
Hand Movement Recognition Based on Fusion of Myography Signals
Shili Wala Eddine
1 a
, Youssef Serrestou
2
, Slim Yacoub
3
, Ali H. Al-Timemy
4 b
and Kosai Raoof
4 c
1
ENSIM- LAUM ENISO, Le Mans University, University of Sousse (ENISO), France
2
Le Mans Univeristy, Le Mans, France
3
INSAT-Carthage University, Tunis, Tunisa
4
Biomedical Eng. Department, Al-Khwarizmi College of Engineeing, University of Baghdad, Iraq
Wala Eddine.Shili.Etu@univ-lemans.fr, youssef.serrestou@univ-lemans.fr, slim.yacoub@insat.ucar.tn,
ali.altimemy@kecbu.uobaghdad.edu.iq, kosai.raoof@univ-lemans.fr
Keywords:
Acousto Myography (AMG), Electromyography (EMG), Mechanomyography (MMG), Support Vector
Machine (SVM).
Abstract:
This article presents a hand movement classification system that combines acoustic myography (AMG) sig-
nals, electromyography (EMG) signals and mechanomyogram signal (MMG) data. The system aims to accu-
rately predict hand movements, with the potential to improve the control of hand prostheses. A dataset was
collected from 9 individuals who repeated 10 times each of 4 hand movements (hand close, hand open, fine
pinch and index flexion). The system, with a Support Vector Machine (SVM) classifier, achieved an accuracy
score of 97%, demonstrating its potential for real-time hand prosthesis control. The combination of AMG,
EMG, and MMG signals proved to be effective in accurately classifying hand movements.
1 INTRODUCTION
Enhancing quality of life for people with impaired
hand mobility is a major public health challenge. Ad-
vanced real-time controlled hand prosthetics offer a
promising solution (Smith, 2020). However, accu-
rately predicting hand movements under real-life con-
ditions remains an unmet need (Johnson and Chen,
2017).
Recent advances in machine learning have created
new possibilities to address this challenge (Hastie
et al., 2009). Prior studies have proposed pre-
diction systems based on electromyography (EMG)
(Li and Zhang, 2013), acoustic myography (AMG)
(Gupta and Patel, 2022) or MMG signals (Castillo
et al., 2021). However, single modalities have limita-
tions—EMG is susceptible to electromagnetic noise
while AMG suffers from motion artifacts (Scheme
and Englehart, 2011). Hybrid systems combining
EMG and MMGs have shown promise (Harrison
et al., 2013) but have not fully mitigated these issues.
To overcome these hurdles, we propose a novel
multi-modal approach by fusing AMG, EMG and
a
https://orcid.org/0009-0004-3815-8361
b
https://orcid.org/0000-0003-2738-8896
c
https://orcid.org/0000-0002-9775-7485
MMG signals. This provides complementary infor-
mation for robust movement prediction: AMG cap-
tures muscle vibrations revealing motor unit recruit-
ment (Mamaghani et al., 2001); EMG measures elec-
trical potentials for high temporal resolution (Shcher-
bynina et al., 2023); MMGs detect limb accelerations
indicating direction and speed (Al-Timemy et al.,
2022). Furthermore, the multi-channel input gives
machine learning algorithms more informative fea-
tures to accurately discriminate movements (Farina
et al., 2014a). Modality-specific artifacts also aver-
age out when the signals are combined, improving
the overall signal-to-noise ratio (Lim et al., 2008). Fi-
nally, ensemble methods leveraging classifiers trained
on each signal lead to higher accuracy than single
modalities alone (Farina et al., 2014b).
In summary, the diversity of signal sources, the
complementary nature of the information they pro-
vide, and the possibility of using ensemble methods
all contribute to the potential for improved accuracy
when combining AMG with EMG and MMGs for
hand movement classification. Extracting discrimi-
native features from different signal channels and ef-
fectively combining them through machine learning
techniques are essential for achieving high accuracy
in this field.
In this paper, we detail the development of a ma-
Eddine, S., Serrestou, Y., Yacoub, S., Al-Timemy, A. and Raoof, K.
Hand Movement Recognition Based on Fusion of Myography Signals.
DOI: 10.5220/0012350000003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 733-738
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
733
chine learning model for classifying hand movements
exploiting the synergistic fusion of AMG, EMG and
MMGs. The proposed model could potentially be ap-
plied for real-time hand prosthesis control to improve
accuracy, pending future implementation and testing.
