
Additive Manufacturing of Nitinol for Smart Personalized Medical
Devices: Current Capabilities and Challenges
Andrés Díaz Lantada
1a*
, Carlos Aguilar Vega
1b
, Rodrigo Zapata Martínez
1c
,
Mónica Echeverry Rendón
2
, Muzi Li
2
, Óscar Contreras-Almengor
2d
, Jesús Ordoño
2
,
William Solórzano-Requejo
1e
, Miroslav Vasic
3
, Juan Manuel Munoz-Guijosa
1
and Jon Molina-Aldareguia
1,2 f**
1
Department of Mechanical Engineering, ETSI Industriales, Universidad Politécnica de Madrid, Madrid, Spain
2
IMDEA Materials Institute, Tecnogetafe, Getafe, Spain
3
Center for Industrial Electronics, ETSI Industriales, Universidad Politécnica de Madrid, Madrid, Spain
Keywords: Additive Manufacturing, Shape-Memory Alloys, Smart Materials and Structures, Medical Devices.
Abstract: Shape-morphing smart medical devices constitute a current research trend and are bound to transform
healthcare thanks to the improved interactions with the human body they enable. 4D printing technologies
facilitate the development of such devices and start to provide innovative solutions like minimally invasive
surgical tools and devices, ergonomic appliances and orthoses, evolutive implants and active in vitro
biodevices, among others. Most studies so far, dealing with 4D printed biodevices, have been focused on
smart polymeric materials and structures, whose biomechanical, biochemical and biological properties cannot
always match those from shape-morphing and shape-memory alloys (SMAs). Considering several recent
synergic breakthroughs in the additive manufacturing of smart alloys, this study presents 4D printing with
SMAs for a new generation of medical devices, illustrated through case studies by our team. The more relevant
strategies under research for enhancing the performance of 4D printed NiTi are illustrated and varied foreseen
directions for achieving a sustainable and equitable impact in healthcare are discussed.
1 INTRODUCTION
Additive manufacturing technologies (AMTs) have
emerged in the last decades, as highly transformative
resources enabling freedom of design, fostering the
personalization of devices and reformulating the
world of design with original design-for-additive-
manufacturing (DfAM) methods (Lipson, 2011,
Yang, 2015 Thompson, 2016). The additive
manufacturing (AM) of lattices, porous structures,
functionally graded materials and multi-material
structures may bring to relevant benefits including
biomimetic and biomechanical design strategies for
enhanced performance through bioinspiration.
a
https://orcid.org/0000-0002-0358-9186
b
https://orcid.org/0000-0003-0291-3041
c
https://orcid.org/0000-0002-2611-7050
d
https://orcid.org/0000-0002-8166-4161
e
https://orcid.org/0000-0002-2989-9166
f
https://orcid.org/0000-0003-3508-6003
Besides, geometrical complexity can be applied
to the integration of varied functionalities, to the
minimization of components and to the elimination of
post-production operations, hence enhancing the
development lifecycle. However, in most cases, 3D
printed objects are passive elements unable to interact
with the environment in a dynamic way, as needed
quite often for biomedical applications and healthcare
products, which should often evolve according to
patients’ growing and healing processes. In general,
passive structures require the incorporation of sensors
and actuators for allowing such interactions and result
suboptimal in terms of integration, direct fabrication,
cost, weight and eco-impacts.
Lantada, A., Vega, C., Martínez, R., Rendón, M., Li, M., Contreras-Almengor, Ó., Ordoño, J., Solórzano-Requejo, W., Vasic, M., Munoz-Guijosa, J. and Molina-Aldareguia, J.
Additive Manufacturing of Nitinol for Smart Personalized Medical Devices: Current Capabilities and Challenges.
DOI: 10.5220/0012363900003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 123-134
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
123

For around two decades now, the AM of shape-
shifting systems or shape-morphing geometries has
been a research topic that has grown in parallel to the
progressive advances in AMTs and related usable
materials. The processing of active, multifunctional
or smart materials, capable of responding to external
stimuli, employing AMTs and rapid prototyping
processes, initiated a field that would be subsequently
referred to as “4D printing”. Synergic integration of
innovative materials, wise designs for self-assembly
or improved deployment, interlocked structures,
printable mechanisms and advanced manufacturing
technologies promoted a steady growth of 4D printing
(Tibbits, 2012, 2014, Ge, 2013, 2014).
Towards higher performance, the AM of smart
alloys has been also a topic of research for more than
a decade, although connections to 4D printed medical
devices are much more recent.
Pioneering studies dealt with the ultrasonic AM
of composites with aluminum matrices and embedded
shape memory nickel-titanium (NiTi or “nitinol”)
alloy, magnetostrictive galfenol and electroactive
PVDF phases, approaching active and self-sensing
structures (Hahnlen, 2010). Ultrasonic consolidation,
a hybrid manufacturing process that embeds fibers
into metal matrices, was also explored and led to NiTi
shape memory alloy (SMA) fibers into aluminum
(Al) matrices, mainly for structural applications
(Friel, 2011).
Regarding more adequate alloys for the
biomedical industry, the precise, efficient and
sustainable AM of highly biocompatible shape-
memory alloys from the NiTi family has been a long-
held dream that is currently at hand. Indeed, in spite
of the tricky processability of NiTi, different studies
have demonstrated that it can be additively processed,
which opens a plethora of applications (Lee, 2017). A
turning point is marked by different technologies
enabling the AM of NiTi, whose limits and potentials
have been analyzed (Van Hunbeeck, 2018).
Healthcare stands out as an extremely interesting
fields for 4D printed NiTi devices, although some
limitations still need to be addressed. Among them,
the finding of final medical applications, in which the
benefits of AMTs combined with the smartness of
NiTi can be exploited, for outperforming the current
gold standards, is also essential.
Accordingly, as presented in this study, exploring
new concepts for personalized, shape-morphing,
minimally invasive and evolutive biomedical devices
seems a very adequate strategy for taking advantage
of the mentioned benefits linked to the printability of
superelastic and shape-memory alloys.
Furthermore, unprecedented design and
manufacturing approaches, like the combined use of
both shape-memory and superelastic properties of
different kinds of NiTi, the employment of special
structures leading to NiTi metamaterials and the
creation of interwoven and interlocked NiTi
structures, to cite a few, may lead to a new generation
of 4D printed high-performance medical devices.
