
Bioinspired Design and Manufacturing Strategies for next
Generation Medical Implants: Trends and Challenges
Andrés Díaz Lantada
a*
, Adrián Martínez Cendrero, Francisco Franco Martínez
b
,
Rodrigo Zapata Martínez
c
, Carlos Aguilar Vega
d
, William Solórzano-Requejo
e
and Alejandro De Blas De Miguel
Department of Mechanical Engineering, ETSI Industriales, Universidad Politécnica de Madrid, Madrid, Spain
Keywords: Bioinspiration & Biomimetics, Medical Devices, Engineering Design, Additive Manufacturing.
Abstract: Bioinspired design and manufacturing strategies are enabling radical innovations in healthcare and medical
devices. The complex, functionally graded, fractal, multifunctional geometries and structures of nature are
inspiring for conceiving highly transformative biomedical engineering solutions, but highly challenging to
replicate. Decades (if not centuries) of research, together with a convergent collection of recently developed
and emergent software and hardware resources, empower our biomimetic design and manufacturing abilities
and render truly bioinspired solutions feasible. Such convergence is analyzed in this study and connected with
the engineering of next generation implants, characterized for their life-like features or even with quasi-living
behaviors. Synergic design and manufacturing technologies with remarkable impact in implants innovation,
tissue engineering, biofabrication and engineered living materials are presented and illustrated by means of
different case studies. Current research trends and challenges are discussed.
1 INTRODUCTION
The complex geometries of nature (Mandelbrot,
1983, Place, 2009), characterized by functionally
graded structures, hierarchical features, multi-
material extracellular matrices, dynamic tissues and
living cells within, lead to highly precious features
from an engineering point of view. Indeed, natural
living entities stand out for being remarkably
lightweight, self-regulated, stimuli-responsive (or
even smart), eco-efficient and truly multifunctional,
which inspires designers (Benyus, 2002).
Achieving some of the mentioned characteristics
has been a long-held dream for many scientists and
technology developers and has made of biomimetics
a fruitful area of study (Bar-Cohen, 2006). The
impact of bioinspiration in healthcare is outstanding
and has led to the birth of research fields like tissue
engineering, biofabrication and, more recently,
engineered living materials (ELMs).
a
https://orcid.org/0000-0002-0358-9186
b
https://orcid.org/0000-0002-7894-7478
c
https://orcid.org/0000-0002-2611-7050
d
https://orcid.org/0000-0003-0291-3041
e
https://orcid.org/0000-0002-2989-9166
In our experience, traditional implants usually
lack carefully conceived biomimetic design features.
Besides, they do not frequently benefit from recent
and ongoing advances in the computational
modelling of complex-shaped objects and fractal,
hierarchical or multi-scale geometries. Seldom are
they manufactured as patient-specific or personalized
medical solutions, as mass-production is currently the
industrial standard. In consequence, the potential
benefits of employing additive manufacturing
technologies (AMTs), also known as solid freeform
fabrication resources, are frequently discarded.
The limited personalization and the shortage of
biomimetic design strategies lead to suboptimal
implants in terms of biomechanical performance. All
kinds of articular implants, acting like thick bulk
metallic nails anchored to the remaining bone, suffer
from dramatic stress shielding phenomena due to the
mechanical mismatches between employed alloys
and bones.
42
Lantada, A., Cendrero, A., Martínez, F., Martínez, R., Vega, C., Solórzano-Requejo, W. and Blas De Miguel, A.
Bioinspired Design and Manufacturing Strategies for next Generation Medical Implants: Trends and Challenges.
DOI: 10.5220/0012363800003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 42-53
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.

The compact structures employed for state-of-the-
art implants have nothing to do with the porous,
vascularized, functionally graded and multi-material
tissues present in bones and joints. Compliant
regions, such as those involving cartilage or
transitions between bones and ligaments or bones and
tendons are extremely complex to repair with mono-
material mass production techniques. In the
cardiovascular and neurological fields, it is common
to find stiff metallic implants interacting with very
soft tissues, which also generates undesired
biomechanical mismatches (Liverani, 2021). In
general, the bulk properties of synthetic materials
cannot match the combination of strength and
flexibility from natural ones. Derived synthetic
structures for potential biomedical implants are either
too stiff or lacking in strength.
Consequently, alternative biomimetic design and
manufacturing technologies are required for
modulating the stiffness of biomedical materials
usable for creating implants, without dramatically
affecting their strength and durability. This has been
a matter for research in the tissue engineering field for
more than two decades now and has been also
addressed, more recently, by the development and
application of biofabrication techniques. Important
advances have been achieved in terms of biomimetic
solutions, but their clinical impact is still very limited.
Next generation medical implants should be
conceived and developed according to new strategies
taking benefit of advanced design and manufacturing
technologies for enhanced biomimetics. In many
ways, these technologies transcend the classical
Bauhaus principle of “form follows function” (Droste,
2019), enabling a new engineering design paradigm.
In this reformulated approach, geometry, structure,
material and function are bound together, become
integral aspects of the same entity, thanks to the
freeform design input and the use of special
manufacturing resources that allow for a precise
definition of matter in three or even four dimensions.
Accordingly, the classical frontiers between
geometry, structure, material and function dissolve,
exactly as in natural living entities, which is also
pursued for next generation implants.
The following section presents some of the most
relevant bioinspired design features for next
generation implants. Subsequently, different design
strategies are presented, and synergic families of
manufacturing technologies discussed through use
cases. These involve AMTs, micro- and nano-
manufacturing resources, robotic technologies and
emergent synthetic biology related techniques.
2 DESIRED FEATURES FOR
“NEXT-GEN” IMPLANTS
2.1 Lightweight and Compliant
Natural materials and structures are consequence of
multi-objective optimizations achieved through
evolution and responses to environmental cues. The
structure of large bones in mammals, with the typical
external cortical region, inner trabecular core and
curvature (Bertram, 1988), are optimized for a
combination of bending and dynamic loads varying in
direction and constitute examples of lightweight
structures. In birds, skeletons have gone through
continuous adaptations to minimize the metabolic
cost of flight (Dumont, 2010). At the same time,
vertebrates count with different means for rendering
their bodies and biological structures compliant for a
better interaction with the external environment.
