
e-Consent in Biomedical Research Registries: A GDPR-Compliant
Approach Explored in the Context of the Australasian Diabetes Data
Network
Zhe Wang
a
, Anthony Stell
b
, Jean Paul Vera Soto
c
and Richard O. Sinnott
d
School of Computing and Information Systems, The University of Melbourne, Melbourne, VIC 3010, Australia
Keywords:
e-Consent, Biomedical Registries, GDPR, ADDN, Type-1 Diabetes.
Abstract:
e-Consent - the digital capture of a patient’s consent to be involved in medical research - is a feature of
biomedical research that is becoming increasingly prevalent with the advance of digital technology to support
clinical/biomedical research and targeted registries. Although there have been many reviews of e-consent over
the past decade - evaluating aspects such as informed consent, engagement, comprehension and data security
- there remain unanswered questions about how e-consent fits in the context of recent data legislation and
privacy demands such as the European General Data Protection Regulation (GDPR). This paper outlines key
aspects of e-consent in the context of GDPR and the specific demands placed on biomedical registries used
for diverse research objectives. We present a practical realisation of GDPR e-Consent in the context of the
Australasian Diabetes Data Network (ADDN) – the national type-1 diabetes registry for Australia.
1 INTRODUCTION
Informed consent is a foundational part of biomedi-
cal research ethics according to the Belmont Report
(1979)
1
. This introduced three key elements: infor-
mation, comprehension and voluntariness that should
be considered for informed consent to ensure the pro-
tection of human subjects in research. Such principles
have guided the evaluation of different consent pro-
cesses (Sugarman et al., 1998)(Del Carmen and Joffe,
2005). Traditionally, this process was dominated by
paper-based methods, where individuals physically
sign documents after discussions with their health-
care providers. However, with the advent of elec-
tronic medical records and advancements in digital
technology, the electronic format of informed con-
sent (e-Consent) is gaining attention. Review papers
of e-Consent have explored multiple domains includ-
ing general healthcare (Chimonas et al., 2023), sur-
gical procedures (Mirza et al., 2023) and biomed-
ical research (Cohen et al., 2023)(De Sutter et al.,
a
https://orcid.org/0000-0001-6054-6468
b
https://orcid.org/0000-0003-4819-9883
c
https://orcid.org/0000-0001-6345-5596
d
https://orcid.org/0000-0001-5998-222X
1
www.hhs.gov/ohrp/regulations-and-policy/belmont-
report/
2020)(Skelton et al., 2020). In healthcare and sur-
gical contexts, e-Consent mainly aims to streamline
administrative undertakings, elevate the standard of
care, and enrich the patient experience (Mirza et al.,
2023).
In the field of biomedical research, different cate-
gories of registries are often used to store and process
electronic medical information extensively. There are
various types of registries including biobanks, clini-
cal trial registries, population registries, and targeted
disease registries. Biobanks are repositories that
store biological samples for use in research. These
are often managed according to professional stan-
dards (Hewitt and Watson, 2013). The NSW Health
Statewide Biobank
2
and Australian Health Biobank
(AHB)
3
are two such exemplar biobanks in Australia.
Biomedical samples are collected to advance future
research discoveries based on access to physical spec-
imens, e.g. for targeted genomic analysis. Clinical
Trial Registries such as the Australian New Zealand
Clinical Trial Registry (ANZCTR)
4
keeps track of
clinical trials being undertaken in diverse research ar-
eas. Disease Registries collect targeted data about
2
https://biobank.health.nsw.gov.au/
3
https://www.csiro.au/en/work-with-us/industries/healt
h/australian-health-biobank
4
https://anzctr.org.au/
Wang, Z., Stell, A., Soto, J. and Sinnott, R.
e-Consent in Biomedical Research Registries: A GDPR-Compliant Approach Explored in the Context of the Australasian Diabetes Data Network.
DOI: 10.5220/0012327200003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 2, pages 45-55
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
45

individuals who have a specific disease together with
how they are being treated. The Australasian Dia-
betes Data Network (ADDN)
5
is a disease registry fo-
cused on collecting type-1 diabetes data from across
Australia and New Zealand. Unlike other registries,
ADDN directly re-uses existing health data from hos-
pitals and centres, i.e., as opposed to requiring man-
ual data entry to a separate registry. Such registries
are used to help researchers observe patterns, under-
stand diseases, and improve treatments and disease
management guidelines at scale. Integrating consent
mechanisms into biomedical registries is increasingly
seen as a pivotal step in ethical research (Win and
Fulcher, 2007).