Considering all the above, this study analyzes the
current state, as regards the additive manufacturing of
NiTi, and the more promising approaches to obtain
4D printed nitinol medical devices. Furthermore,
different biomedical application concepts, benefiting
from DfAM methods are presented and illustrated
through printed prototypes. Main strategies for
empowering the shape-morphing performance of
these and related biodevices are also exemplified. At
the end of the paper, current limitations are described
in detail and a research roadmap is provided.
2 ADDITIVE MANUFACTURING
OF NITINOL: CURRENT STATE
2.1 Shape-Memory and Superelastic
Nitinol as Biomedical Materials
SMAs constitute a family of high-performance smart
materials capable of recovering an original shape
after being significantly deformed, just by heating
over the transformation shape temperature (shape-
memory effect). At the high temperature phase, the
deformation can be recovered just by releasing the
applied stress (superelasticity). Transformations
between the low temperature phase (martensite) and
the high temperature phase (austenite) or between
austenite and stress-induced martensite are involved.
NiTi, FeMnSi, CuZnAl and CuAlNi stand out as
polycrystalline SMAs with the previously described
transformations (Huang, 2002, De la Flor, 2005).
However, due to its special relevance, outstanding
thermomechanical properties (including shape-
memory and superelasticity) and biomedical aptitude,
our team is focusing on the enhanced AM of the
nitinol family and its application to biomedical
devices. Indeed, NiTi has been widely used in
healthcare, for instance in the cardiovascular field in
blood-contacting devices (i.e. stents, heart valves), in
orthopedics as bone substitutes and in orthodontics as
orthodontic fixators, among other applications (e.g.
otolaryngology, neurosurgery, ophthalmology,
urology, gynecology) (Auricchio, 2021).
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
124

Growing evidence suggests that NiTi alloys are
biocompatible due to the protective titanium-based
oxide layer on its surface (Elsisy, 2021). Nonetheless,
one major drawback impairing these alloys
biocompatibility is the content of Ni that can be
released from the surface into the surrounding
microenvironment and their associated toxicity
(Nasakina, 2019). Another concern that has been
associated with the use NiTi is surface-induced
thrombus formation (Gegenschatz-Schmid, 2022). In
this sense, several surface treatments have been
studied in order to improve NiTi biocompatibility.
The additive processability of NiTi may lead to a
very promising freedom of creation for highly
innovative medical devices, but necessasrily brings
also new unknowns to its biocompatibility, which
should be carefully addressed, as further discussed in
the research outline.
Regarding biomedical applicability, both
superelastic and shape-memory nitinol alloys have
their own application areas:
On the one hand, thermally activated nitinol can
be employed for the controlled deployment of a
surgical tool that may perform mechanical and
thermal ablation at the same time. Although the high
activation temperature is normally inadequate in
healthcare, composition modifications may lead to
activations at room temperature, thus opening a wider
set of applications.
On the other hand, superelastic nitinol has led to
several minimally invasive procedures based on self-
expanding surgical tools and implants. A current
research challenge is the sensitivity of these
outstanding thermomechanical properties to the
actual composition and processing conditions, as
described in sections 5.1 and 5.2.
2.2 Additive Manufacturing Strategies
for 3D/4D Printed Nitinol
Biodevices
Selective laser sintering (SLS) and selective laser
melting (SLM) or laser powder bed fusion (LPBF)
have transformed metals’ research and enabled solid
freeform fabrication with a wide set of metals and
alloys and with new design metallic materials
including high entropy and superalloys. For example,
both shape-memory and superelastic NiTi samples
have been achieved by selective laser melting
(Shayesteh Moghaddam, 2019, Obeidi, 2021). Other
laser-based processes such as laser engineering net
shaping (LENS) and laser cladding have been also
reported as options for additively processing smart
alloys (Van Hunbeeck, 2018).
To some extent, other processes like electron
beam melting (EBM), fused deposition modeling
(FDM) and wire arc additive manufacturing
(WAAM) are also applicable to the processing of
smart alloys (Van Hunbeeck, 2018), yet without the
achieved impact of PBLF. Recently, electron beam
freeform fabrication (EBF3) has been highlighted as
technology capable of mitigating some of the
common challenges involved in the selective laser
melting of NiTi, such as impurity pick-up (C, O and
N) and part size limitation (Paiotti, 2019).
Among key enabling technologies, not yet
explored for the printing of shape-memory alloys, but
with remarkable potential considering their recent
impact for extreme quality parts in high performance
materials, it is important to highlight lithography-
based methods. These lithographic techniques evolve
from the widespread digital light processing (DLP) of
photopolymers but employ slurries, in which a
polymeric matrix additively processed includes high
quantities of ceramic or metallic particles.
For instance, lithography-based ceramic
manufacturing technique (LCM) provides the most
remarkable precision and versatility in ceramic AM,
as it can process several kinds of ceramics, including
smart piezoceramics, magnetic ceramics and multi-
ceramic components (Schwentenwein, 2014). The
same principle is being applied to metallic slurries, as
an emergent process and could prove viable for the
AM of NiTi, even in the micro/nano-scale using two-
photon polymerization of metal (Vyatskikh, 2018).
2.3 Other Key Enabling Technologies
for 3D/4D Printed Nitinol
Biodevices
There are several methods by which SMAs can be
heated and activated. Among the most widely used
for this purpose are: 1) Joule, resistive heating or
direct Ohmic heating, 2) convective heating, and 3)
inductive heating (Qiu, 2001). These methods have
been validated with both conventional NiTi alloys
and with additively manufactured ones, employing
powder bed laser fusion, by our team.
Resistive heating is the most widely used heating
methodology as SMA are conductive alloys with low
resistance, but such a method is generally current-
limited to small and slim geometries. It depends on
the electrical resistivity of the SMA actuator. To cite
a biomedical example, an artificial urethral valve was
developed with NiTi wires as actuators that replace
the urinary sphincter muscles and by resistive heating
the opening and closing functions are controlled
(Chonan, 1997).
Additive Manufacturing of Nitinol for Smart Personalized Medical Devices: Current Capabilities and Challenges
125

Convective heating depends on the heat transfer
coefficient of the SMA. Convective heating using
water for rapid heating and cooling was applied to an
artificial muscle designed based on a spring bundle
actuator (Park, 2019) and to a bioinspired design of
vascular “blood vessel” that includes within a wet
SMA actuator (Mascaro, 2003). Microvascular
actuators demonstrated adequate for the convective
heating of shape-memory polymers (Díaz Lantada,
2016), which could be directly translated to SMAs,
once their high-precision 3D printing is mastered.