Kinematic chains of bones connected through
ligaments, like the spine and extremities, or the
cushion-like features of cartilage contribute to such
compliance. However, the medical device industry
has traditionally relied on highly stiff materials, like
steel or titanium alloys, and used mainly 100%
compact structures. These lack the desired
lightweight properties and compliance that biological
structures exhibit. Hence, innovative design and
manufacturing approaches are needed.
2.2 Functionally Graded
Biological structures also stand out for their usual
functional gradients of properties. The already
mentioned cortical-trabecular structure of bone is an
example of density, stiffness and strength gradients,
which also renders bone multifunctional by enabling
vascularization. Entheses, connective tissues between
tendon or ligament and bone, modulate stiffness
through functionally graded structures and by
combining different fibres, arrangements of extra-
cellular matrices and cells. Functional gradients of
properties are also found in synthetic biomechanical
replacements and are normally achieved by wise
geometrical designs, combinations of materials or
through functional coatings (Leong, 2008, Phillips,
2008). However, in order to perfectly mimic the
functionally gradients of biological materials and
structures, additional research is required. Taking
mechanobiology into account (Boccaccio, 2016,
Perier-Metz, 2022) constitutes a relevant design trend
for improved results.
Bioinspired Design and Manufacturing Strategies for next Generation Medical Implants: Trends and Challenges
43

2.3 Multi-Scale and Multi-Material
Essential functional gradients are also achieved in
nature thanks to the hierarchical, multi-scale or fractal
geometry that characterizes living tissues, organs and
systems. Our cells decode and transcribe DNA, our
lungs perform gas exchange, and our fingers play an
electric guitar or a piano thanks to the hierarchical
organization of our body. Nevertheless, multi-scale
features are not so common in classical medical
implants, apart from their increasingly frequent
hierarchical surfaces that lead to enhanced biological
interactions. These features indicate an interesting
path for bioinspired implant development.
At the same time, composites are very common in
biological structures. In the case of the human body,
ceramics and polymers co-exist in bones, whose
ceramic structure is interpenetrated by a polymeric
vascular network, and joints, with their osteochondral
transitions, to mention a couple of examples. Multi-
material articular prostheses, with ultra-high
molecular weight polyethylene capsules in between
metallic components, are common but their structures
and radical transitions not yet truly biomimetic.
2.4 Multifunctional and Smart
Functional gradients, combinations of constructive
building blocks and hierarchical structures render
biological systems multifunctional and smart, in the
sense of adequately responding or adapting to
external stimuli. Tissues perform several functions at
the same time: structural support, thermal stability,
energetic management, self-sensing, information
processing, acting, among others. These features are
seldom found in biomechanical replacements like
prostheses or orthoses. In fact, biological multi-
scaling and multifunctionality allows both for “plenty
of room at the bottom” (Feynman, 1959) and for
“plenty of room right here” (Bongard, 2023).
2.5 Dynamic and Living
Possibly the most challenging bioinspired properties
for next generation or “next-gen” implants are the
dynamism and liveness of biological entities. The
self-healing properties of biological structures, based
on extremely complex surveillance and repair
strategies; the natural mechanisms of growth and
biodegradation, which would be fundamental for
paediatric biomedical prostheses; the reconfigurable
and shape-morphing nature of several organs, to cite
a few, feature dynamism and liveliness. Still, they
prove extremely challenging to replicate.
3 BIOINSPIRED DEVELOPMENT
STRATEGIES
3.1 Lattices, Meshes and Woven
Structures
Straightforward combinations of computer-aided
design operations can rapidly lead to biomimetic
geometrical complexity built upon simple geometries
like cylindric trusses, square-section bars, spheric
pores, among others. Extrusions, lofts along splines,
Boolean and matrix-based design tools let us achieve
lattices, meshes and woven structures that imitate the
porous and compliant structures of several tissues. In
many cases, these geometries can be employed as
load bearing scaffolds for tissue repair, which have
set the foundations of tissue engineering and
biofabrication (Hutmacher, 2000, Harley, 2021). The
scaffolds, being porous, should allow for three-
dimensional cell culture, access to nutrients in vitro,
elimination of debris and vascularization in vivo. In
general, meshes and woven structures may be usable
for soft tissue repair, while lattices, depending on the
properties of raw materials employed, may lead to
advanced multipurpose implants.
3.2 FGMs and Hierarchical Structures
Despite the benefits of quasi-periodic repetitions for
easily designing biomedical constructs with some
biomimetic features, in many cases an additional
level of complexity involving hierarchical and
functionally graded materials (FGMs) can lead to
enhanced biomechanical and biological performance.