The results demonstrate promising performance to-
wards more intuitive prosthetic control in the future.
2 DATA ACQUISITION
Dataset was collected from individuals performing
four distinct hand movements: hand close, hand open,
fine pinch, and index finger flexion. The integra-
tion of AMG, EMG, and MMG signals, also known
as Mechanomyography (MMG) resulted in a multi-
dimensional dataset primed for advanced processing.
2.1 Materials
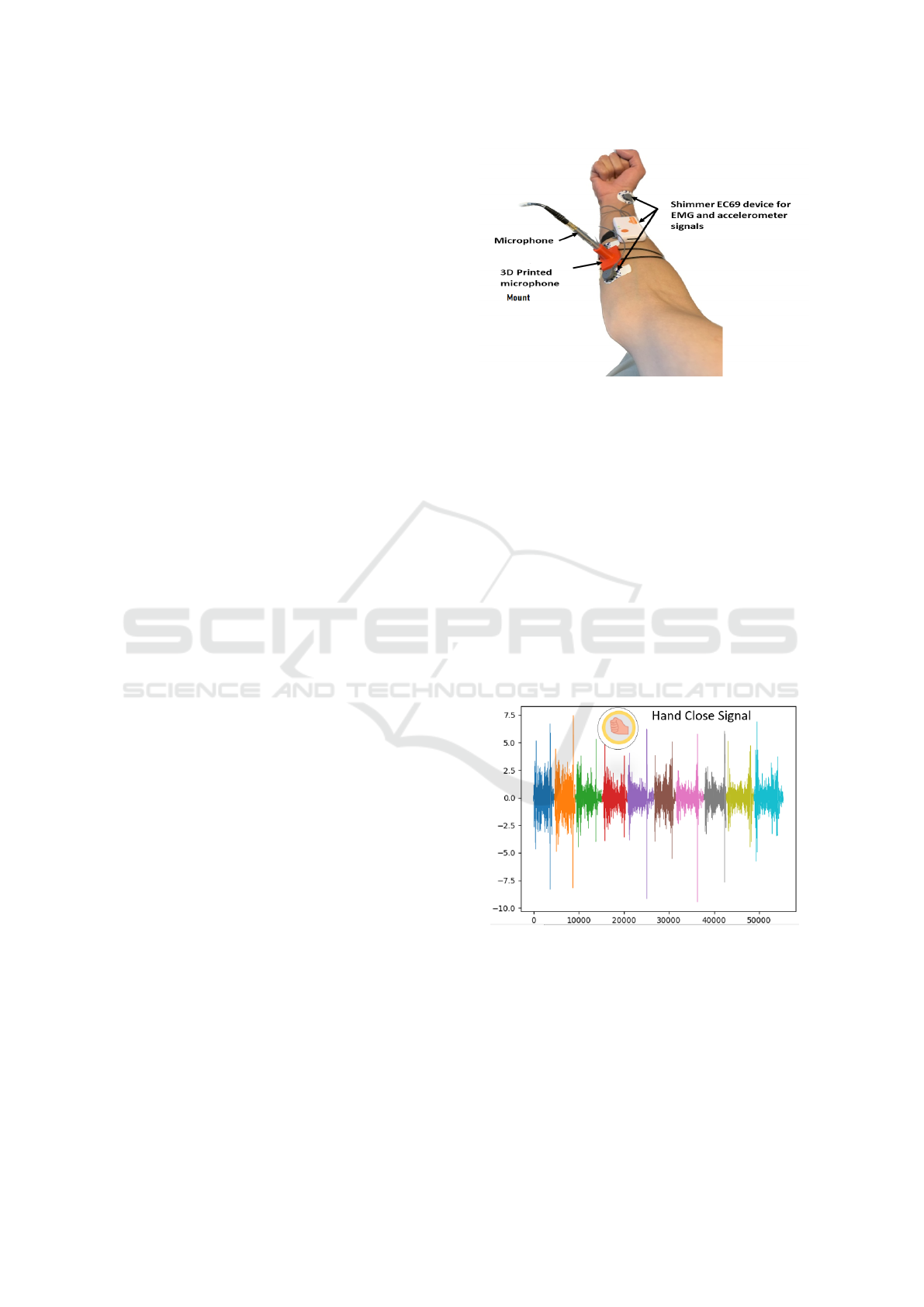
The acoustic myography (AMG) signal was recorded
using a microphone model MPA416, with a frequency
range of 20-20kHz and sensitivity of 50mV/Pa. Pre-
vious literature (Orizio et al., 1989)-(Beck and et al.,
2005) has shown that the frequency range of AMG
signal is 5-100 Hz. To securely position the micro-
phone and reduce signal noise (see Fig 1.), a custom
3D-printed apparatus was developed to fix the micro-
phone on the skin surface over the target forearm mus-
cles (Harrison et al., 2013). This minimized motion
artifacts and ensured consistent AMG capture during
hand movements. AMG signals were sampled at 1024
Hz.