Induction heating seems to be a very good option
for the future of the medical field, since contactless
heating may be accomplished. With induction heating
SMA can be uniformly heated resulting in better
thermal control and higher heating rate. It depends
mostly on the magnetic permeability of the SMA, size
of the sample and applied frequency and magnetic
field intensity (Saunders, 2016). For instance,
transcutaneous induction heating was used to heat an
orthopedic shape memory implant within biological
tissue (Pfeifer, 2013, Müller, 2014). In addition,
safety studies, where stents were inducted heated,
were assessed as a potentially new method to treat
esophageal cancer (Zhou, 2009).
3 BIOMEDICAL APPLICATION
CONCEPTS FOR 3D/4D
PRINTED NITINOL
3.1 Materials
To explore the printability of NiTi and its versatility
for developing innovative medical technologies, two
powder compositions obtained by gas atomization
were acquired (Fort Wayne Metals). Mean particle
diameter was c.a. 30μm for both powders. One batch
had a composition closer to the equiatomic, for
achieving shape-memory properties, the other with
around an additional 1% of Ni to reach superelastic
properties.
3.2 Methods
Design of a collection of test probes and medical
devices concepts, such as vascular stents, heart valve
structures, surgical tools…, were carried out at UPM
employing a combination of software resources,
including: NX (Siemens PLM Solutions) for
geometrical modeling and n-Topology (nTop) for
topology/topography optimization.
Manufacturing of the different test probes and
conceptual biodevices presented in this study was
performed at IMDEA Materials Institute by laser
powder bed fusion. A Renishaw AM400 was
employed as additive manufacturing system. Final
post-processing included cleaning and electro-
polishing to achieve an adequate surface roughness,
whose impact in biocompatibility is further analyzed
in section 5.1. Overall, the process followed
recommendations from previous studies with slight
modifications due to specific software/hardware
constraints (Gan, 2021, Mani, 2022).
3.3 Innovative Concepts for 3D/4D
Printed Nitinol Biodevices
Figure 1 presents a collection of stents and heart valve
structures with systematic variations to illustrate the
viability and versatility of the additive processing of
Ni-Ti alloys.
Figure 1: Collection of stents and structures for heart valve
replacement obtained by LPBF of superelastic NiTi.
Designs: UPM, prototypes: IMDEA Materials Institute, as
in all figures presented in the study (iMPLANTS-CM
“Synergy” project from Comunidad de Madrid, Spain).
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
126
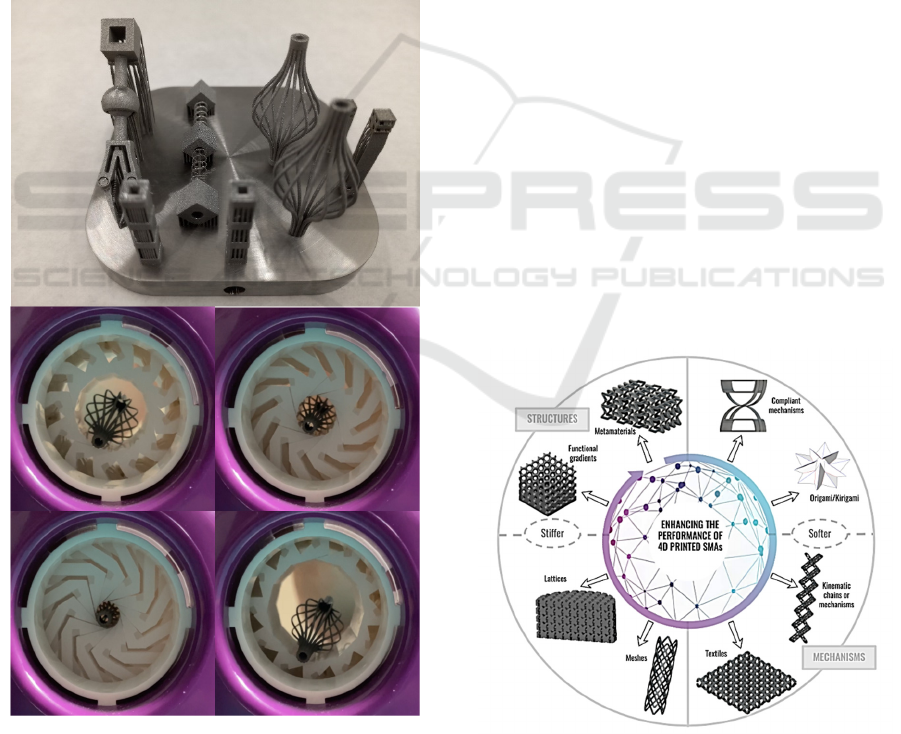
Classical manufacturing processes have led to a
very successful implementation of superelastic Ni-Ti
in medical practice, especially for self-expandable
vascular stents and valve structures. However, those
mass-produced devices do not really account for
some patients with unique morphological features. It
is precisely in this field, in which AM Ni-Ti would
prove competitive, for example for heart valve
structure with slightly elliptical cross-sections to
minimize perivalvular leakage or for Y-shaped stents
for the treatment of aneurysms.
After demonstrating the viability of obtaining the
more classical vascular devices other concepts
presented below in figure 3 and in section 4 are also
explored, such as: surgical manipulators or grippers,
functionally graded lattices as tissue engineering
scaffolds and minimally invasive kidney stone
retrieval baskets.
Figure 2: Proof-of-concept surgical actuators in NiTi.
Crimping test of a superelastic NiTi mesh conceived as
minimally invasive kidney stone retrieval basket.
4 EMPOWERING THE
SHAPE-MORPHING
PERFORMANCE
4.1 Overview of Strategies
Different principles applied to promoting the shape-
morphing behavior of shape-memory & superelastic
NiTi, are schematically presented in figure 3 and
experimentally demonstrated in the supporting videos
provided as companion for current paper. These will
be presented in the Biodevices conference and are
available for readers, on reasonable request, in case
the final publication will not share them openly. In
short, the supporting videos illustrate examples of 4D
printed actuators related to medical devices concepts
from previous section. S1 presents the successive
crimping and compliant response of a 4D printed
NiTi mesh, inspired by medical devices designed for
extracting strange bodies from inside the organism in
a minimally invasive way, such as kidney surgical
baskets and thrombectomy devices (as also presented
in figure 2). S2 presents a similar geometry to that of
S1, but 4D printed employing shape-memory NiTi
powder; the shape-memory training and recovery
processes are illustrated. S3 deals with a shape-
memory NiTi structure, counting with two structural
cubes connected by a compliant spring, which is
trained by pseudoplastic deformation and recovered
through heating. Finally, S4 and S5 present scaffolds
connected by compliant strips, respectively with
shape-memory and superelastic properties.