A recent review (Skelton et al., 2020) shed light
on the current e-Consent landscape by presenting a
workflow of the informed consent processes (shown
in Figure 1. The right-hand side of the workflow illus-
trates how digital informed consent typically works in
biomedical research. It comprises three elements of
informed consent: information, comprehension and
voluntariness which are supported by different digital
tools. However, the workflow is abstract and does not
consider the fine-grained and evolving privacy regula-
tions of different countries. For example, in the U.S.,
the HIPAA (Health Insurance Portability and Ac-
countability Act)
6
was issued by the US Department
of Health and Human Services (HHS) in 1996. It in-
troduced HIPAA Authorization for Research
7
which
requires researchers and healthcare organisations to
obtain written authorisation from individuals before
using their health information for research purposes.
The European Union’s General GDPR (General Data
Protection Regulation)
8
, which was put into effect in
2018, offers the most advanced privacy legal frame-
work. It takes the protection of personal data, in-
cluding health-related information, to a much finer-
grained and patient-oriented perspective. The consent
conditions for GDPR require that consent is freely
given, specific, informed, unambiguous, and easy to
withdraw (also known as the right to be forgotten).
GDPR is more structured and stringent compared to
HIPAA and empowers individuals in how “their data”
might be used or not as the case might be. In Aus-
tralia, the health data sharing landscape has largely
been shaped by the Privacy Act 1988 (The Act)
9
. This
is currently being amended to align with GDPR and
5
www.addn.org.au
6
https://www.hhs.gov/hipaa/index.html
7
https://privacyruleandresearch.nih.gov/authorization.a
sp
8
https://eur-lex.europa.eu/legal-content/EN/TXT/?uri
=CELEX%3A32016R0679
9
https://www.legislation.gov.au/Details/C2014C00076
other international frameworks (Australian Govern-
ment, 2022).
In this context, biomedical research registries
need to design their electronic consent processes to be
aligned with stringent legal requirements while keep-
ing a delicate balance between individual privacy, re-
search safety, and broader public interest (Win and
Fulcher, 2007). Our work aims to augment the e-
Consent workflow (Figure 1) delineated by (Skelton
et al., 2020) with concrete examples of what it looks
like to capture and enforce e-Consent in a GDPR-
compliant manner in a specific biomedical registry:
the Australasian Diabetes Data Network (ADDN) that
is used for diverse research interests.
2 BACKGROUND
2.1 ADDN Background
Australia has one of the world’s highest rates of type-
1 diabetes (T1D). By September 2022, over 134,000
individuals with T1D were registered with the Na-
tional Diabetes Service Scheme (NDSS)
10
in Aus-
tralia. To better understand and manage this health
challenge, the Australasian Diabetes Data Network
(ADDN - www.addn.org.au) was launched. It was
funded by the Juvenile Diabetes Research Foundation
(JDRF – www.jdrf.org.au) in 2012. The ADDN reg-
istry consolidates longitudinal data from 59 diabetes
centres from across Australia and New Zealand, cap-
turing extensive records from more than 20,000 pa-
tients. This includes over 250,000 hospital visits.
ADDN’s primary mission is to collate T1D health
data from various centres into a single platform.
This unified database helps monitor long-term pa-
tient outcomes, advance T1D research, and enhance
clinical care across Australasia. The University of
Melbourne’s Melbourne eResearch Group (MeG–
www.eresearch.unimelb.edu.au) maintains and sup-
ports the ADDN registry.
