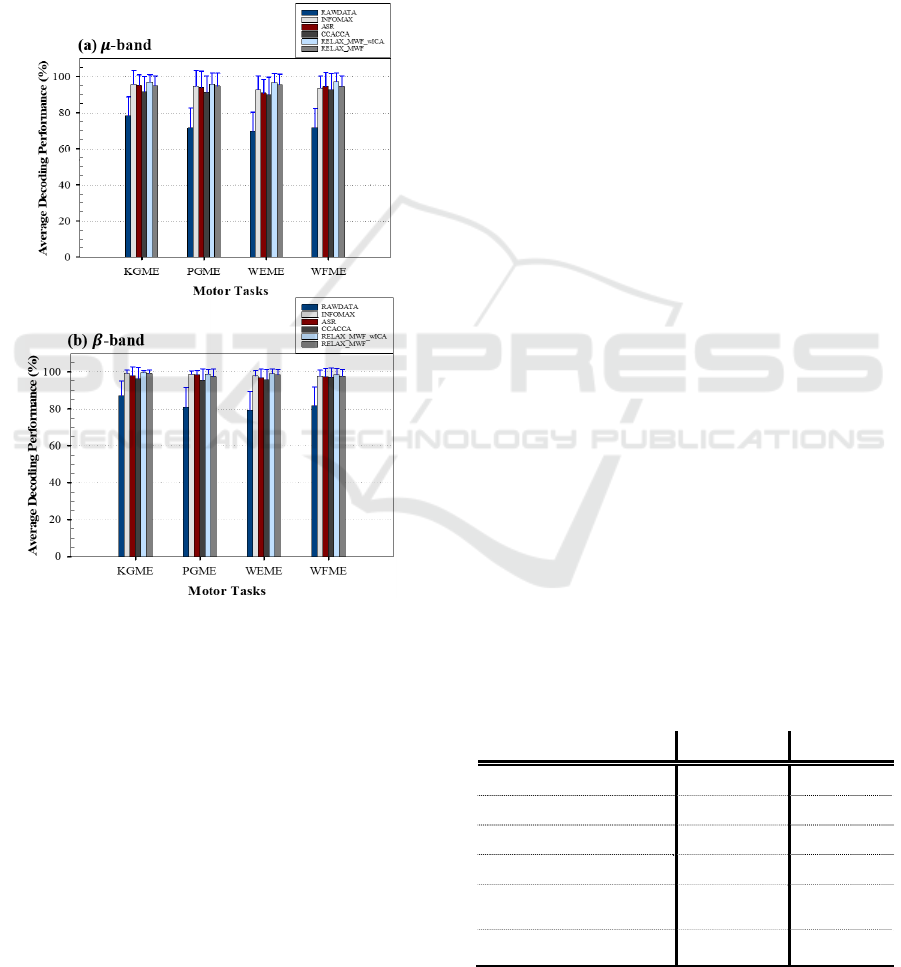
This section presents the individual ME task
recognition rate for LDA classifier because of its
performance in section 3.2. The obtained result in the
μ and β bands is presented in Figure 4a-b using a bar
plot graph.
Figure 4: Class-wise task decoding performance for (a) μ
(b) and β bands using LDA classifier.
The error bar on each preprocessing method
represents the standard deviation across participants.
From the results, the β band performs better than the
μ band, and it is clear that all artifact removal methods
were able to eliminate artifacts from the signals,
yielding varying classification performance.
Looking at the performance of each method, the
RELAX_MWF_wICA (p-value: 0.0022 for μ and β
bands) yielded the best average accuracies.
Specifically, μ: 96.98 ± 8.40%, 95.91 ± 6.05%, 96.49
± 5.00%, and 97.27 ± 4.70% for KGME, PGME,
WEME and WFME respectively. For the β band,
accuracies of KGME: 99.63 ± 1.02%, PGME: 98.77
± 2.54%, WEME: 98.97 ± 2.75% and WFME: 98.49
± 3.46%.
On the other hand, the least performance was
obtained by the raw signal with mean accuracies of
78.13 ± 10.68%, 71.62 ± 10.92%, 69.66 ± 10.60%,
71.79 ± 10.58%, for μ band. The β band recorded
87.14 ± 7.76%, 80.94 ± 10.79%, 79.30 ± 10.15%,
81.68 ± 10.29% KGME, PGME, WEME and WFME
respectively.
4 DISCUSSION AND
CONCLUSION
In this study, we demonstrated the impact of artifacts
on μ and β oscillations detected in the motor cortex
region of the brain during the execution of upper limb
motor tasks. Five automated artifact removal
methods, including single (INFOMAX) and multiple
artifact removal capabilities (ASR, CCACCA,
RELAX_MWF_wICA, and RELAX_MWF), were
individually employed to attenuate the artifacts
present in both bands. After the Independent
Component Analysis (ICA) decomposition of the
processed signal, an automated IC classifier, namely
IC_LABEL (Pion-Tonachini et al., 2019), was
utilized to detect and flag artifact-contaminated ICs
for removal from the processed signal.