The 3D-printed microphone enclosure (Yacoub
et al., ) was specifically designed to house micro-
phone. The base of the enclosure has a tapered shape,
and the opposite end of the microphones snugly fits
into the mount, thereby eliminating any distortions
caused by microphone movements. Inadequate fix-
ation of the microphone can cause alterations of the
muscle signals.
The Shimmer EC69 device was used to record
both the electromyography (EMG) and MMG sig-
nals. Sensors were strategically positioned on the up-
per arm to capture muscle activation potentials and
limb acceleration. EMG signals were also sampled at
1024 Hz.
Fig. 1 shows the locations of shimmer device that
was used to record both of EMG and MMG signals,
with microphone which record AMG signal.
Figure 1: Channel locations on the upper forearm of the
right hand of the subject.
2.2 Data Collection
To evaluate the effectiveness of combing signals for
hand movement classification, a dataset was collected
from 9 subjects (9 males). The mean age of partic-
pants is 23 years. Prior to participating in the study,
all participants provided informed consent and signed
consent forms. The exprimental protocol was done
according to the declaration of Helsinki. Fig.1 il-
lustrate the position of each sensors. Subjects are
asked to repeat 10 times four distinct hand move-
ments: hand close, hand open, fine pinch, and index
finger flexion. For exemple Fig.2 illustrates an ex-
ample of a signal where one person’s hand is closed,
repeated 10 times.
Figure 2: Example of ten repetitions of hand closure AMG
Signal.
In total, the dataset comprises data from 9 subjects
executing 4 distinct hand movements: close, open,
fine pinch, and index finger flexion. Each movement
was repeated 10 times, recorded across 5 channels
(AMG, EMG, and 3 MMG), with a sampling rate of