Progressively, along the last decade, CAD modelling
resources have been complemented with specific
modules or with dedicated packages aimed at
performing very relevant design operations from a
biomimetic point of view. Nowadays, topology
optimization resources, conformal lattice design
tools, algorithmic CAD modelling software, to cite
some options, enable the generation of networks and
porous structures within computational models, the
application of lattices to desired working volumes and
the use of recursive approaches to reach multi-scale
hierarchical structures. These are already making a
remarkable impact in biomedical implants innovation
(Wang, 2016). In parallel, classical CAD modelling
and methodical procedures can also lead to multi-
scale features. Some examples of lattices and meshes
with functional gradients and hierarchical structures
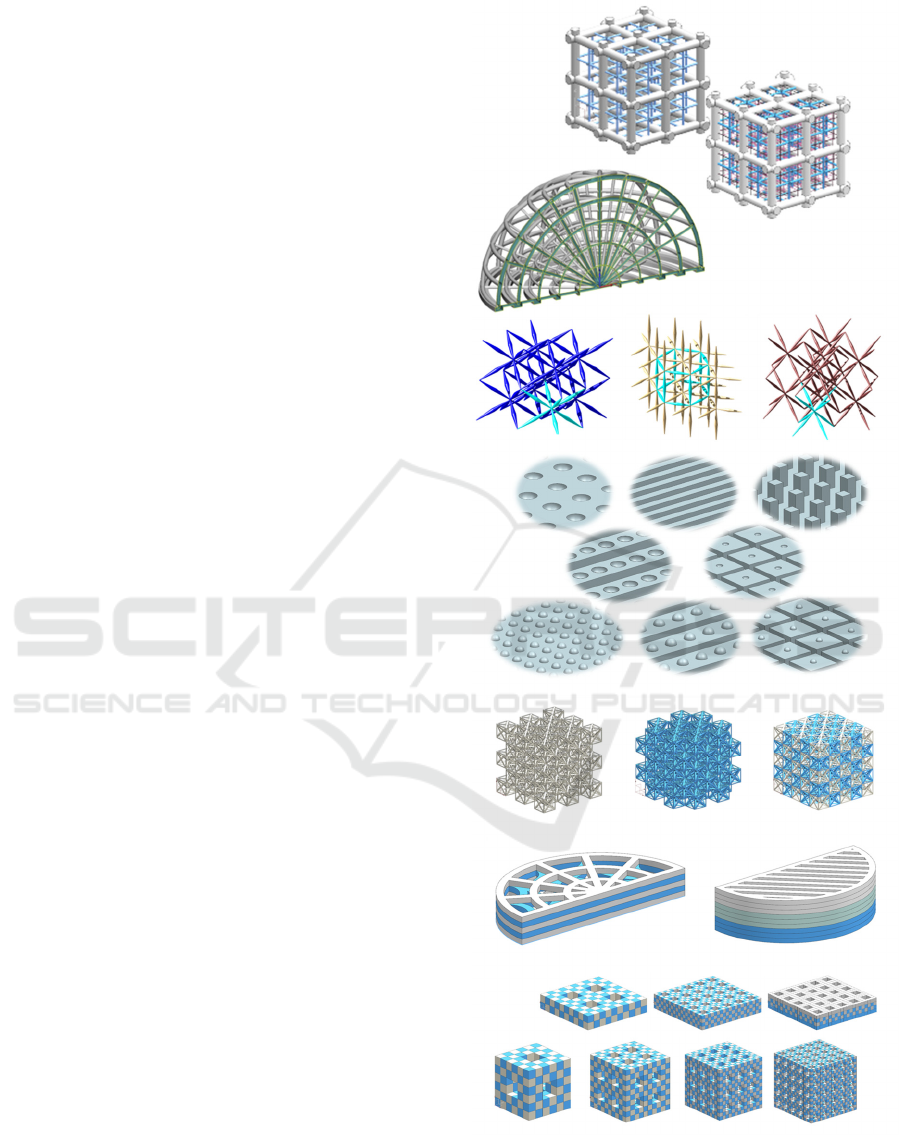
are shown in figure 1 to illustrate the already
achievable geometrical complexity.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
44
3.3 Metamaterials and Metasurfaces
The unconventional properties of biological materials
(stress-stiffening behaviours, unusual Poisson ratios,
stimuli-responsive abilities…) are challenging to
replicate with traditional materials. Metamaterials
and metasurfaces, thanks to their microstructures
being designed on purpose to achieve very unique
structural properties or surface interactions, constitute
an emergent path for creating biomimetic biodevices.
Their properties depend on their CAD-modelled
designed features more than on the raw materials
employed, which is remarkable. The advent of high-
performance AMTs is enabling their conceptual
application to healthcare (Zadpoor, 2019, 2020).
3.4 Composites, Digital Materials and
Voxelated Matter
Composite materials are also being reinvented by
computational design and manufacturing means,
which has led to concepts like digital materials and
voxelated matter (Bader, 2018, Skylar-Scott, 2019),
in which both structure and chemical composition are
precisely defined in 3D or 4D. Consequently, relevant
opportunities arise for biomedical implants better
imitating the composite and intricate structures and
compositions of living tissues.
3.5 Smart Materials and Structures
Smart materials and structures contribute to the final
biomimetic performance through enhanced multi-
functionality. In fact, smart, stimuli-responsive or
multi-functional materials incorporated to advanced
implants, may act as transducers for enabling self-
sensing and acting abilities. The possibility of
processing many of these families, such as shape-
memory polymers and alloys, piezoelectric materials,
electroactive polymers…, using solid freeform
fabrication technologies is bound to make their
incorporation to biodevices quite direct (Gardan,
2019).
3.6 Engineered Living Materials
Probably the ultimate degree of biomimicry may only
be achieved by resorting to biohybrid solutions, in
which synthetic materials, biological extracellular
matrices and living cells synergize, as in the case of
tissue engineering scaffolds, biofabricated constructs
and emergent engineered living materials (ELMs)
(Nguyen, 2018, Srubar III, 2020, Díaz Lantada,
2022).
Figure 1: Examples of CAD models showcasing different
bioinspired design strategies: multi-material and multi-
scale lattices, functionally graded structures, mechanical
metamaterials, microtextured biointerfaces, interwoven and
layered materials and voxelated matter.
Bioinspired Design and Manufacturing Strategies for next Generation Medical Implants: Trends and Challenges
45
4 ADVANCED COMPUTATIONAL
RESOURCES
4.1 CAD Modelling and Simulations
The bioinspired development strategies from section
3, as regards advanced computational resources for
design purposes, are illustrated in this section and
exemplified through different examples connected to
the design, simulation and optimization of different
biomedical implants. To start with, CAD modelling
supported by simulations constitutes the statu quo for
designing and optimizing engineering components.