Figure 3: Examples of designed geometries, metamaterials,
mechanisms, textiles and other structures for empowering
the shape-memory performance of smart alloys, especially
shape-memory and superelastic NiTi.
Additive Manufacturing of Nitinol for Smart Personalized Medical Devices: Current Capabilities and Challenges
127

4.2 Biomechanical Metamaterials
Mechanical metamaterials, through designed
microstructures and solid freeform manufacturing
technologies, which are capable of defining matter in
3D, can be created with unique or exotic properties
not found in traditional synthetic materials (Kadic,
2012, Frenzel, 2017). Control over the elasticity
tensor, auxetics with negative Poisson ratio, atypical
thermal expansion coefficients and thermal
conductivity, unusual interactions with sound and
shock waves, among others, can be achieved for a
wide set of application fields (Kadic, 2019). Some
biological and biomechanical materials’ properties
can be mimicked with ad hoc designed metamaterials
and with combinations of metamaterials, lattices and
porous networks, which puts forward the interest of
these innovative geometries-structures-materials for
the development of biomedical implants. AMTs have
proven to be a fundamental booster for research in
this exciting area of metamaterials, whose properties
depend more on the actual designed microstructure
than on the characteristics of the raw materials
employed for their materialization. Interestingly,
shape-memory materials may synergize with
metamaterials’ structures to achieve enhanced shape-
morphing ability or to promote their superelastic
behavior. In consequence, investigating the
manufacturing of metamaterials employing SMAs is
proposed, as strategy for enhancing the performance
of 4D printed SMAs and devices based on them.
4.3 Origami Principles, Compliant,
Bistable & Multi-Stable Structures
Obtaining deployable structures with sufficient
stiffness in the deployed state is a very frequent
requirement in different types of applications: they
allow the transport in folded configuration of space
load-bearing structures, the minimally invasive
introduction by catheterization of medical devices
such as stents or valves, or the change of wing
geometry during the takeoff and landing of aircrafts.
Additionally, the large deflections involved in the
deployment process can be used to obtain compliant
actuators, very useful in applications such as robotic
manipulators and surgical biodevices. The energy
stored in the folding process and/or provided during
deployment can also allow high actuation forces with
very low control forces. Obtaining deployable
structures with these features and functionality
suitable for each application is based on three
fundamental considerations:
First, the design of stable structures in the
deployed state; second, the flexibility of the folding
zones; and third, the energy supply for the
deployment process. Traditionally, the physical
materialization of any of these concepts has been
subject to important restrictions, associated with the
use of plastic deformation or subtractive processes,
such as casting, folding, electrical discharge
machining or chip removal. These processes are
associated with important limitations for the
manufacture of deployable structures and compliant
actuators, both in size and geometry, being
complicated to obtain structures with characteristic
lengths less than 10
-2
m, with variable thicknesses or
morphological gradients, and usually being necessary
the assembly of different components to reach the
final system. The characteristics associated to
additive manufacturing and 4D printed components
make it possible to overcome the described
restrictions, which expands the horizons for
developing smart medical devices with
unprecedented functionalities and features. In
particular:
1) The manufacture of very small thickness features
and continuously variable thickness elements -
which allows for the tuning of the local flexural
stiffness and prevents the formation of stress
concentrators- which, in turn, facilitates:
a) Integral manufacturing of high performance
deployable structural concepts without the
need for assembly or mechanical hinges, such
as articulated, reticulated and chiral structures,
based on both flexibility and bistability, with
improved precision and repeatability.
b) The optimization of flexure hinges, and the
use of origami and auxetic geometries, that
could lead to deployable structures with
greater stiffness as well as a longer fatigue life.
2) The possibility of manufacturing along-the-
thickness morphological gradients would allow
asymmetrical sheets without the need for the use
of composite materials (Riley, 2020). Those
sheets, when cooled, would acquire bistable or
multi-stable characteristics. The same could be
achieved by using shape memory materials, which
would acquire these characteristics once the
transition temperature is reached.
3) The option of multi-material manufacturing
would allow, for example, the integration of shape
memory materials into bistable structural
concepts, giving rise to low-energy activation
actuators (Liu, 2019, Puthanveetil, 2022).
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
128

4.4 Functionally Graded Geometries
and Structures
In connection with the above, the AM of shape-
memory alloys can, not only lead to a biomimetic
performance of the developed medical devices, but
also help to reach singular smart properties like
increased shape-morphing ability, step-by-step
actuations or even quasi-autonomous responses.
Thanks to the careful design of functionally
graded geometries and structures, such features are
possible. For example, it is feasible to create
structural and functional units in the same device, to
employ topology optimization procedures to fine-
tune the compliance of different regions of the device,
and to use conformal lattice design methods to map
different unit cells of well-know properties within the
actual medical device (Feng, 2018). These methods
are already impacting the field of tissue engineering
with different alloys, ceramics and polymers, whose
additive processing and designs including
functionally graded structures lead to biomimetic
structures for repairing tissues with nonhomogeneous
density like bone. The processability of superelastic
NiTi can provide an extra design freedom and
bioinspired implants for replacing bones and bones
connected to other bones through cartilaginous joints
may be possible. The regeneration of the sternal-rib
complex and innovative spine tissue engineering
approaches can also benefit from these ongoing
research directions. Besides, functionally gradients of
porosity could even be applied to the creation of
biomimetic microvascular structures within the smart
medical devices, for enabling convective heating /
cooling from within, for enhanced control of the
activation, as already demonstrated with shape-
memory polymer concepts for surgical devices (Díaz
Lantada, 2016).
Through additive manufacturing, function and
structure become more interwoven than ever before
and multifunctional structures are achievable. The use
of smart alloys increases the number of achievable
functionalities. Thinking about the future, as
discussed in section 5.2, compliant regions for
biobots with active SMA wires or “muscles”
integrated in their structures, may lead towards smart
and living materials and structures with NiTi chassis.
Related schematic concepts and prototypes for an
innovative robotic hand (both showing a single finger
and an index-thumb couple) are presented in figure 4.