1024 Hz. The duration of each repetition was approx-
imately 5 seconds, and there was a rest interval of 40
to 60 seconds between movements as Fig.3 illustrates.
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
734
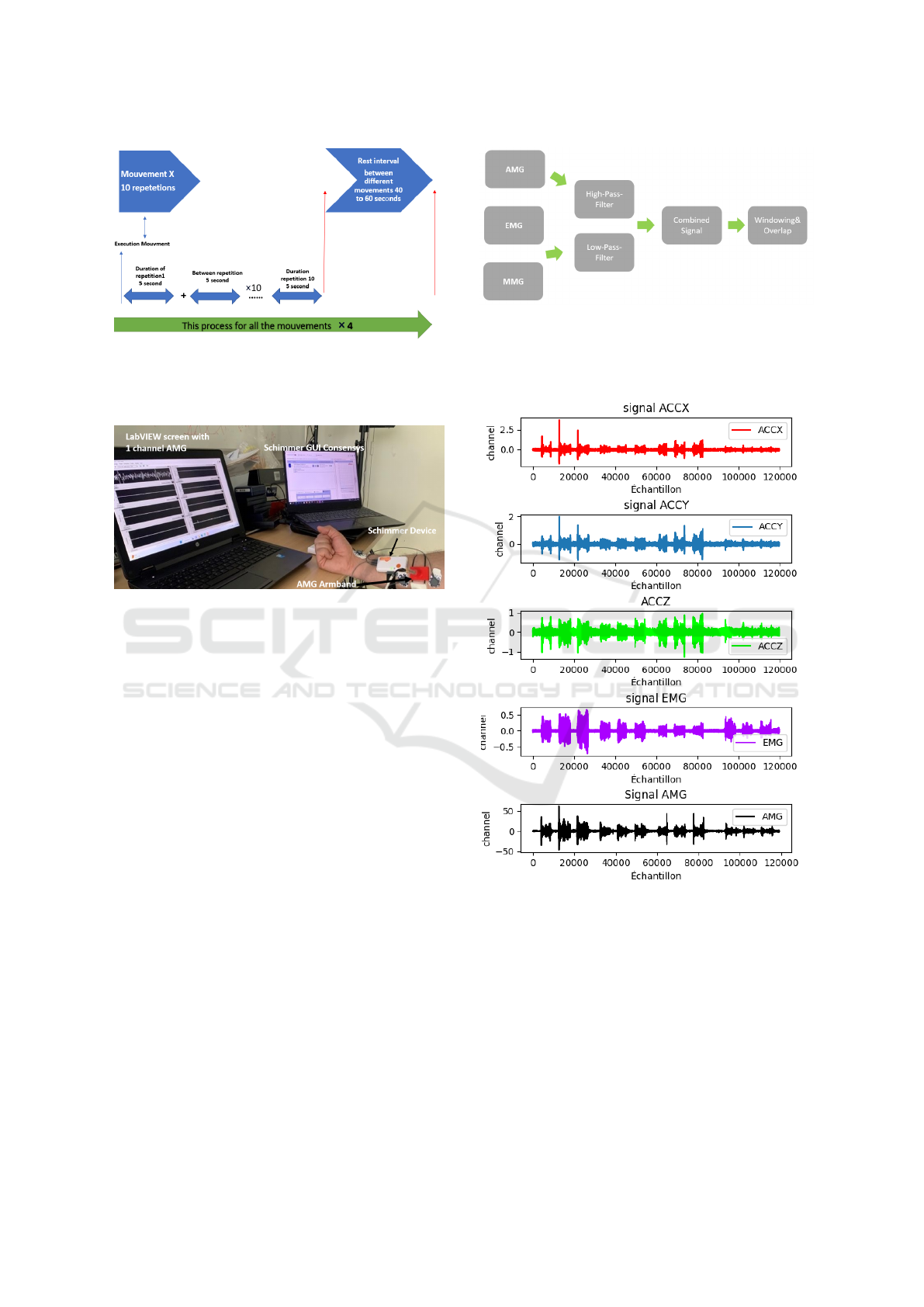
Figure 3: Execution mouvement Process.
Fig.4 illustrates the full experimental setup which
integrates signals from AMG, EMG, and MMG.
Figure 4: The setup of combining AMG with EMG and
MMG signals.
3 FEATURE EXTRACTION
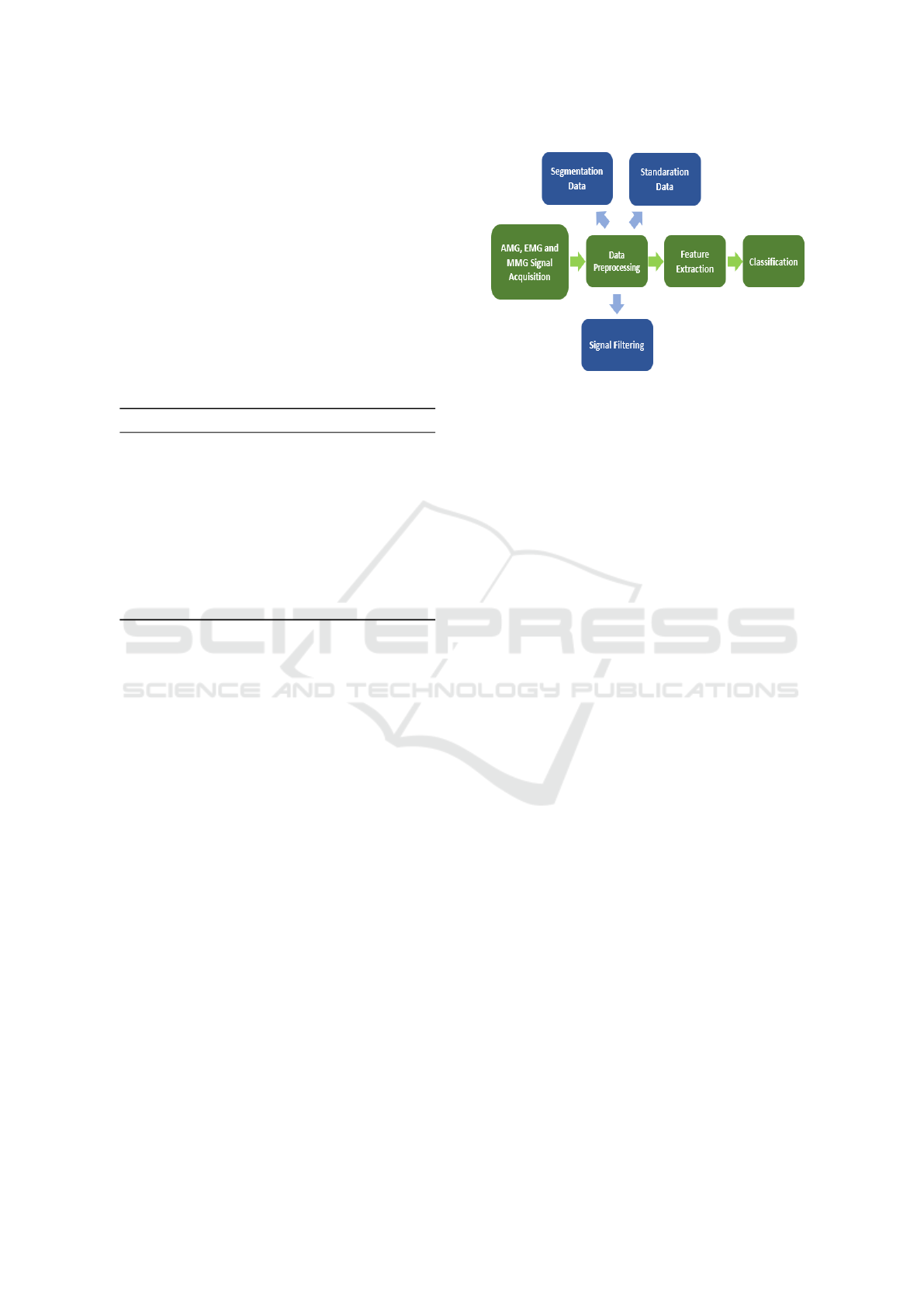
3.1 Data Processing
The acquisition of AMG and EMG-MMG signals was
done by different devices. A synchronization pro-
cess is necessary to harmonize these signals. Ini-
tially, AMG data was loaded, followed by the ac-
quisition of data from MMGs and EMG sensors (via
a Shimmer device). Subsequently, the EMG signal
underwent high-pass filtering, and the signal lengths
among AMG, MMG, and EMG data streams were
harmonized to achieve synchronization. These syn-
chronized signals were combined into a singular vari-
able, yielding a cohesive dataset suitable for advanced
processing. As shown in Fig.5, the data streams were
first loaded separately and underwent pre-processing
such as filtering. They were then aligned in length and
combined into a single matrix for further analysis.
Signal segmentation employed a window size of
100 samples, facilitating the division of the signal
stream into discrete segments. To ensure continuous
and overlapping signal segments, an intentional over-
lap of 50 was strategically applied, enhancing the ro-
bustness and completeness of subsequent analyses.
Figure 5: Preprocessing signals.
Fig. 6, it displays an example of the 5 channels
(AMG, 3-channels MMG and EMG) signals recorded
simultaneously during a hand movement.
Figure 6: Example of the 5 channels AMG EMG and MMG