Figure 2 shows an example of a subset of the pa-
tient data based on the ADDN schema. As noted,
ADDN re-uses existing health data from hospital sys-
tems. The health data is de-identified at source before
it is populated into the ADDN registry, a Unique Sub-
ject Identifier (USI) is generated using the BioGrid
data linkage platform (www.biogrid.org.au). This
identifier replaces personal identifiers whilst ensur-
ing that data remains traceable for clinical or research
10
https://www.ndss.com.au/about-the-ndss/diabetes-fac
ts-and-figures/
HEALTHINF 2024 - 17th International Conference on Health Informatics
46
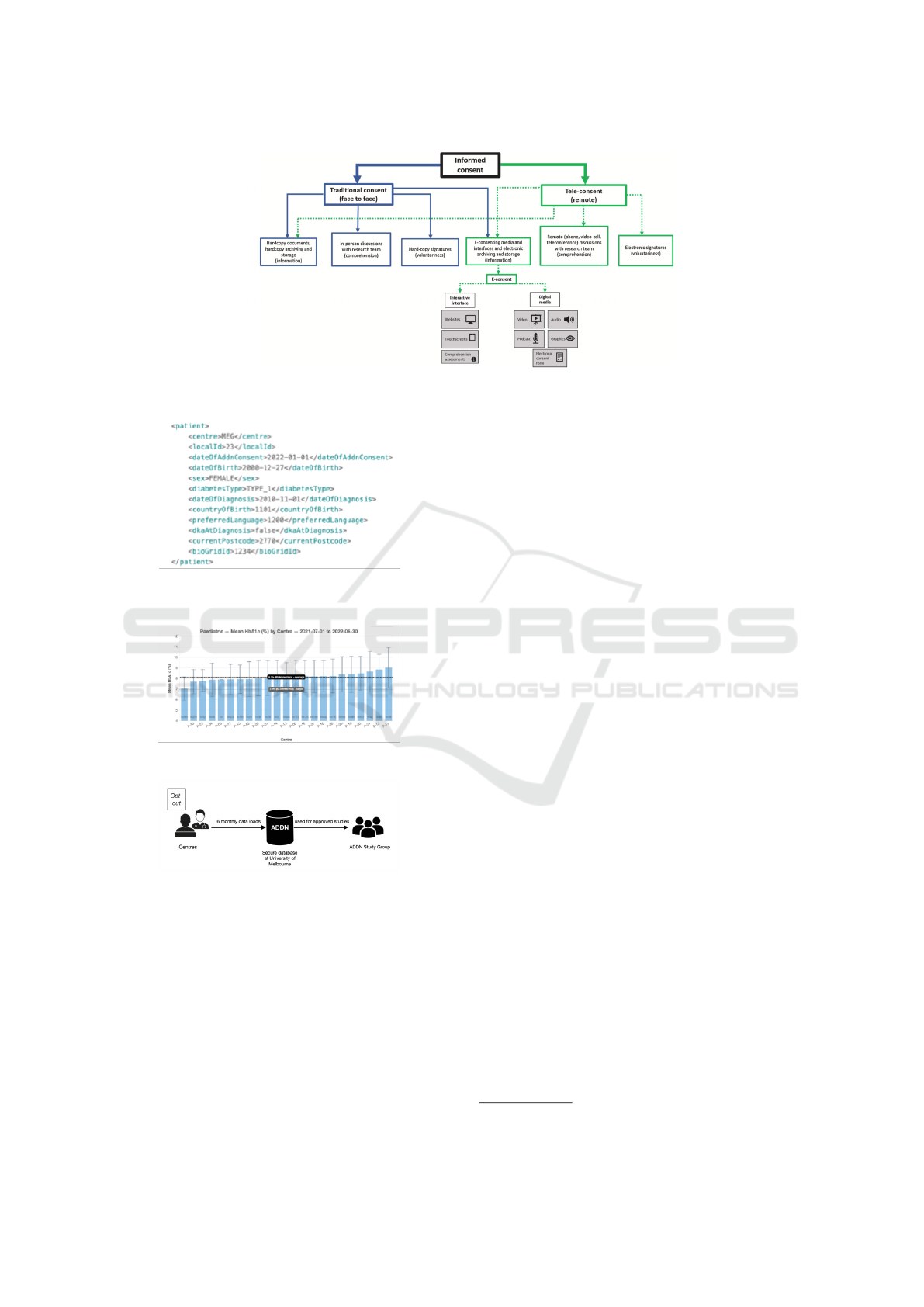
Figure 1: Workflow of informed consent processes from (Skelton et al., 2020).
Figure 2: Example of patient data based on the ADDN
schema.
Figure 3: Example of a site-specific benchmarking report.
Figure 4: ADDN consent process diagram.
purposes without compromising the privacy of the in-
dividuals.
Figure 4 shows the basic process of the current
ADDN consent mechanism. Rather than seeking ex-
plicit permission, e.g., signing a consent form or tick-
ing a consent box from each individual (opt-in), pa-
tients are automatically included in the registry unless
they actively choose to opt-out. The opt-in/opt-out
consent status is captured by clinicians and/or nurses
who provide them with healthcare during hospital vis-
its. As part of this process, they are presented with all
of the details of the ADDN project and explanations
of the consent process. This consent capture has his-
torically been realised by a signed letter. If a partici-
pant chooses not to opt-out after being provided with
this information, the date on which an individual de-
cides not to opt-out (and thereby passively provides
consent) is documented. This is shown as ”dateO-
fAddnConsent” in Figure 2.