Geometries from implants can be designed, even in
personalized ways using input from patients’ medical
images, and their biomechanical performance
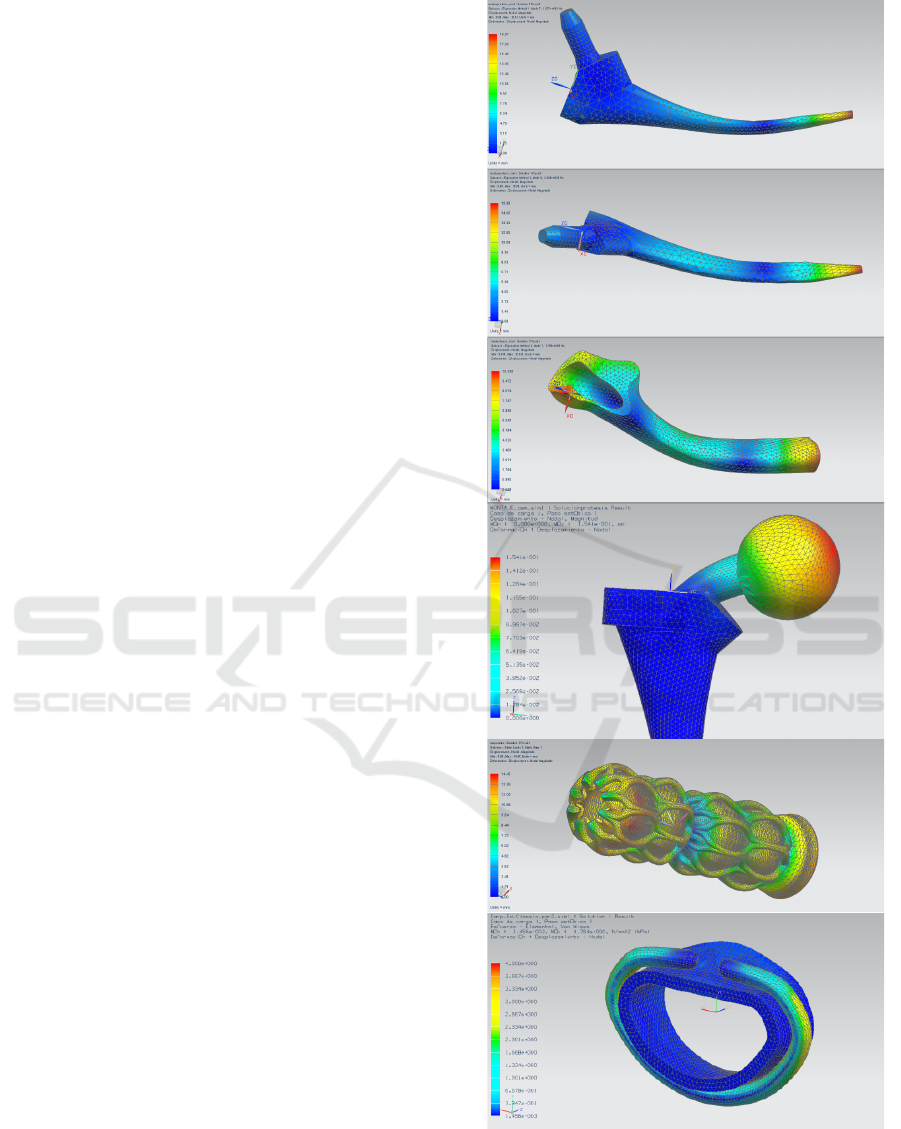
evaluated by simulations. Figure 2 presents examples
of the finite element method applied to assessing and
validating in silico different designs, before eventual
in vitro or ex vivo trials. These have been performed
with NX (Siemens PLM Solutions) as computational
modelling software. In fact, in silico methods (i.e.
simulations, digital twins…) are becoming more and
more relevant as an alternative to in vivo testing, even
for certification purposes, in a clear alignment with
the 3Rs principles (Tannenbaum, 2015).
4.2 Topology and Topography
Optimization
For an increased degree of biomimicry, the porous
intricate networks that conform human tissues and
their functional surface topographies should be taken
into account. To this end, topology and topography
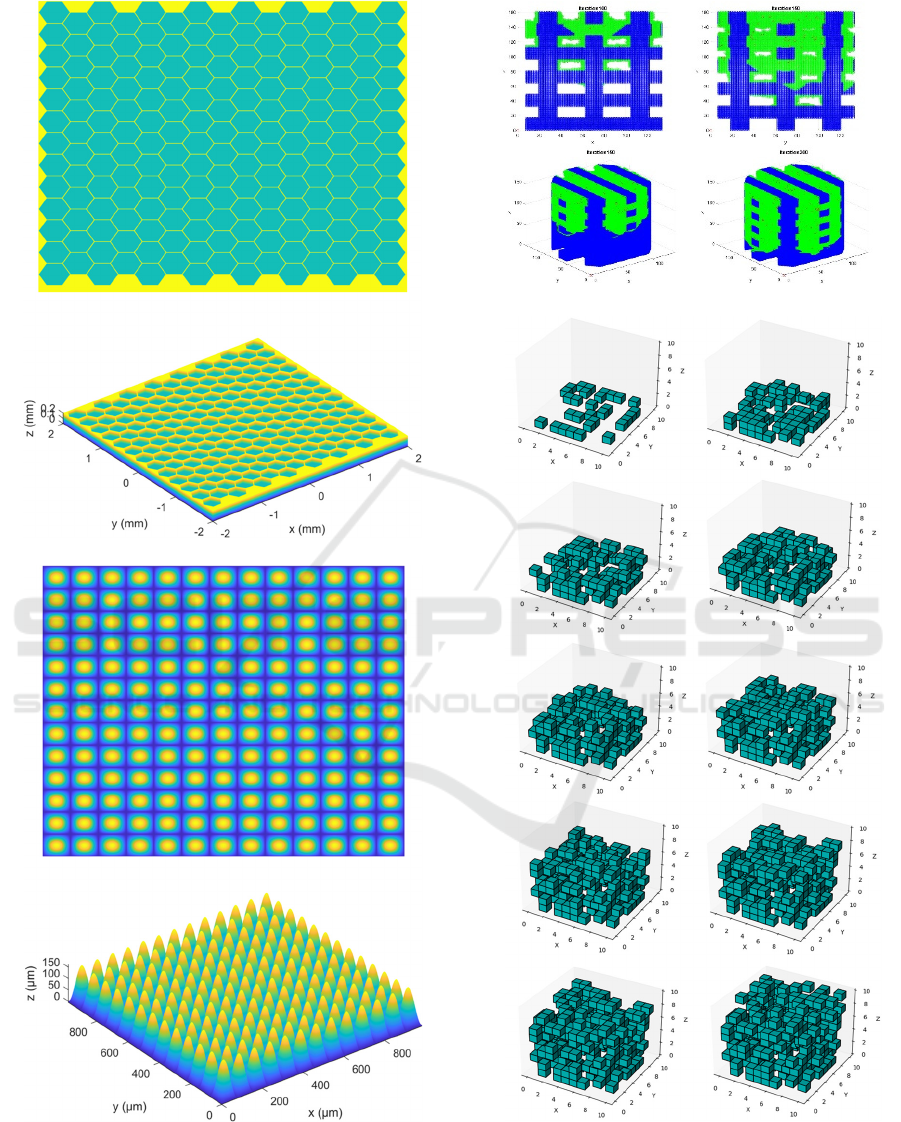
optimization resources, such as n-Topology and 3D
Coat, are a right choice. For instance, n-Topology (n-
Top) is applied to obtain the functionally graded and
bioinspired porous scaffolds designs of figure 3.