signals.
3.2 Feature Extraction
The feature extraction process was performed using
windowing and signal characteristics such as min,
max, and standard deviation. The window size used
was 100 samples, and the overlap was 50 to increase
the amount of data for training. Each window was
considered as a data sample.
Given that the signals were recorded from 5 chan-
nels (AMG, 3 MMGs, EMG), and 4 features (mean,
Hand Movement Recognition Based on Fusion of Myography Signals
735
standard deviation, min, max) were extracted from
each channel per window, the total number of features
extracted per window was 5 channels × 4 features =
20 features.
Therefore, the total number of samples (win-
dows) multiplied by the number of features per sam-
ple yielded the final feature space/dimension of the
dataset.
The features extracted from each window were the
signal mean, standard deviation, minimum and max-
imum values from each of the 5 channels. These 20
features per window for each person were then nor-
malized using standard scaling to prepare them for the
machine learning model.
Summary of Feature Extraction Process
The feature extraction in totaling 20 features per win-
dow. Multiplying this by the number of windows pro-
vided the dataset’s final feature space. Each window’s
20 features, capturing signal characteristics from each
channel, were normalized using standard scaling for
machine learning preparation
Dataset Specifications:
Number of Persons 9
Number of Movements 4
Number of Channels 5
Number of Features per Window per Movement 20
Number of Windows per Movement 1000
4 HAND MOVEMENT
RECOGNITION SYSTEM
The methodology involves the use of sensitive micro-
phones and EMG and MMG signals to combine sig-
nal quality. We will divide each movement into fixed-
size windows and extract features from each window.