After recording the ”dateOfAddnConsent”, pa-
tient data is transmitted to the ADDN registry by cen-
tres. This occurs twice per year. This action indi-
cates “by default” that the data is authorised for use
in subsequent research studies. Such downstream use
of the data is unbeknownst to the patients. This is
not aligned with GDPR, however. As part of ADDN,
centres receive a site-specific benchmarking report as
shown in Figure 4 illustrating how their centre com-
pares to other centres across a range of metrics, e.g.,
average HbA1c for patients at their centre compared
to other centres for example).
Centres and researchers more generally also have
the opportunity to propose studies using the ag-
gregated T1D data from ADDN. Projects undergo
an ADDN-specific approval process decided by an
ADDN Study Group
11
. However, patients are not in-
formed about these projects, hence there is a lack of
patient awareness regarding research initiatives using
their data.
2.2 Background to Consent
Based on a systematic review conducted by (de Man
et al., 2023), it was found that opt-out procedures tend
to yield higher consent rates and result in more repre-
sentative participant samples when compared to opt-
in procedures. This is because opt-out procedures do
not require individuals to take proactive steps to pro-
vide consent. However, it may raise ethical concerns
about personal autonomy when participants are un-
11
https://www.addn.org.au/governance
e-Consent in Biomedical Research Registries: A GDPR-Compliant Approach Explored in the Context of the Australasian Diabetes Data
Network
47

aware of their participation and the subsequent, down-
stream use of their data. (Williams et al., 2015) dis-
cuss the legality of opt-out consent, which can de-
crease potential participation due to unwarranted mis-
conceptions and associated risks thereby impacting
the validity of research outcomes.
Additionally (de Man et al., 2023) identified that
opt-in studies utilizing broad consent tend to achieve
higher consent rates compared to study-specific con-
sent projects. Study-specific consent has been criti-
cised for potentially causing consent fatigue (Dankar
et al., 2020)(Ploug and Holm, 2015)(Holm and Ploug,
2017), since participants receive high volumes of con-
sent requests so that the choice will become routinised
and/or cause them to refuse or withdraw consent due
to the volume of requests (Dankar et al., 2020)(Holm
and Ploug, 2017).
However, it is important to note that while broad
consent offers advantages in terms of a lower admin-
istrative burden, (Haas et al., 2021) identified that
broad consent models raise privacy issues regarding
personal data, potentially resulting in reduced partici-
pation rates in studies. This reduction in participation
rates could also lead to participants feeling as though
they have less control over their data, ultimately giv-
ing rise to trust and ethical concerns (Mamo et al.,
2020).
The one-off opt-out consent model provided to
ADDN patients remains in place unless patients
proactively contact the ADDN project manager to
make changes to their registry status. Patients have
the option to leave the information collected so far
but not permit further collection (partial opt-out) or
request deletion of all information collected (full opt-
out). This approach lacks ongoing patient engage-
ment and involvement in dynamic decision-making.
When centres send their data to the ADDN registry,
patients are not informed about it, nor do they have ac-
cess to the data being collected on them, or the oppor-
tunity to express their preferences regarding the data
use in specific downstream studies.
This lack of ongoing interaction between patients
and the broader research community and the use of
one-off static consent overlooks the dynamic nature
of biomedical research for several reasons. For ex-
ample, the notion of “personal data” is not static
and data can be easily repurposed (Ausloos, 2012).
Some biomedical research registries such as longitu-
dinal studies require the ongoing collection of bio-
logical samples and health-related records (Lee and
Lee, 2022). The future usage of data is often not
static following initial data collection (Kaye et al.,
2015)(Mamo et al., 2020), particularly in rapidly
evolving fields like biotechnology. As a result, par-
ticipant preferences may evolve with changing values
and aspirations (Mascalzoni et al., 2022) and legality
across different jurisdictions in cross-border research
may evolve.
The existing opt-out, broad, one-off consent
framework for ADDN gives rise to many issues, es-
pecially with regard to frameworks such as GDPR.
When assessed against the three pillars of the Bel-
mont Report
12
— information, comprehension, and
voluntariness—the current mechanism is predomi-
nantly aligned with the left-hand side of the work-
flow depicted in Figure 1., as shown in the consent
process diagram (Figure 4), patients receive informa-
tion through a physical patient letter, comprehension
is facilitated by discussions between the clinician and
patients during regular visits, and the exercise of vol-
untariness is limited to opt-out options.
These challenges are further compounded by re-
search demands such as linking ADDN data with
external datasets such as the Australian Institute of
Health and Welfare’s
13
national death index, the Aus-
tralian medical benefits scheme
14
, and the Australian
pharmaceutical benefits scheme
15
. To facilitate this,
introducing more personal identification into ADDN
is essential to avoid exacerbating technical, ethical,
and legal issues.