4.3 Math-Based Designs and
Algorithmic CAD
In a complementary way, math-based designs and
algorithmic CAD modelling are also usable for
achieving lattices, meshes, woven structures, textures
and metamaterials with biomimetic features. These
methods also apply to rapidly modifying parametric
designs for personalization purposes. By means of
example, figure 3 includes the algorithmic design of
a woven mesh for a stent-like device, while figure 4
presents the math-based design of innovative
biointerfaces (Franco Martínez, 2023).
Figure 2: FEM simulations upon CAD models (adapted
from Díaz Lantada, 2013): study of resonances in hip
prosthesis and femur, biomechanical performance of hip
replacement, interactions between transcatheter stent,
annuloplasty ring and surrounding tissues.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
46

Figure 3: Topology optimized and functionally graded
tissue engineering scaffolds (upper images). Algorithmic
design of woven meshes for stent-like medical devices with
improved mechanical compliance (lower images).
4.4 Agents Based Modelling and
AI-Based Approaches
The hierarchical fractal features of the extracellular
matrices, the presence of living entities in biological
structures and their particular responses to a myriad
of environmental cues lead to irregular and random
features, which are almost impossible to imitate with
classical design software. To account for these
particular characteristics, the employment of agents-
based modelling -in which cells, pixels or voxels
iteratively and autonomously evolve in a sort of
“game of life”- adequately integrated with CAD
modelling and artificial intelligence (AI) methods,
can be an interesting solution (Von Neumann, 1966,
Gardner, 1970).
As an example, figure 5a presents the cellular
automata-based modelling (Matlab, The Mathworks
Inc.) of cells colonizing a 3D scaffolding structure, an
approach that can be applied with some modifications
to the modelling of porous networks and biomimetic
structures for biomedical devices (Díaz Lantada,
2023). Through this approach, it is possible to model
the influence of cell-material interactions and predict
aspects related to cellular colonization of scaffolds,
vascularization within porous implants, eventual
biodegradation of the implanted structures, among
other issues relevant for predicting the long-term
biocompatibility and understanding the interactions
between the abiotic structures and the living cells.
Another case study is presented in Figure 5b,
which illustrates the automated design of a porous
scaffolding structure employing cellular automata. It
has been programmed using Python and interactions
1 to 10 are presented. Initial seeds, iteration by
iteration, thanks to the defined growth rules, lead to a
voxelated structure. Different biomimetic properties
like porosity, functional gradients of stiffness,
eventual outer textures… can be achieved by minor
modifications of the growth rules.
A relevant aspect of these agents-based methods
is their adequacy for mimicking the randomness of
nature and their applicability to designing self-similar
fractured fractal geometries common in nature. Once
connected to artificial intelligence methods, which
are capable of screening biomechanical properties
and biointerfaces performance from the design stage
(Bermejillo Barrera 2021, Díaz Lantada, 2020),
automated design and optimization procedures can be
implemented.
Bioinspired Design and Manufacturing Strategies for next Generation Medical Implants: Trends and Challenges
47
a)
b)
Figure 4: a-b) Math-based design of micro- and nano-
textured biointerfaces for special cellular interactions
(adapted from Franco Martínez, 2023). Top and isometric
views. a) Hexagonal-based texture and b) lotus flower
leave-like pattern.
a)
b)
Figure 5: a) Cellular automata modelling of cells colonizing
scaffolds, (adapted from: Díaz Lantada, 2023). b)
Automated design of porous scaffolding structure
employing cellular automata: iterations 1 to 10.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
48

5 ADVANCED
MANUFACTURING
RESOURCES
5.1 Additive Manufacturing
Technologies
The degree of geometrical complexity achieved with
advanced computational resources can only be
materialized thanks to the advent of some special
families of manufacturing technologies analyzed in
this section and schematically illustrated in figure 6.
Among these advanced resources, additive
manufacturing technologies, most of them invented
during the 1980s and 1990s, and importantly
improved in terms of resolution, precision and
processable materials along the first two decades of
the 21
st
Century, stand out for the freedom of creation
they enable. Indeed, AMTs (a.k.a. 3D printing
technologies) usually work on a layer-by-layer
fashion, depositing or changing the physical/chemical
state of the raw materials being processed, employing
written lines, pixels or voxels as building blocks. In a
way, material, structure and product are being created
at the same time, which leads on many occasions to
an integration of functions through geometrical
complexity. The additive approach enables the
creation of meshes, lattices, porous structures,
interwoven geometries, metamaterials, common in
nature, but impossible or very challenging to achieve
with traditional methods. An additional benefit of
AMTs is the autonomous processing directly from the
computational models.
From the very beginning, AMTs were applied to
the biomedical field. At first, they were used for
creating surgical training and planning models, and as
a complement to medical diagnostic technologies, but
progressively also for the direct fabrication of
orthoses and prostheses (Díaz Lantada, 2012).
Nevertheless, the expansion of their materials
portfolio, especially the increasing possibility of
manufacturing with a wide set of biomedical
materials including polymers, metals and ceramics,
has recently led to very relevant transformations in
the medical industry. Among them, the increase of
personalized implants is a clear industrial trend.
Furthermore, AMTs have helped to set the
foundations of biomedical research fields like tissue
engineering and biofabrication, which are radically
reformulating the therapeutic strategies for
biomechanical tissue repair and regeneration
(Hutmacher, 2000, Harley, 2021).
5.2 Robotic-Assisted Manufacturing
Progresses in robotics synergize with AMTs and
contribute to healthcare innovation. 5-axis, 6-axis, 7-
axis robots, with the possibility of moving along the
x, y, z axes and performing additional movements,
like roll, pitch and yaw, and of being mounted upon
linear paths in production facilities, may outperform
AMTs in some aspects. The use of robots for freeform
fabrication by deposition of material, taking
inspiration from 3D printing, has led to the concepts
of “5D-, 6D-, 7D-printing”, depending on the number
of axes employed (Haleem, 2019, Vasiliadis, 2022).
Biomedical applications are indeed being explored,
especially in fields like tissue engineering and
biofabrication, in which non-planar deposition paths
may be biomechanically remarkable compared to
those achievable by 3D printing.