Figure 4: Innovative concepts for lightweight and smart
robotic hand: CAD models and prototypes by LPBF of
shape-memory NiTi. A single finger and an index-thumb
couple are illustrated. Both benefit from the use of
functional gradients of porosity and from the incorporation
of flexible hinges between phalanxes for enhanced
compliance and consequent actuation range.
4.5 Functionally Graded Alloys
Another way of achieving functional gradients, to
obtain design-controlled properties at every point of
a smart SMA-based biodevice, is the employment of
functional gradients of composition across the
structure. This leads to functionally graded alloys,
which combined with functionally graded geometries
and structures increase the freedom of design and
actuation of smart systems based on SMAs. The
potential creation of well-defined shape-memory and
superelastic regions in a single device, thanks to
controlling the composition of deposited NiTi powder
or the processing conditions layer-by-layer, or even
within each single layer, is of special relevance.
Pioneering examples have demonstrated that it is
possible to modify the processing conditions of
selective laser melting to obtain superelastic NiTi
without postprocessing. Arguably this effect could be
employed to spatially control the phases of NiTi
within complex printed parts. Another option may be
to resort to laser-based multiple metallic material
additive manufacturing with different compositions
of NiTi raw powders (Shayesteh Moghaddam, 2019,
Wei, 2021).
Additive Manufacturing of Nitinol for Smart Personalized Medical Devices: Current Capabilities and Challenges
129

4.6 Printed Kinematic Joints
(Mechanisms) and Textiles
An additional degree of compliance can be obtained
by printing mechanisms or textiles, for achieving
highly deformable NiTi structures, to which active
NiTi elements can be incorporated as driving
actuators. Arguable this can lead to empowering the
shape-morphing magnitude of the smart biodevices
and promote precise micromanipulation. In this way,
the traditional MEMS-related processes, applied to
the creation of NiTi surgical tools and micro-
manipulators, could be complemented with 3D/4D
printed micro-mechanisms, having the desired
number of degrees of freedom depending on the
application, and aimed at providing innovative
configurations for more versatile interactions with the
human body. The topology optimized shape-memory
NiTi actuator of figure 5, which includes a spherical
joint with 3 degrees of freedom and distal grippers
with revolute joints and shape-memory actuation,
provides an example of the achievable complexity.
Figure 5: Design and prototype of shape-memory NiTi
gripper. LPBF-printed biodevices (stents and actuarors).
Similar design principles applied to textiles can
lead to innovative NiTi meshes for implants like
stents, gastric bands and septal occluders. Surgical
devices like thrombectomy and lithoextraction
systems can also rely on SMA textiles. Interestingly,
the use of woven or knitted 3D printed meshes
importantly increases the compliance of superelastic
biodevices, as illustrated in figure 6 with a conceptual
superelastic NiTi structure mimicking an esophageal
stent.
Figure 6: Prototypes of computational-driven design of a
knitted mesh: LPBF-printed superelastic NiTi biodevices
imitating the size and features of esophageal stents. Simple
manipulation by hand illustrates the remarkable compliance
of the achieved woven structure.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
130

5 TOWARDS THE FUTURE
5.1 Current Limitations and
Challenges
The potentials of AMTs for the personalization of
healthcare are evident and involve varied subfields,
such as patient-oriented surgical training and
planning, support to personalized surgical practice,
development of patient specific implants for soft and
hard tissues, and personalized solutions for tissue
engineering, regenerative medicine and
biofabrication. However, most experiences still are
experimental and rely on a written prescription,
which limits their incorporation to the standard
procedures at hospitals, in part due to a current lack
of standardization (and almost absent regulation
harmonization) surrounding patient-specific medical
devices obtained additively from patients’ medical
images. The incorporation of active materials, such as
shape-memory alloys, to patient-specific implants
brings new concerns, which should be analyzed and
discussed by the communities of researchers,
materials and technology developers, medical devices
manufacturers, healthcare practitioners, end users and
patients, standardization entities, regulators and
regulatory bodies. Only through the definition of
shared good practices will these medical solutions,
based on 4D printed smart alloys, deploy their
transformative potentials. The creation of specific
technical committees for standardization, the sharing
of advances through open publications and the
involvement of users in these biomedical
transformations, are among the good practices that
should be promoted.
Among technological challenges, one of the most
critical for LPBF of NiTi alloys is to identify the
optimum processing window for fabricating parts
with desirable mechanical and functional properties.
This has been widely studied in the literature. A wide
range of volumetric energy densities (VED) have
been reported in the literature by manipulating the
input laser power, scanning velocity, hatch spacing
and layer thickness to find optimal combinations,
from as low as 38 J/mm
3
(Gan, 2021) to as high as
750 J/mm
3
(Zhao, 2020). Hence, due to the high
sensitivity of NiTi SMAs and the possible hardware
and software differences in each commercial AM
system, process parameter sets generated by one
group might be rendered unusable by other
researchers (Xue, 2021). Nevertheless, a relatively
low energy density range (between 55 and 100
J/mm
3
) is commonly observed to provide the right
combination of desired properties.
Furthermore, the surface finish required by most
biomedical applications is another one of the critical
postprocessing issues that must be faced in future.
The printed parts are characterized by a rough
surface, including the presence of un-melted powder
particles left by LPBF. Surface modifications will be
thus required both to smooth the rough finish left by
LPBF and to impose the appropriate oxide layer to
optimize corrosion resistance and minimize Ni
release, which can be toxic, allergenic, and
carcinogenic depending on the amount released and
length of exposure. This is common to any
manufacturing method of nitinol implantable devices
and several reviews can be found in literature about
finishing methods, including chemical etching,
mechanical polishing, electropolishing and/or
thermal treatments (Mani, 2022). However, the
optimization of these surface treatments for LPBF
produced parts remains a challenge that must be
tackled in future.
Deepening into biocompatibility, a successful
medical device should not rely on the simple use of a
biocompatible material. The in vivo response and the
success of an implant in the body depends on multiple
variables, including aspects from the design,
composition, microstructure, surface properties, and
interaction of the material with cells and body fluids.
In addition, the applications also play a crucial role in
the material's performance. Static and dynamic
systems, for instance, consider different variables and
phenomena where mass transfer and diffusion of
elements help to compensate and equilibrate the
complete system.