Given the shift to more rigorous privacy regu-
lations in Australia, it is imperative to design the
consent workflow with GDPR compliance in mind,
to offer participants better control over downstream
use of their personal data. The existing ADDN
consent model, as represented on the left-hand side
of the workflow in Figure 1, is incompatible with
GDPR standards. In the subsequent sections, we
delve deeper into the specific conditions of GDPR
consent and highlight gaps in the current framework
and how to augment the right-hand side of the work-
flow (e-consent) to better capture consent in a GDPR-
compliant manner for biomedical research registries
such as ADDN.
2.3 The GDPR Context
Article 4.7 and 4.8 of GDPR provides definitions for
two crucial roles within the data processing ecosys-
tem. The ’Data Controller’ is the entity responsible
for determining the ’why’ and ’how’ of processing
personal data. In the case of a biomedical research
registry, for example, the institution overseeing the
12
www.hhs.gov/ohrp/regulations-and-policy/belmont-r
eport/
13
https://www.aihw.gov.au/
14
www.mbsonline.gov.au
15
www.pbs.gov.au
HEALTHINF 2024 - 17th International Conference on Health Informatics
48

registry’s operations would be considered the Data
Controller, as it decides the purposes and methods for
collecting and storing biological data.
The ’Data Processor’ is the entity performing ac-
tual actions on behalf of the Data Controller. In a
biomedical research registry scenario, an IT or data
management company contracted by the project to
process associated data would typically assume the
role of Data Processor.
GDPR broadly defines a ’data subject’ as any liv-
ing individual whose personal data is collected, held,
or processed by a particular organisation.
In the context of ADDN, the roles of data con-
troller and data processor are delineated among var-
ious collaborating entities. The direction and pur-
pose of ADDN operations are primarily shaped by
the ADDN governance team. This team comprises
independent external investigators, ADDN investiga-
tors, representatives from JDRF
16
, and a group of pa-
tient advisors. Meanwhile, the actual processing of
patient data — transferring it from hospital systems
and ensuring its alignment with ADDN’s governance
policies — is managed by the software developers of
the Melbourne eResearch Group (MeG). Within the
GDPR’s framework, every living patient registered
within ADDN falls under the definition of a ’data sub-
ject’.
According to Article 7 of GDPR, consent only be-
comes legally valid when it satisfies the five condi-
tions listed below:
“(1) Freely Given - the data subjects must not be
cornered into agreeing, noting that the imbalance be-
tween the data subject and controller can often mak-
ing unencumbered consent difficult, e.g., patients may
feel obliged or have concerns that the treatments they
receive may be inferior if they do not agree. Further-
more, each use of personal data should be given sep-
arate consent.
(2) Specific – the consent must be collected for
certain agreed activities or purposes unless explicitly
identified as “general” research.
(3) Informed - the data subject must fully under-
stand the implications of consent before making a de-
cision. This includes an understanding of data pro-
cessing activities, their purpose and any associated
risks or consequences.
(4) Unambiguous – it should be immediately
clear whether a data subject has consented. Consent
under GDPR cannot be implied or assumed, rather ex-
plicit opt-in consent is required.
(5) Withdrawal – individuals can withdraw their
consent at any time, and this withdrawal should be
made as easy as obtaining the original consent. This
16
www.jdrf.org.au
should result in the removal/deletion of their data
from the registry.”
Based on the GDPR’s five consent conditions, an
ideal consent process in the realm of biomedical re-
search should evolve into a patient-centric, contin-
uous, opt-in, and dynamic engagement mechanism.
This not only maintains GDPR compliance but also
grants individual’s autonomy over use of their data.
2.4 Australian Privacy Law
In Australia, the health data sharing landscape has
predominantly been shaped by the Privacy Act of
1988 (The Act)
17
. Recently in September 2023, the
Australian Government unveiled its response
18
to the
Privacy Act Review Report (Feb 2023)
19
. This is
the culmination of two years of extensive consulta-
tion and review resulting in the release of Issues Paper
(Oct 2020)
20
and Discussion Paper (Oct 2021)
21
.
In this response, the government’s stance on pri-
vacy has been clarified, showing alignment with nu-
merous proposals designed to enhance privacy safe-
guards for individuals.