These features will be used to train an SVM classifier
with grid search for hyperparameter tuning (Scheme
and Englehart, 2011).
Fig.7 summarizes the overall workflow. It
shows the data acquisition described in Section 2,
pre-processing steps of filtering and segmenting the
signals detailed in Section 3.1, feature extraction
covered in Section 3.2, and the classification model
training. As described in Section 2, the dataset
consists of signals from 9 subjects performing 4 hand
movements repeated 10 times each. The data will be
divided into training and test sets for each person.
4.1 Machine Learning Model
The machine learning model used in this study is a
support vector machine (SVM) classifier. The SVM
Figure 7: Model Workflow.
classifier was chosen because it has proven to be ef-
fective in various classification tasks and can han-
dle high-dimensional feature spaces. To optimize
the SVM classifier’s hyperparameters, we used grid
search with a range of C and gamma values. The
best hyperparameters were chosen based on the high-
est accuracy score. To ensure the SVM classifier’s
optimal performance, hyperparameters such as C and
gamma are fine-tuned using grid search (Hastie et al.,
2009). C represents the regularization parameter that
controls the trade-off between achieving a wide mar-
gin and minimizing the classification error. Gamma
determines the influence of a single training exam-
ple, with low values leading to a broader influence and
high values causing localized influence. Grid search
involves systematically searching through a prede-
fined hyperparameter grid to find the combination that
yields the best performance. In our study, we experi-
mented with various C and gamma values to find the
hyperparameters that result in the highest accuracy
score on our dataset. application of a SVM classi-
fier for signal data classification. By fine-tuning hy-
perparameters through grid search, we enhanced the
classifier’s accuracy, highlighting the significance of
proper hyperparameter optimization.
5 RESULTS AND DISCUSSION
The performance metrics used to evaluate the sys-
tem’s performance were accuracy, precision, recall,
and F1 score. After training the SVM model with
grid search, we obtained the following best hyper-
parameters: C=10, kernel=’rbf’, and degree=2. The
model achieved an accuracy of 97%, a recall of 97%,
an F1 score of 97%, and a well-balanced confusion
matrix. The analysis of results highlights the vary-
ing performance of the SVM model for different in-
dividuals and movement classes. Some individuals
achieved accurate classification with high accuracy
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
736

and F1-scores, while others exhibited errors in spe-
cific classes. The performance of certain individuals
indicates the model’s capability to generalize well for
those specific individuals, while larger errors for oth-
ers underscore the need for finer personalization. It
should be noted that the utilization of the SVM model
for movement classification based on signal data has
demonstrated promising performance. The results of
confusion matrices and performance measures pro-
vide valuable insights into the strengths and limita-
tions of the model. Detailed analyses for each individ-
ual offer specific insights for targeted model improve-
ment, such as hyperparameter tuning or individual-
specific preprocessing methods. This study under-
scores the importance of customization and optimiza-
tion of models for optimal performance.
Figure 8: Confusion Matrix for Hand Movement Classifi-
cation Using AMG, EMG, and MMG Signals of 9 subjects.
This table illustrates the performance metrics, in-
cluding accuracy, precision, recall, and F1 score, for
different individuals in the context of hand gesture
recognition. It provides a comparative view of the
classifier’s effectiveness in recognizing various hand
gestures across multiple people.
Table 1: Performance Metrics for Hand Gesture Recogni-
tion.
Person Accuracy Precision Recall F1Score
1 1.00 1.00 1.00 1.00
2 0.93 0.94 0.93 0.93
3 0.94 0.94 0.94 0.94
4 0.99 0.99 0.99 0.99
5 0.91 0.92 0.91 0.91
6 0.95 0.95 0.95 0.95
7 0.90 0.91 0.90 0.90
8 0.74 0.74 0.74 0.74
9 0.93 0.94 0.93 0.93
6 CONCLUSION
This study demonstrated the feasibility of classify-
ing hand movements from a combination of AMG,
EMG, and MMG signals using machine learning. An
SVM model was able to effectively classify move-
ments with high accuracy and precision of 97% on
the test set.
The results validate the multi-model approach
of fusing complementary information from different
sensors. By capturing muscle vibrations, electrical
potentials, and limb movements, the combined sig-
nals provided rich discriminative features for the clas-
sification model. Individual signal streams were also
shown to average out artifacts when combined, im-
proving robustness.