The impact of artifacts were considered on
cortical activation strength and motor task
recognition. The brain activation response was
evaluated using z-score power. From the obtained
results, the average z-score values across participants
varied from task to task and between methods. Table
1 presents the average z-score power values across
participants and tasks for μ and β frequency bands.
When comparing the values between the bands, the μ
band showed better brain activation during the tasks
for all the methods compared to the β band. The ASR
Table 1: Average z-score power values all across
participants and tasks for the 𝜇 and 𝛽 bands.
Methods 𝝁 Band 𝜷 Band
RAWDATA -0.0314 -0.0182
INFOMAX -0.0302 -0.0197
ASR -0.0421 -0.0238
CCACCA -0.0244 -0.0157
RELAX_MWF_
w
ICA
-0.0370 -0.0231
RELAX_MWF -0.0353 -0.0202
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
760

method exhibited the highest z-score values in both
bands, followed by RELAX_MWF_wICA.
CCACCA recorded the least z-score value compared
to the raw data and other methods in the μ and β
bands. In other words, these methods, except
CCACCA, enhanced the brain response through the
mitigation of artifacts.
The outcome from the False Discovery Rate
(FDR) and Positive Predictive Value (PPV) and
Negative Predictive Value (NPV) validation metrics
shows that the RELAX_MWF_wICA-based method
is an accurate and effective model for processing
EEG signals compared to other methods. Similarly, in
individual task classification performance, the
RELAX_MWF_wICA method outperformed other
methods and the raw data in both bands.
Examining the performance of CCACCA, though
it has a low z-score power value compared to the raw
data, it recorded better decoding performance
compared to the raw data. One possible reason for this
could be that a strong brain response during the ME
task may not necessarily correlate with high
classification performance.
Overall, considering the impact of artifacts on
brain activation response and motor task
classification, the RELAX_MWF_wICA
demonstrated better performance, albeit with no
significant difference when compared with ASR and
RELAX_MWF methods. It performance could be
attributed to its status as a hybrid artifact attenuation
method that incorporates the advantages of MWF and
wICA.
The outcome of this work provides valuable
insights into the significance of using appropriate
methodology in the EEG signal-processing pipeline
to obtain precise estimations of motor brain activity,
thereby avoiding biased signal analyses and
interpretation.
It's important to note that this study is preliminary
and confined to a dataset consisting solely of healthy
subjects. The analysis utilized Z-score power
quantifier and statistical metrics. In our forthcoming
research, we plan to recruit stroke patients and
acquire EEG signals from them to validate our
findings. Furthermore, we will employ noteworthy
quantifiers to thoroughly investigate and analyze
EEG oscillatory rhythms.
ACKNOWLEDGEMENTS
The research work was supported in part by the
Ministry of Science and Technology of China under
grants (STI2030-Brain Science and Brain-Inspired
Intelligence Technology-2022ZD0210400), National
Natural Science Foundation of China under grant
(#62150410439), Ministry of Science and
Technology, Shenzhen (#QN2022032013L), and
Guangdong Basic and Applied Research Foundation
(#2023A1515011478).
The authors appreciates Zhengxiang Jing and
Yixin Ma, for their support in the data acquisition.
Thanks to all the recruited subjects who volunteered
to participate in the experiment.
REFERENCES
Ward, N. S. (2015). Does neuroimaging help to deliver
better recovery of movement after stroke?. Current
Opinion in Neurology, 28(4), 323-329.
Gui, X. U. E., Chuansheng, C. H. E. N., Zhong-Lin, L. U.,
& Qi, D. O. N. G. (2010). Brain imaging techniques and
their applications in decision-making research. Xin li
xue bao. Acta psychologica Sinica, 42(1), 120.
Jee, S. (2021). Brain Oscillations and Their Implications for
Neurorehabilitation. Brain & Neurorehabilitation,
14(1).
Wu, J., Srinivasan, R., Burke Quinlan, E., Solodkin, A.,
Small, S. L., & Cramer, S. C. (2016). Utility of EEG
measures of brain function in patients with acute stroke.
Journal of neurophysiology, 115(5), 2399-2405.
Lin, C. T., Chuang, C. H., Cao, Z., Singh, A. K., Hung, C.
S., Yu, Y. H., ... & Wang, S. J. (2017). Forehead EEG
in support of future feasible personal healthcare
solutions: Sleep management, headache prevention,
and depression treatment. IEEE Access, 5, 10612-
10621.
Asogbon, M. G., Samuel, O. W., Li, X., Nsugbe, E.,
Scheme, E., & Li, G. (2021). A linearly extendible
multi-artifact removal approach for improved upper
extremity EEG-based motor imagery decoding. Journal
of Neural Engineering.
Anupama, H. S., Cauvery, N. K., & Lingaraju, G. M.
(2012). Brain computer interface and its types-a study.
International Journal of Advances in Engineering &
Technology, 3(2), 739.
Maura, R. M., Rueda Parra, S., Stevens, R. E., Weeks, D.