One of the key points has been the area of con-
sent. Aiming to alleviate burdens on individuals and
avoid consent fatigue, the government has given in-
principle agreement to Proposal 11.1 which seeks to
refine the definition of consent, emphasising that it
must be voluntary, informed, current, specific, and
unambiguous. Moreover, with Principle 11.3, indi-
viduals are given the clear empowerment to withdraw
their consent, insisting that the withdrawal process
should be as straightforward as giving the consent
process.
These developments in Australian privacy stan-
dards align with the consent conditions of the GDPR,
underscoring their global relevance and prominence.
Given these evolving legal landscapes, it is paramount
for Australasia-based research initiatives such as
ADDN, to improve their consent mechanisms. By
aligning with these contemporary standards, not only
will they be adhering to domestic regulations but
this will also ensure compatibility with international
norms, driven by GDPR.
To realise this vision, a digital platform — poten-
17
www.legislation.gov.au/Details/C2014C00076
18
www.ag.gov.au/rights-and-protections/publications/
government-response-privacy-act-review-report
19
www.ag.gov.au/integrity/consultations/review-priva
cy-act-1988
20
www.ag.gov.au/rights-and-protections/publications/re
view-privacy-act-1988-cth-issues-paper
21
https://consultations.ag.gov.au/rights-and-protections
/privacyact-review-discussion-paper/
e-Consent in Biomedical Research Registries: A GDPR-Compliant Approach Explored in the Context of the Australasian Diabetes Data
Network
49
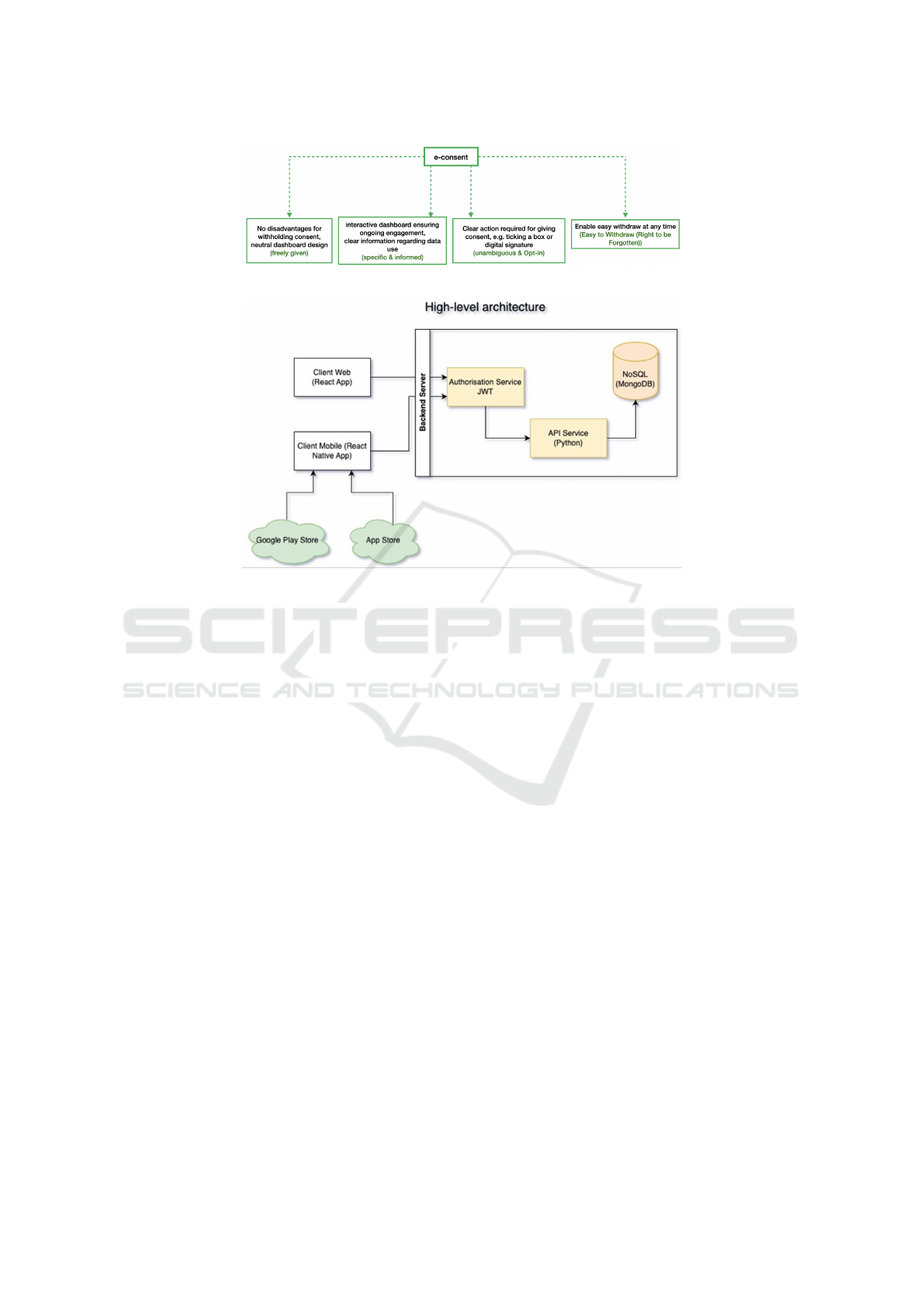
Figure 5: Augmented e-Consent workflow.