SMAs are a promising tool to obtain engineered
tissue constructs and to treat tissues with complex
geometry and restricted access. However, there are
still some concerns about this topic regarding the
biological response (Wen, 2018). Among other
biomaterial properties, cell-material interactions may
be influenced by surface roughness, wettability and
chemical composition, which are well known to
determine the fate of cells. In any case, this will be
highly dependent on the cell type and the tissue to
regenerate. For instance, materials with high
roughness have been reported as suitable for inducing
osteointegration for bone implants since the surface
area to anchor the bone is more considerable, favoring
the cell adhesion. In the case of stents, roughness has
greatly influenced hemocompatibility (Wang, 2010)
or modulated endothelial and smooth muscle cell
functions (Khang, 2010), thus arising as an important
parameter to consider for the design and manufacture
of vascular stents.
Additive Manufacturing of Nitinol for Smart Personalized Medical Devices: Current Capabilities and Challenges
131
Another big challenge of 4D bioprinted structures
is their in situ delivery and activation. NiTi implants
activation or self-organization by external stimulus
without affecting the cell environment is still a big
challenge. The responsive effect of these materials to
multiple physiological signals can be modified in a
controlled way (inducing growth tissue or destroying
it, if necessary, as in the case of tumoral tissue). The
quid of the question is to identify the proper stimulus
to activate the system at the right moment, precisely,
and without causing acute side effects by affecting
multiple physiological processes (Sinha, 2020). One
promising application for these NiTi SMAs structures
is as drug delivery systems (Lukin, 2019). Through
these devices, it is possible to have precise control not
only on the spatial distribution, by the material
organization of the implant, but also on the release of
drugs or cells in a programable manner.
Among other biological-related aspects, it is
important to consider the ability to form complex
structures with multi-materials and structures that can
stimulate a stratified tissue considering the different
cell groups and specifications (Ionov, 2018). Arteries
and osteochondral regions are among the functionally
graded tissues potentially benefiting from additively
manufacturing SMAs.
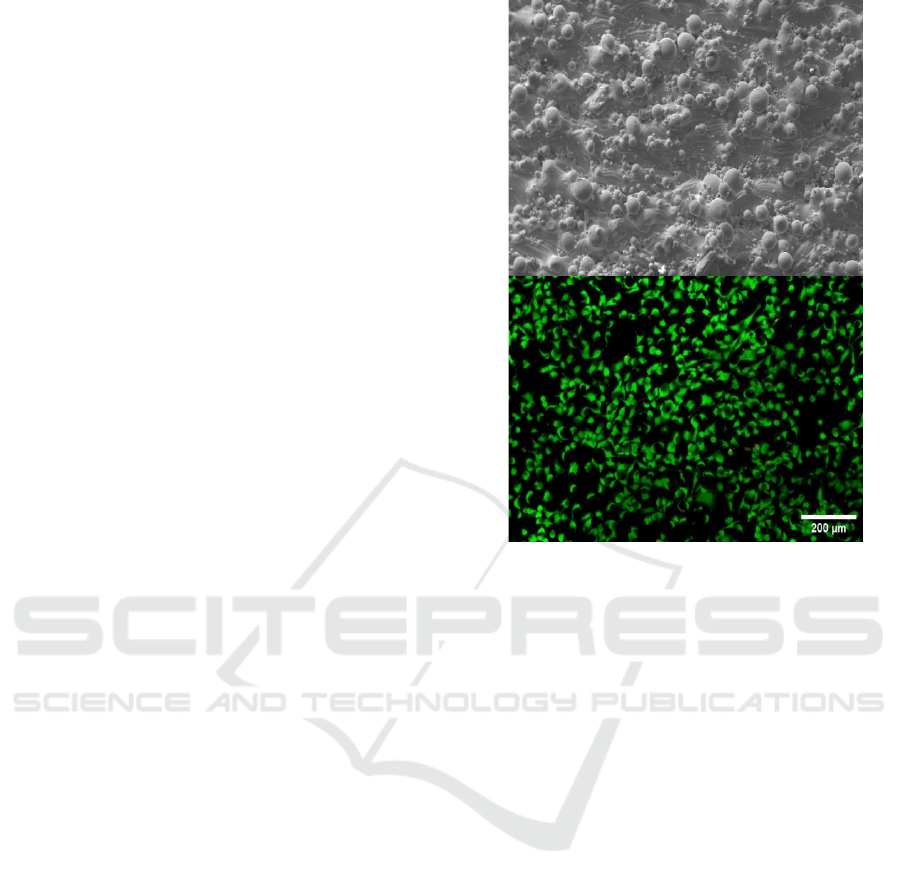
Summarizing, although preliminary results are
promising regarding the biocompatibility of
additively processed NiTi alloys for future
biomedical applications (f.e. figure 7), it is necessary
to further study several aspects, as detailed below.
5.2 Research Perspective
AMTs are continuously advancing and are expected
to lead to the processing of a wider range of NiTi
compositions and other SMAs. These combinations
may lead to high-performance devices, although their
biomedical applicability should be cautiously
analyzed, following guidelines from ISO Standard
10993.
In parallel, apart from increasing the portfolio of
additively processable alloys, other interesting
combinations between polymers and alloys, both with
shape-memory properties, should be studied for
achieving more versatile systems, capable of multiple
or stepped actuations, triggered at different
temperatures or by a variety of external stimuli, to
reach a new generation of smarter medical devices.
Among examples of the benefits of combining soft
polymeric phases with embedded SMA actuators it is
important to highlight the research by Akbari and cols
(Akbari, 2021).
Figure 7: Direct viability test, in which endothelial cells
(EA.hy926) are seeded upon a directly 3D printed surface
of NiTi (SEM image). The lower image shows live cells in
green and dead cells in red.
Together with the urgent need for internationally
accepted standards dealing with the safe and
straightforward development of high-quality
personalized medical implants, other ethical, legal
and social aspects demand careful attention for
making 4D printed SMAs truly transformative for
healthcare. Among them, healthcare technology
equity is probably the most remarkable. The key
question is: how do we accomplish that game-
changing technologies, such as high-performance
AMTs, come closer to the patients and users, in both
physical and economic terms, hence ensuring
accessible medical devices for all?
Challenging though it may be, some innovative
and inspiring equity-fostering approaches to
healthcare and production, in which additive
manufacturing technologies are key players, can be
highlighted. Do-it-yourself communities of “makers”
have already transformed several industrial fields and
their creative power, adequately canalized and
mentored to comply with internationally accepted
standards and applicable regulations, in connection
with open-source medical device initiatives, is
becoming a remarkable trend.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
132

Thanks to open-innovation environments and to
the promotion of open-source approaches, the whole
development life-cycle can be arguably optimized
and reach to safer products by enhanced design peer-
review (De Maria, 2020). Regarding production and
supply chain issues, interconnected global networks
of additive manufacturing systems, working with
similar quality control procedures, are bound to
promote the print-on-demand of personalized
medical devices and to bring the production closer to
the point-of-care. As regards the accessibility of
expensive AM systems, strategies for making them
available to the community should be also explored.