L., Wolbrecht, E. T., & Perry, J. C. (2023). Literature
review of stroke assessment for upper-extremity
physical function via EEG, EMG, kinematic, and
kinetic measurements and their reliability. Journal of
NeuroEngineering and Rehabilitation, 20(1), 1-32.
Bartur, G., Pratt, H., & Soroker, N. (2019). Changes in mu
and beta amplitude of the EEG during upper limb
movement correlate with motor impairment and
structural damage in subacute stroke. Clinical
Neurophysiology, 130(9), 1644-1651.
Cassidy, J. M., Wodeyar, A., Srinivasan, R., & Cramer, S.
C. (2021). Coherent neural oscillations inform early
stroke motor recovery. Human Brain Mapping, 42(17),
5636-5647.
Investigation of Artifact Contamination Impact on EEG Oscillations Towards Enhanced Motor Function Characterization
761

Keser, Z., Buchl, S. C., Seven, N. A., Markota, M., Clark,
H. M., Jones, D. T., ... & Lundstrom, B. N. (2022).
Electroencephalogram (EEG) with or without
transcranial magnetic stimulation (TMS) as biomarkers
for post-stroke recovery: a narrative review. Frontiers
in Neurology, 13, 827866.
Thibaut, A., Simis, M., Battistella, L. R., Fanciullacci, C.,
Bertolucci, F., Huerta-Gutierrez, R.,... & Fregni, F.
(2017). Using brain oscillations and corticospinal
excitability to understand and predict post-stroke motor
function. Frontiers in neurology, 8, 187.
López-Larraz, E., Figueiredo, T. C., Insausti-Delgado, A.,
Ziemann, U., Birbaumer, N., & Ramos-Murguialday,
A. (2018). Event-related desynchronization during
movement attempt and execution in severely paralyzed
stroke patients: An artifact removal relevance analysis.
NeuroImage: Clinical, 20, 972-986.
Jutten, C., & Herault, J. (1991). Blind separation of sources,
part I: An adaptive algorithm based on neuromimetic
architecture. Signal processing, 24(1), 1-10.
Comon, P. (1994). Independent component analysis, a new
concept?. Signal processing, 36(3), 287-314.
Bloniasz, P. (2022). Artifact Subspace Reconstruction
(ASR) for electroencephalography artifact removal
must be optimized for each unique dataset.
Chang, C. Y., Hsu, S. H., Pion-Tonachini, L., & Jung, T. P.
(2019). Evaluation of artifact subspace reconstruction
for automatic artifact components removal in multi-
channel EEG recordings. IEEE Transactions on
Biomedical Engineering, 67(4), 1114-1121.
Blum, S., Jacobsen, N. S., Bleichner, M. G., & Debener, S.
(2019). A Riemannian modification of artifact subspace
reconstruction for EEG artifact handling. Frontiers in
human neuroscience, 13, 141.
Gómez-Herrero, G., De Clercq, W., Anwar, H., Kara, O.,
Egiazarian, K., Van Huffel, S., & Van Paesschen, W.
(2006, June). Automatic removal of ocular artifacts in
the EEG without an EOG reference channel. In
Proceedings of the 7th Nordic signal processing
symposium-NORSIG 2006 (pp. 130-133). IEEE.
De Clercq, W., Vergult, A., Vanrumste, B., Van Paesschen,
W., & Van Huffel, S. (2006). Canonical correlation
analysis applied to remove muscle artifacts from the
electroencephalogram. IEEE transactions on
Biomedical Engineering, 53(12), 2583-2587.
Bailey, N., Biabani, M., Hill, A. T., Miljevic, A., Rogasch,
N. C., McQueen, B., ... & Fitzgerald, P. (2022).
Introducing RELAX (the Reduction of
Electroencephalographic Artifacts): A fully automated
pre-processing pipeline for cleaning EEG data-Part 1:
Algorithm and Application to Oscillations. BioRxiv,
2022-03.
Somers, B., Francart, T., & Bertrand, A. (2018). A generic
EEG artifact removal algorithm based on the multi-
channel Wiener filter. Journal of neural engineering,
15(3), 036007.
Castellanos, N. P., & Makarov, V. A. (2006). Recovering
EEG brain signals: Artifact suppression with wavelet
enhanced independent component analysis. Journal of
neuroscience methods, 158(2), 300-312.
Pion-Tonachini, L., Kreutz-Delgado, K., & Makeig, S.
(2019). ICLabel: An automated
electroencephalographic independent component
classifier, dataset, and website. NeuroImage, 198, 181-
197.
Delorme, A., & Makeig, S. (2004). EEGLAB: an open
source toolbox for analysis of single-trial EEG
dynamics including independent component
analysis. Journal of neuroscience methods, 134(1), 9-
21.
The MathWorks Inc. (2019). MATLAB version: 9.7.0
(R2019b), Natick, Massachusetts: The MathWorks Inc.
https://www.mathworks.com
Leonardi, G., Ciurleo, R., Cucinotta, F., Fonti, B., Borzelli,
D., Costa, L., ... & Alito, A. (2022). The role of brain
oscillations in post-stroke motor recovery: An
overview. Frontiers in Systems Neuroscience, 16,
947421.
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
762