Figure 6: High-level architecture of ADDN Consent app.
tially in the form of a web or mobile app — is essen-
tial. This app should not only provide patients with an
interactive interface but also grant them direct access
to their data. They could then comprehend their in-
formation, and based on this, decide if they consent to
its use in specific research projects on an ongoing ba-
sis. The traditional consent process represented in the
left-hand side of (Skelton et al., 2020)’s workflow for
e-consent in Figure 1 would never support such dy-
namic and evolving systems. Furthermore, different
from the right-hand side of Figure 1, this model goes
beyond just digital signatures or list of digital media.
Rather it is about designing a continuous, informed,
and interactive relationship between the patient and
the ongoing use of their data.
3 E-CONSENT WORKFLOW
As discussed above, we have refined the workflow
presented by (Skelton et al., 2020) as shown in Fig-
ure 1. The augmented tree diagram in Figure 5 un-
derscores the foundational pillars of GDPR consent,
essential for an e-Consent platform. Extended from
the “information, comprehension and voluntariness”
requirements, each branch of the tree represents one
of the main GDPR consent conditions and the critical
requirements and features that e-Consent platforms
should integrate to ensure GDPR compliance. This il-
lustration offers practical implementation suggestions
suitable for real-world applications. Guided by our
augmented workflow, the subsequent sections detail
the specific requirements of the e-Consent platform
in the ADDN context.
4 ADDN IMPLEMENTATION
4.1 Overview
To improve the current consent process as described
in section 2, we introduce the ADDN eConsent mo-
bile app, as detailed in (Wang et al., 2022)(Wang
et al., ). Figure 6 shows the high-level architecture
of the application. At the heart of the backend sys-
tem lies the Authorisation Service JWT (JSON Web
Tokens). This service controls the authentication pro-
cesses, ensuring that only authorized users and ser-
vices can access the required data.
Building on this foundation, the platform sup-
ports a Python-based API Service. This service
serves as a conduit between the Authorisation Ser-
vice and the underlying data store, which is realised
as a NoSQL MongoDB. The MongoDB database pro-
vides the scalability and flexibility needed to store
vast amounts of data pertaining to users, consent data,
HEALTHINF 2024 - 17th International Conference on Health Informatics
50
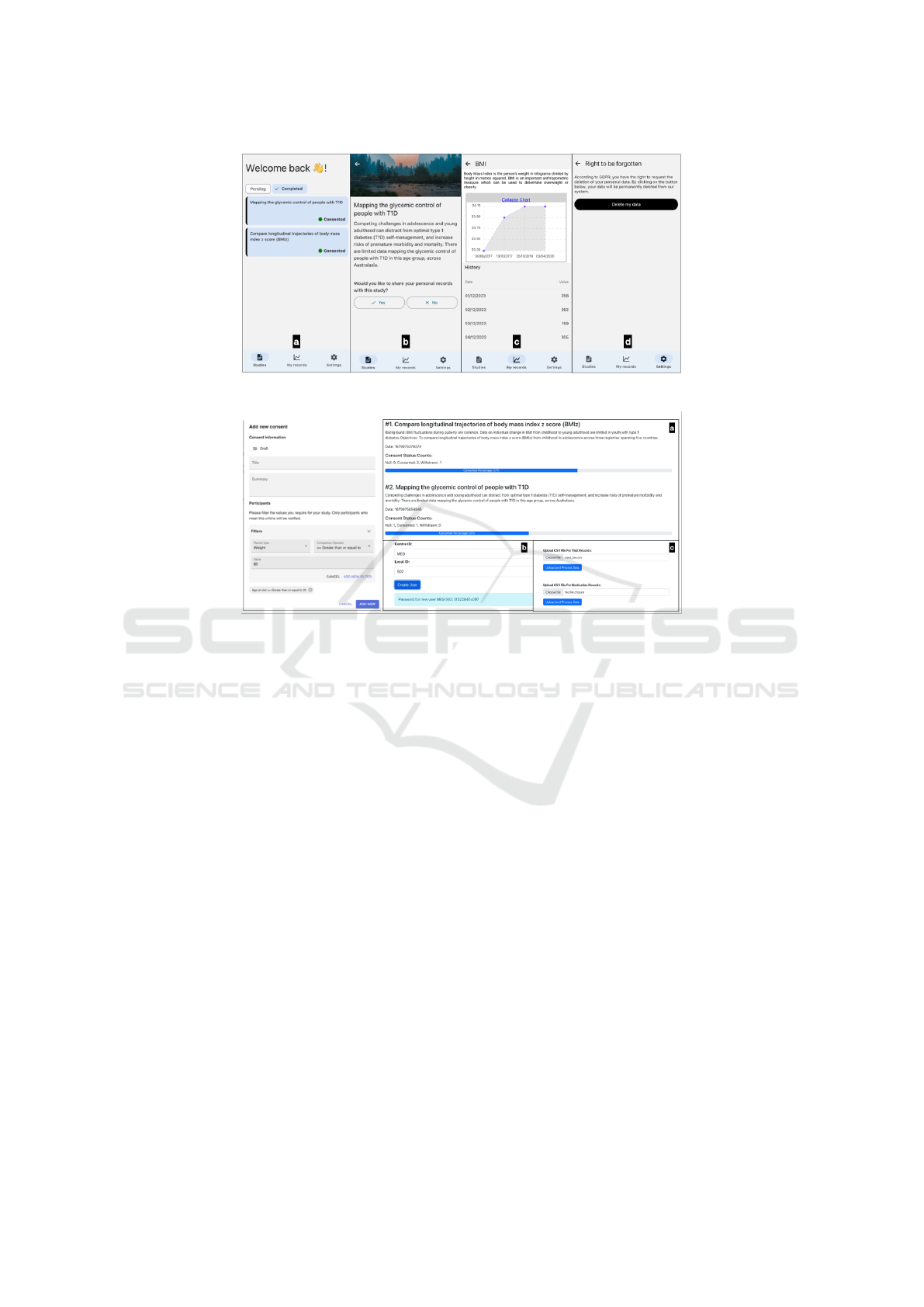
Figure 7: Screenshot of ADDN Consent Mobile Application.