ACKNOWLEDGEMENTS
The research presented has been supported by the
following research and innovation project:
“iMPLANTS-CM”, from the “Convocatoria 2020 de
ayudas para la realización de proyectos sinérgicos de
I+D en nuevas y emergentes áreas científicas en la
frontera de la ciencia y de naturaleza interdisciplinar”
funded by Comunidad Autónoma de Madrid
(reference: Y2020/BIO-6756).
REFERENCES
H. Lipson, “The Shape of Things to Come: Frontiers in
Additive Manufacturing,” 2011.
S. Yang and Y. F. Zhao, “Additive manufacturing-enabled
design theory and methodology: a critical review,” The
International Journal of Advanced Manufacturing
Technology, vol. 80, no. 1–4, pp. 327–342, Sep. 2015.
M. K. Thompson et al., “Design for Additive
Manufacturing: Trends, opportunities, considerations,
and constraints,” CIRP Annals, vol. 65, no. 2, pp. 737–
760, 2016.
S. Tibbits, “Design to Self-Assembly,” Architectural
Design, vol. 82, no. 2, pp. 68–73, Mar. 2012.
S. Tibbits and K. Cheung, “Programmable materials for
architectural assembly and automation,” Assembly
Automation, vol. 32, no. 3, pp. 216–225, Jul. 2012.
Q. Ge, H. J. Qi, and M. L. Dunn, “Active materials by four-
dimension printing,” Appl Phys Lett, vol. 103, no. 13,
p. 131901, Sep. 2013.
Q. Ge, C. K. Dunn, H. J. Qi, and M. L. Dunn, “Active
origami by 4D printing,” Smart Mater Struct, vol. 23,
no. 9, p. 094007, Sep. 2014.
R. Hahnlen and M. J. Dapino, “Active metal-matrix
composites with embedded smart materials by
ultrasonic additive manufacturing,” Mar. 2010, p.
76450O.
R. J. Friel, “Investigating the effect of ultrasonic
consolidation on shape memory alloy fibres,” 2011.
A. Y. Lee, J. An, and C. K. Chua, “Two-Way 4D Printing:
A Review on the Reversibility of 3D-Printed Shape
Memory Materials,” Engineering, vol. 3, no. 5, pp.
663–674, Oct. 2017.
J. Van Humbeeck, “Additive Manufacturing of Shape
Memory Alloys,” Shape Memory and Superelasticity,
vol. 4, no. 2, pp. 309–312, Jun. 2018.
W. Huang, “On the selection of shape memory alloys for
actuators,” Mater Des, vol. 23, no. 1, pp. 11–19, Feb.
2002.
S. de la Flor, “Simulación numérica y correlación
experimental de las propiedades mecánicas en las
aleaciones con memoria de forma.,” Tesis, Universitat
Politècnica de Catalunya, Barcelona, 2005.
F. Auricchio, E. Boatti, M. Conti, and S. Marconi, “SMA
biomedical applications,” in Shape Memory Alloy
Engineering, Elsevier, 2021, pp. 627–658.
M. Elsisy, M. Shayan, Y. Chen, B. W. Tillman, C. Go, and
Y. Chun, “Assessment of mechanical and
biocompatible performance of ultra-large nitinol
endovascular devices fabricated via a low-energy laser
joining process,” J Biomater Appl, vol. 36, no. 2, pp.
332–345, Aug. 2021.
Nasakina, Sudarchikova, Sergienko, Konushkin, and
Sevost’yanov, “Ion Release and Surface
Characterization of Nanostructured Nitinol during
Long-Term Testing,” Nanomaterials, vol. 9, no. 11, p.
1569, Nov. 2019.
K. Gegenschatz-Schmid et al., “Reduced thrombogenicity
of surface-treated Nitinol implants steered by altered
protein adsorption,” Acta Biomater, vol. 137, pp. 331–
345, Jan. 2022.
M. A. Obeidi et al., “Laser beam powder bed fusion of
nitinol shape memory alloy (SMA),” Journal of
Materials Research and Technology, vol. 14, pp. 2554–
2570, Sep. 2021.
N. Shayesteh Moghaddam et al., “Achieving superelasticity
in additively manufactured NiTi in compression
without post-process heat treatment,” Sci Rep, vol. 9,
no. 1, p. 41, Dec. 2019.
R. Paiotti Marcondes Guimaraes, F. Pixner, G. Trimmel,
and S. T. Amancio-Filho, “4-D Printing of NiTi Shape
Memory Alloys,” Sep. 2019. Accessed: Sep. 14, 2022.
M. Schwentenwein, P. Schneider, and J. Homa,
“Lithography-Based Ceramic Manufacturing: A Novel
Technique for Additive Manufacturing of High-
Performance Ceramics,” Oct. 2014, pp. 60–64.
A. Vyatskikh, S. Delalande, A. Kudo, X. Zhang, C. M.
Portela, and J. R. Greer, “Additive manufacturing of 3D
nano-architected metals,” Nat Commun, vol. 9, no. 1, p.
593, Dec. 2018.
J. Qiu, J. Tani, D. Osanai, Y. Urushiyama, and D.
Lewinnek, “High-speed response of SMA actuators,”
International Journal of Applied Electromagnetics and
Mechanics, vol. 12, no. 1–2, pp. 87–100, Feb. 2001.
S. Chonan et al., “Development of an artificial urethral
valve using SMA actuators,” Smart Mater Struct, vol.
6, no. 4, p. 004, Aug. 1997.
C. H. Park, K. J. Choi, and Y. S. Son, “Shape Memory
Alloy-Based Spring Bundle Actuator Controlled by
Additive Manufacturing of Nitinol for Smart Personalized Medical Devices: Current Capabilities and Challenges
133

Water Temperature,” IEEE/ASME Transactions on
Mechatronics, vol. 24, no. 4, pp. 1798–1807, Aug.
2019.
S. A. Mascaro and H. Harry Asada, “Wet Shape Memory
Alloy Actuators for Active Vasculated Robotic Flesh”.