5.3 Manufacturing of Advanced
Micro/Nano-Composites
Micro and nanomanufacturing technologies, such as
chemical and physical vapour deposition, UV-photo-
lithography, electrochemical deposition, to cite a few,
synergize with the aforementioned technologies in
the quest for enhanced implants. As advanced, most
tissues have a functionally graded and composite
nature, for which the synthetic creation of graded,
multi-layered and composite materials and structures
is fundamental. Functionalized biomaterials that can
be additively processed to achieve micro/nano-
composites and the use of multi-material printing
technologies creating voxelated composites are also
becoming relevant for smart implants (Velu, 2019).
5.4 Synthetic Biology, Tissue
Engineering, Biofabrication
Last but not least, methods from synthetic biology,
tissue engineering and biofabrication enable the
processing of living cells and their employment,
together with biomaterials, as building blocks for
highly innovative healthcare products. Scaffolds with
cells are advanced medicinal products, not just
medical devices, and enter the realm of engineered
living materials (ELMs) (Srubar III, 2020, Díaz
Lantada, 2022). The boundaries of biomimicry are
hence expanded and may even lead to living
biomaterials as biomaterials factories (Niemeyer,
2018, Nguyen, 2018).
Bioinspired Design and Manufacturing Strategies for next Generation Medical Implants: Trends and Challenges
49
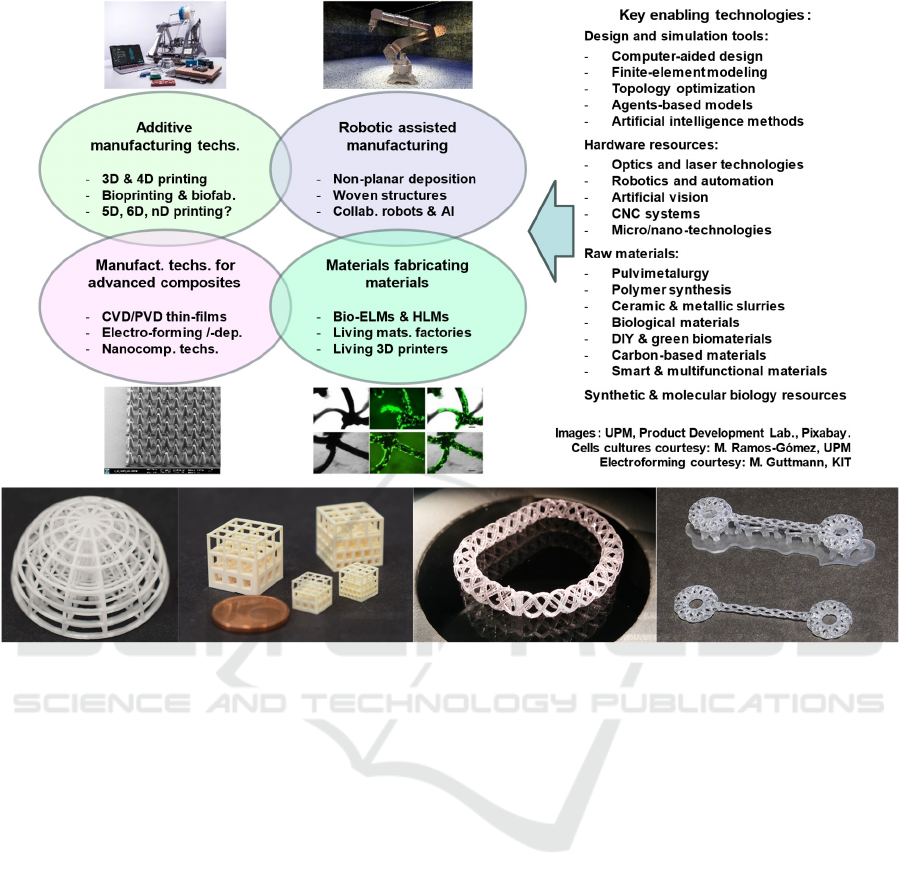
a) b) c) d)
Figure 6: Schematic representation of synergic families of advanced manufacturing technologies enabling the engineering of
biomimetic biodevices: additive manufacturing technologies, robotic assisted manufacturing, technologies for advanced
composites and micro-/nano-composites and resources derived from synthetic biology. Key enabling technologies and raw
materials for these families of advanced manufacturing technologies are also presented. Illustrative examples of achievable
complexity include prototypes of: b) functionally graded tissue engineering ceramic scaffolds (courtesy of Lithoz GmbH,
Tomax project), c) concepts for annuloplasty reconstruction and d) tendon repair lattices (UPM, Product Development Lab).
6 APPLICATION CASES
6.1 Bioinspired Hip Prosthesis
Two conceptual application cases are presented in
this section to illustrate synergies between varied
design strategies aiming at enhanced biomimicry.
First, a bioinspired hip prothesis stem is designed,
as schematically shown in figure 7. The design stands
out for combining: 1) a biomechanical short structure
for minimizing stress-shielding; 2) a topology
optimization for achieving a graded network that
mimics the trabecular and cortical regions; and 3) a
selective application of bioinspired biointerfaces to
different regions, in which osseointegration and
vascularization should be selectively promoted.
6.2 Bioinspired Vascular Stent
Second, a bioinspired vascular stent is designed, as
illustrated in figure 8. Its compliant mesh is surface
functionalized by means of two microtextures. The
external one is aimed at the improved interaction with
the endothelial cells by using a pattern that imitates
the extracellular matrix of the blood vessels. The
internal biointerface is conceived for simultaneously
promoting blood flow and minimizing blood clotting
by employing a bioinspired shark skin design.