Further optimization of the system is still needed.
Personalizing the model for each individual could
help address limitations in accuracy for some move-
ment classes and subjects. Refining feature engineer-
ing techniques may also enhance classification perfor-
mance.
A key future direction is to translate this work di-
rectly into intuitive prosthetic devices. Integrating the
developed hand movement recognition system could
enable more natural control for upper limb prosthet-
ics. This has potential to significantly improve quality
of life for individuals with limb impairments.
REFERENCES
Al-Timemy, A., Serrestou, Y., Khushaba, S. R., Yacoub,
S., and Raoof, K. (2022). Hand gesture recognition
with acoustic myography signals and Wavelet scatter-
ing Transform. IEEE Access, 10:107526–107535.
Beck, T. W. and et al. (2005). Mechanomyographic ampli-
tude and frequency responses during dynamic muscle
actions: a comprehensive review. Biomed. Eng. On-
line, 4(1):1–27.
Castillo, C., Wilson, S., Vaidyanathan, R., and Atashzar, S.
(2021). Wearable MMG-Plus-One Armband: Evalu-
ation of normal force on mechanomyography (MMG)
to enhance human–machine interfacing. IEEE Trans.
Neural Syst. Rehabil. Eng., 29:196–205.
Farina, D., Aszmann, O., Rosiello, E., and Pratesi, R.
(2014a). Noninvasive rehabilitation technologies for
upper extremity motor recovery in stroke patients:
State of the art and future directions. Rev. Neurosci.,
25(1):155–170.
Farina, D., Negro, F., and Dideriksen, J. (2014b). The ef-
fective neural drive to muscles is the common synaptic
input to motor neurons. J. Physiol., 592(4):743–757.
Gupta, R. and Patel, M. (2022). Real-time hand prosthe-
sis control using combined acoustic myography and
MMG signals. In Proc. IEEE Int. Conf. Robot. Au-
tom. (ICRA), pages 1234–1240.
Hand Movement Recognition Based on Fusion of Myography Signals
737

Harrison, A., Danneskiold-Samsøe, B., and Bartels, E.
(2013). Portable acoustic myography – a realistic
noninvasive method for assessment of muscle activ-
ity and coordination in human subjects in most home
and sports settings. Physiol. Rep., 1(2).
Hastie, T., Tibshirani, R., and Friedman, J. (2009). The
Elements of Statistical Learning. Springer.
Johnson, R. and Chen, L. (2017). Acoustic myography
for real-time prosthetic hand control. IEEE Trans.
Biomed. Eng., 45(3):320–327.
Li, Y. and Zhang, L. (2013). Support vector machines
for hand movement classification based on EMG and
MMG data. J. Neuroeng. Rehabil., 10(11).
Lim, B., Lee, S., Jang, D., and Kim, Y. (2008). Classi-
fication of forearm EMG signals using time-domain
feature and artificial neural network. Biomed. Eng.
Online, 7(1).
Mamaghani, N., Shimomura, Y., Iwanaga, K., and Kat-
suura, T. (2001). Changes in surface EMG and acous-
tic myogram parameters during static fatiguing con-
tractions until exhaustion: Influence of elbow joint
angles. J. Physiol. Anthropol. Appl. Human Sci.,
20(2):131–140.
Orizio, C., Perini, R., and Veicsteinas, A. (1989). Muscular
sound and force relationship during isometric contrac-
tion in man. Eur. J. Appl. Physiol. Occup. Physiol.,
58(5):528–533.
Scheme, E. and Englehart, K. (2011). Electromyogram
pattern recognition for control of powered upper-limb
prostheses: State of the art and challenges for clinical
use. J. Rehabil. Res. Dev., 48(6):643–659.
Shcherbynina, M., Gladun, V., and Sarana, V. (2023).
Acoustic Myography as a Noninvasive Technique
for Assessing Muscle Function: Historical Aspects
and Possibilities for Application in Clinical Practice.
Physiol. Rep., 11(6).
Smith, J. (2020). Acoustic Myography: Principles and Ap-
plications. Springer.
Yacoub, S., Al-Timemy, A., Serrestou, Y., S.E., Khushaba,
R., and Raoof, K. An Investigation of Acoustic Cham-
ber Design for Recording AMG Signal. In 2023 IEEE
International Conference on Advanced Systems and
Emergent Technologies (IC
ASET
).
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
738