A. Díaz Lantada, A. De Blas Romero, and E. C. Tanarro,
“Micro-vascular shape-memory polymer actuators with
complex geometries obtained by laser
stereolithography,” Smart Mater Struct, vol. 25, no. 6,
p. 065018, Jun. 2016.
R. Pfeifer et al., “Noninvasive induction implant heating:
An approach for contactless altering of mechanical
properties of shape memory implants,” Med Eng Phys,
vol. 35, no. 1, pp. 54–62, Jan. 2013.
C. W. Müller et al., “Transcutaneous electromagnetic
induction heating of an intramedullary nickel–titanium
shape memory implant,” Int Orthop, vol. 38, no. 12, pp.
2551–2557, Dec. 2014.
J. Zhou et al., “Hyperthermia by a nitinol stent in an
alternating magnetic field: Safety and feasibility in
rabbit esophageal cancer,” Progress in Natural Science,
vol. 19, no. 12, pp. 1713–1719, Dec. 2009.
R. N. Saunders, J. G. Boyd, D. J. Hartl, J. K. Brown, F. T.
Calkins, and D. C. Lagoudas, “A validated model for
induction heating of shape memory alloy actuators,”
Smart Mater Struct, vol. 25, no. 4, p. 045022, Apr.
2016.
M. Kadic, T. Bückmann, N. Stenger, M. Thiel, and M.
Wegener, “On the practicability of pentamode
mechanical metamaterials,” Appl Phys Lett, vol. 100,
no. 19, p. 191901, May 2012.
T. Frenzel, M. Kadic, and M. Wegener, “Three-
dimensional mechanical metamaterials with a twist,”
Science (1979), vol. 358, no. 6366, pp. 1072–1074,
2017.
M. Kadic, G. W. Milton, M. van Hecke, and M. Wegener,
“3D metamaterials,” Nature Reviews Physics, vol. 1,
no. 3, pp. 198–210, Mar. 2019.
K. S. Riley, K. J. Ang, K. A. Martin, W. K. Chan, J. A.
Faber, and A. F. Arrieta, “Encoding multiple permanent
shapes in 3D printed structures,” Mater Des, vol. 194,
p. 108888, Sep. 2020.
Y. Liu et al., “Synergistic effect enhanced shape recovery
behavior of metal-4D printed shape memory polymer
hybrid composites,” Compos B Eng, vol. 179, p.
107536, Dec. 2019.
S. Puthanveetil, W. C. Liu, K. S. Riley, A. F. Arrieta, and
H. le Ferrand, “Programmable multistability for 3D
printed reinforced multifunctional composites with
reversible shape change,” Compos Sci Technol, vol.
217, p. 109097, Jan. 2022.
J. Feng, J. Fu, Z. Lin, C. Shang, and B. Li, “A review of the
design methods of complex topology structures for 3D
printing”, doi: 10.1186/s42492-018-0004-3.
A. Díaz Lantada, A. De, B. Romero, and E. C. Tanarro,
“Micro-vascular shape-memory polymer actuators with
complex geometries obtained by laser
stereolithography,” 2016, doi: 10.1088/0964-
1726/25/6/065018.
N. Shayesteh Moghaddam et al., “Achieving superelasticity
in additively manufactured NiTi in compression
without post-process heat treatment,” Sci Rep, vol. 9,
no. 1, p. 41, Dec. 2019.
C. Wei, Z. Zhang, D. Cheng, Z. Sun, M. Zhu, and L. Li,
“An overview of laser-based multiple metallic material
additive manufacturing: from macro- to micro-scales,”
International Journal of Extreme Manufacturing, vol. 3,
no. 1, p. 012003, Jan. 2021.
J. Gan et al., “Effect of laser energy density on the evolution
of Ni4Ti3 precipitate and property of NiTi shape
memory alloys prepared by selective laser melting,” J
Alloys Compd, vol. 869, p. 159338, Jul. 2021.
C. Zhao, H. Liang, S. Luo, J. Yang, and Z. Wang, “The
effect of energy input on reaction, phase transition and
shape memory effect of NiTi alloy by selective laser
melting,” J Alloys Compd, vol. 817, p. 153288, Mar.
2020.
L. Xue et al., “Controlling martensitic transformation
characteristics in defect-free NiTi shape memory alloys
fabricated using laser powder bed fusion and a process
optimization framework,” Acta Mater, vol. 215, p.
117017, Aug. 2021.
G. Mani, D. Porter, K. Grove, S. Collins, A. Ornberg, and
R. Shulfer, “Surface finishing of Nitinol for implantable
medical devices: A review,” J Biomed Mater Res B
Appl Biomater, Jun. 2022.
C. Wen et al., “Mechanical behaviors and biomedical
applications of shape memory materials: A review,”
AIMS Mater Sci, vol. 5, no. 4, pp. 559–590, 2018.
B. L. Wang, L. Li, and Y. F. Zheng, “In vitro cytotoxicity
and hemocompatibility studies of Ti-Nb, Ti-Nb-Zr and
Ti-Nb-Hf biomedical shape memory alloys,”
Biomedical Materials, vol. 5, no. 4, p. 044102, Aug.
2010.
J. Lu, D. Khang, and T. J. Webster, “Greater endothelial
cell responses on submicron and nanometer rough
titanium surfaces,” J Biomed Mater Res A, vol. 9999A,
p. NA-NA, 2010.
S. K. Sinha, “Additive manufacturing (AM) of medical
devices and scaffolds for tissue engineering based on
3D and 4D printing,” in 3D and 4D Printing of Polymer
Nanocomposite Materials, Elsevier, 2020, pp. 119-160.
I. Lukin et al., “Can 4D bioprinting revolutionize drug
development?,” Expert Opin Drug Discov, vol. 14, no.
10, pp. 953–956, Oct. 2019.
L. Ionov, “4D Biofabrication: Materials, Methods, and
Applications,” Adv Healthc Mater, vol. 7, no. 17, p.
1800412, Sep. 2018.
S. Akbari, A. H. Sakhaei, S. Panjwani, K. Kowsari, and Q.
Ge, “Shape memory alloy based 3D printed composite
actuators with variable stiffness and large reversible
deformation,” Sens Actuators A Phys, vol. 321, p.
112598, Apr. 2021.
C. de Maria, L. di Pietro, A. Ravizza, A. D. Lantada, and A.
D. Ahluwalia, “Open-source medical devices:
Healthcare solutions for low-, middle-, and high-
resource settings,” Clinical Engineering Handbook,
Second Edition, pp. 7–14, Jan. 2020.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
134