In both cases, materialization of the presented
designs would rely on ultra high-performance AMTs
capable of processing the adequate biomedical
materials with the desired precision, which is still a
current challenge, as happens with in vitro validation.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
50
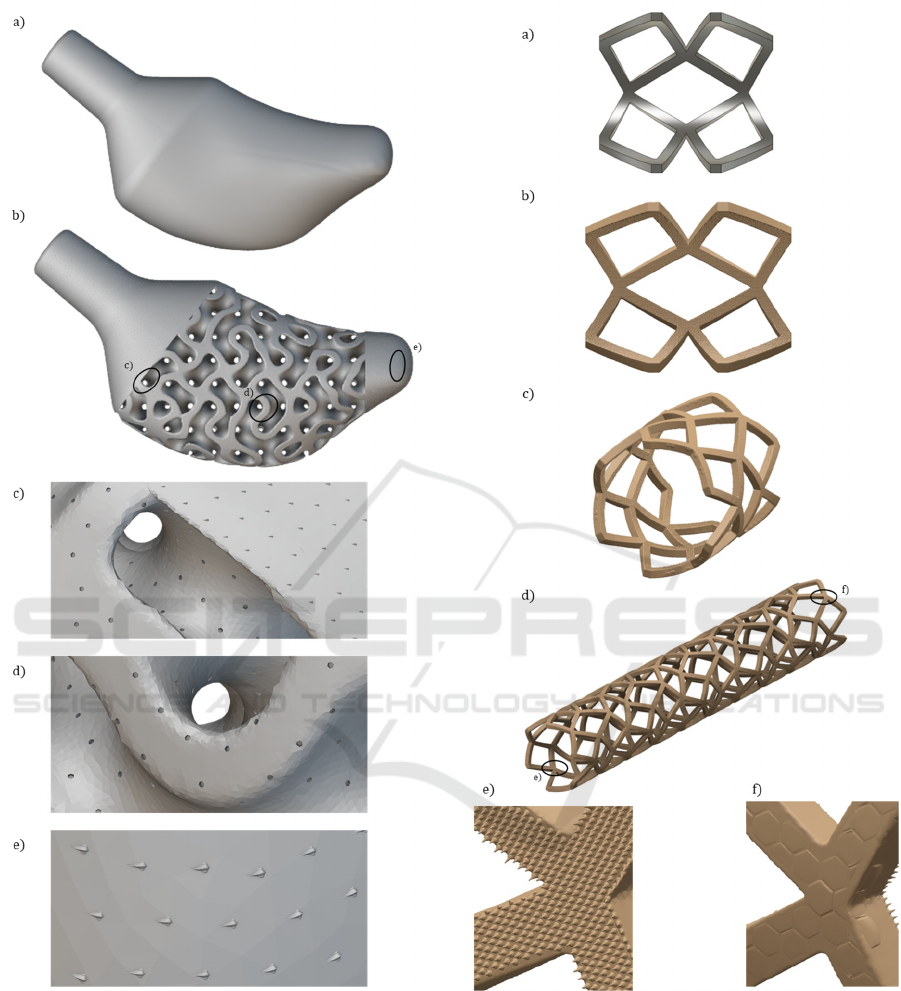
Figure 7: Multifunctional topography optimization of a
short stem hip prosthesis. a) Biomechanically optimized
short stem for femoral implantation. The conceptual design
counts with different biointerfaces defined from the design
stage according to desired biological features. b) Topology
and topography-optimized solution. c-e) Topography
optimization with textures mimicking the shark skin in
regions where different flow orientations would be desired.
d) Bone-like surface topography for enhanced
osseointegration and increased primary stability.
Figure 8: Innovative design for vascular stent with
bioinspired surface topographies. The design process
includes: a) Creation of unit cell. b) Application of
topography optimizations. c) Design of basic ring. d)
Replication towards complete stent. e) Detailed inner
texture imitating shark skin for enhanced hemodynamics. f)
Detailed external texture in contact with arterial wall for
improved adhesion and long-term stability of the stent
(avoiding slippery and preventing migration).
Bioinspired Design and Manufacturing Strategies for next Generation Medical Implants: Trends and Challenges
51

7 CONCLUSIONS
The geometrical and material complexity of living
biological structures has been traditionally extremely
challenging to imitate, which used to derive in
suboptimal biomedical devices and implants, whose
biomechanical behavior and biological interaction
properties were not truly biomimetic.
Fortunately, bioinspired development strategies
and advanced computational and manufacturing
resources, as explained and exemplified in this study,
are already synergizing in a highly stimulating way to
solve the riddles of natural materials and biological
structures. The quest for next generation bioinspired
implants is just starting and requires integrative
research efforts from as many fields as possible.
Towards the future, further expanding the
biomaterials portfolio of advanced manufacturing
technologies and exploring new ways of jointly
processing biomaterials and living entities like cells
and bacteria, in clear alignment with the nascent field
of engineered living materials, can contribute to
bringing biomimicry a step beyond.
In addition, if the implants of the future may rely
on biohybrid solutions, there is a need for updated
regulations and standards. In the European Union, to
take an example, implants and tissue engineering
scaffolds without cells are usually Class III medical
devices, according to the Medical Device Regulation
2017/745, while scaffolds with cells are still
considered advanced therapy medicinal products
according to regulation 1394/2007. Further efforts in
regulation and standardization harmonization are
needed in this continuously evolving field.
Arguably, through expanded bioinspired and
biomimetic development strategies and technological
capabilities the biomedical implants of the future will
importantly outperform the state-of-the-art and,
hopefully, become the perfect solutions for users’
biological structures needing repair or regeneration.
ACKNOWLEDGEMENTS
The research presented has been supported by the
following research and innovation projects:
“iMPLANTS-CM”, from the “Convocatoria 2020 de
ayudas para la realización de proyectos sinérgicos de
I+D” funded by Comunidad Autónoma de Madrid
(reference: Y2020/BIO-6756). “INKplant” funded
by the European Union’s Horizon 2020 Research and
Innovation Programme under grant agreement No.
953134.
REFERENCES
Bader, C. et al. (2018) ‘Making data matter: Voxel printing
for the digital fabrication of data across scales and
domains’, Science Advances, 4(5), p. eaas8652.
Bar-Cohen, Y. (2006) ‘Biomimetics--using nature to
inspire human innovation’, Bioinspiration &
Biomimetics, 1(1), pp. 1–12.
Benyus, J.M. (2002) ‘Biomimicry: innovation inspired by
nature’, Harper Perennial, Harper Collins, New York,
USA.
Bermejillo Barrera, M.D., Franco-Martínez, F., Díaz
Lantada, A. (2021) ‘Artificial Intelligence Aided
Design of Tissue Engineering Scaffolds Employing
Virtual Tomography and 3D Convolutional Neural
Networks’. Materials, 14(18), 5278.
Bertram, J.E. and Biewener, A.A. (1988) ‘Bone curvature:
sacrificing strength for load predictability?’, Journal of
Theoretical Biology, 131(1), pp. 75–92.
Boccaccio, A. et al. (2016) ‘Geometry Design Optimization
of Functionally Graded Scaffolds for Bone Tissue
Engineering: A Mechanobiological Approach’, PLOS
ONE, 11(1), p. e0146935.
Bongard, J. and Levin, M. (2023) ‘There’s Plenty of Room
Right Here: Biological Systems as Evolved,
Overloaded, Multi-Scale Machines’, Biomimetics,
8(1), p. 110.
Dumont, E.R. (2010) ‘Bone density and the lightweight
skeletons of birds’, Proceedings of the Royal Society B:
Biological Sciences, 277(1691), pp. 2193–2198.
Díaz Lantada, A. (ed.) (2013) Handbook on Advanced
Design and Manufacturing Technologies for
Biomedical Devices. Boston, MA: Springer US.
Díaz Lantada, A., Franco-Martínez, F., Hengsbach, S.,
Rupp, F., Thelen, R., Bade, K. (2020) Artificial
Intelligence Aided Design of Microtextured Surfaces:
Application to Controlling Wettability. Nanomaterials,
10(11), 2287.
Díaz Lantada, A., Korvink, J.G. and Islam, M. (2022)
‘Taxonomy for engineered living materials’, Cell
Reports Physical Science, 3(4), p. 100807.
Díaz Lantada, A. and Morgado, P.L. (2012) ‘Rapid
Prototyping for Biomedical Engineering: Current
Capabilities and Challenges’, Annual Review of
Biomedical Engineering, 14(1), pp. 73–96.
Díaz Lantada, A., Sánchez, M.U. and Fernández, D.F.
(2023) ‘In silico Tissue Engineering and Cancer
Treatment Using Cellular Automata and Hybrid
Cellular Automata-Finite Element Models’, in. 16th
International Conference on Biomedical Electronics
and Devices, pp. 56–63.
Droste, M. (2019) ‘Bauhaus 1919-1933. Updated Edition’.
Taschen, commemorative edition for the 100th
anniversary of Bauhaus.
Feynman, R.P. (1959) ‘Plenty of Room at the Bottom’.
Lecture at the annual American Physical Society
meeting at Caltech.
Franco Martínez, F. (2022) ‘Artificial intelligence aided
design of microstructured surfaces for tribology and
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
52

biointerfaces engineering’, PhD thesis, Universidad
Politécnica de Madrid.
Gardan, J. (2019) ‘Smart materials in additive
manufacturing: state of the art and trends’, Virtual and
Physical Prototyping, 14(1), pp. 1–18.
Gardner, M. (no date) ‘Conway’s Game of Life: Scientific
American, October 1970’, Scientific American, 223,
pp. 120–123.
Haleem, A., Javaid, M. and Vaishya, R. (2019) ‘5D printing
and its expected applications in Orthopaedics’, Journal
of Clinical Orthopaedics and Trauma, 10(4), pp. 809–
810.
Harley, W.S. et al. (2021) ‘Advances in biofabrication
techniques toward functional bioprinted heterogeneous
engineered tissues: A comprehensive review’,
Bioprinting, 23, p. e00147.
Hutmacher, D.W. (2000) ‘Scaffolds in tissue engineering
bone and cartilage’, Biomaterials, 21(24), pp. 2529–
2543.
Leong, K.F. et al. (2008) ‘Engineering functionally graded
tissue engineering scaffolds’, Journal of the Mechanical
Behavior of Biomedical Materials, 1(2), pp. 140–152.
Liverani, E., Rogati, G., Pagani, S., Brogini, S., Fortunato,
A. and Caravaggi, P. (2021) ‘Mechanical interaction
between additive-manufactured metal lattice structures
and bone in compression: implications for stress
shielding of orthopaedic implants’, Journal of the
Mechanical Behavior of Biomedical Materials, 121,
104608.
Mandelbrot, B.B. (1983) The Fractal Geometry of Nature.
Henry Holt and Company.
Neumann, J.V. and Burks, A.W. (1966) Theory of Self-
Reproducing Automata. USA: University of Illinois
Press.
Nguyen, P.Q. et al. (2018) ‘Engineered Living Materials:
Prospects and Challenges for Using Biological Systems
to Direct the Assembly of Smart Materials’, Advanced
Materials (Deerfield Beach, Fla.), 30(19), p. e1704847.
Niemeyer, C.M. et al. (2018) ‘White Paper on the
Biologization of Materials Research’. Preprints.
Perier-Metz, C., Duda, G.N. and Checa, S. (2022) ‘A
mechanobiological computer optimization framework
to design scaffolds to enhance bone regeneration’,
Frontiers in Bioengineering and Biotechnology, 10.
Phillips, J.E. et al. (2008) ‘Engineering graded tissue
interfaces’, Proceedings of the National Academy of
Sciences, 105(34), pp. 12170–12175.
Place, E.S., Evans, N.D. and Stevens, M.M. (2009)
‘Complexity in biomaterials for tissue engineering’,
Nature Materials, 8(6), pp. 457–470.
Skylar-Scott, M.A. et al. (2019) ‘Voxelated soft matter via
multimaterial multinozzle 3D printing’, Nature,
575(7782), pp. 330–335.
Srubar III, W.V. (2020) ‘Engineered living materials:
taxonomies and emerging trends’, Trends in
Biotechnology, 39(6), pp. 574-583.
Tannenbaum, J. and Bennett, B.T. (2015) ‘Russell and
Burch’s 3Rs Then and Now: The Need for Clarity in
Definition and Purpose’, Journal of the American
Association for Laboratory Animal Science, 54(2).
Vasiliadis, A.V., Koukoulias, N. and Katakalos, K. (2022)
‘From Three-Dimensional (3D)- to 6D-Printing
Technology in Orthopedics: Science Fiction or
Scientific Reality?’, Journal of Functional
Biomaterials, 13(3), p. 101.
Velu, R. et al. (2019) ‘A Comprehensive Review on Bio-
Nanomaterials for Medical Implants and Feasibility
Studies on Fabrication of Such Implants by Additive
Manufacturing Technique’, Materials, 13(1), p. 92.
Wang, X. et al. (2016) ‘Topological design and additive
manufacturing of porous metals for bone scaffolds and
orthopaedic implants: A review’, Biomaterials, 83, pp.
127–141.
Zadpoor, A.A. (2019) ‘Mechanical performance of
additively manufactured meta-biomaterials’, Acta
Biomaterialia, 85, pp. 41–59.
Zadpoor, A.A. (2020) ‘Meta-biomaterials’, Biomaterials
Science, 8(1), pp. 18–38.
Bioinspired Design and Manufacturing Strategies for next Generation Medical Implants: Trends and Challenges
53