Figure 8: (Left) Screenshot of web application: create a research study (right) Screenshots of web application: Consent
statistics (a), create a user (b) and upload user’s data.
and details of research studies. The Python API Ser-
vice handles the communication between the database
and the client-side applications. In addition, its ar-
chitecture allows connections with external databases,
which can be a valuable feature in future iterations.
For the front end, there are two clients: the Re-
act Web Application (Figure 8) is primarily designed
for the ADDN administrative team clinicians, and the
React Native Mobile Application is tailored for the
end-users and is where the actual consent processes
take place.
During a patient’s regular visit, the clinician will
explain the ADDN project and the usage of the
ADDN consent app. Once the patient agrees to use
the app, the clinician will prepare it for the patient, as
shown in Figure 8(b), with an activation code being
generated for security log-in.
When an ADDN research study is approved, the
administrator can dispatch consent tasks to target
groups as shown in Figure 8 (left) and a notification
indicating a new consent task will be sent to the tar-
get users via their mobile application. They can also
monitor the progress of different consents across var-
ious research studies Figure 8 (right(a)). The inter-
face ensures that admins can keep track of all ongo-
ing activities and make decisions swiftly. At the same
time, patients are fully aware of the projects that are
requesting access to and use of their data and can ac-
cept or refuse such requests on an ongoing basis.
4.2 The Consent App
In this section, we use a table (shown in the Ap-
pendix) to outline the specific consent requirements
associated with the five GDPR conditions based on
the augmented e-Consent workflow presented in Sec-
tion 3. These requirements inform the design of a
compliant and functional e-Consent system.
The e-Consent app offers the ”My Records” dash-
board, where patients can access and review their clin-
ical data. As illustrated in Figure 9, patients can re-
trieve their visit and medication data directly on their
devices. This provides them with direct access to the
data captured about them that exists within the ADDN
registry. This not only reinforces the principle of data
accessibility but also serves as an added incentive for
patients to engage with the app. For example, they
can track key health indicators such as their HBA1c
levels over time or their BMI.
It is noted that to comply with the freely given
e-Consent in Biomedical Research Registries: A GDPR-Compliant Approach Explored in the Context of the Australasian Diabetes Data
Network
51
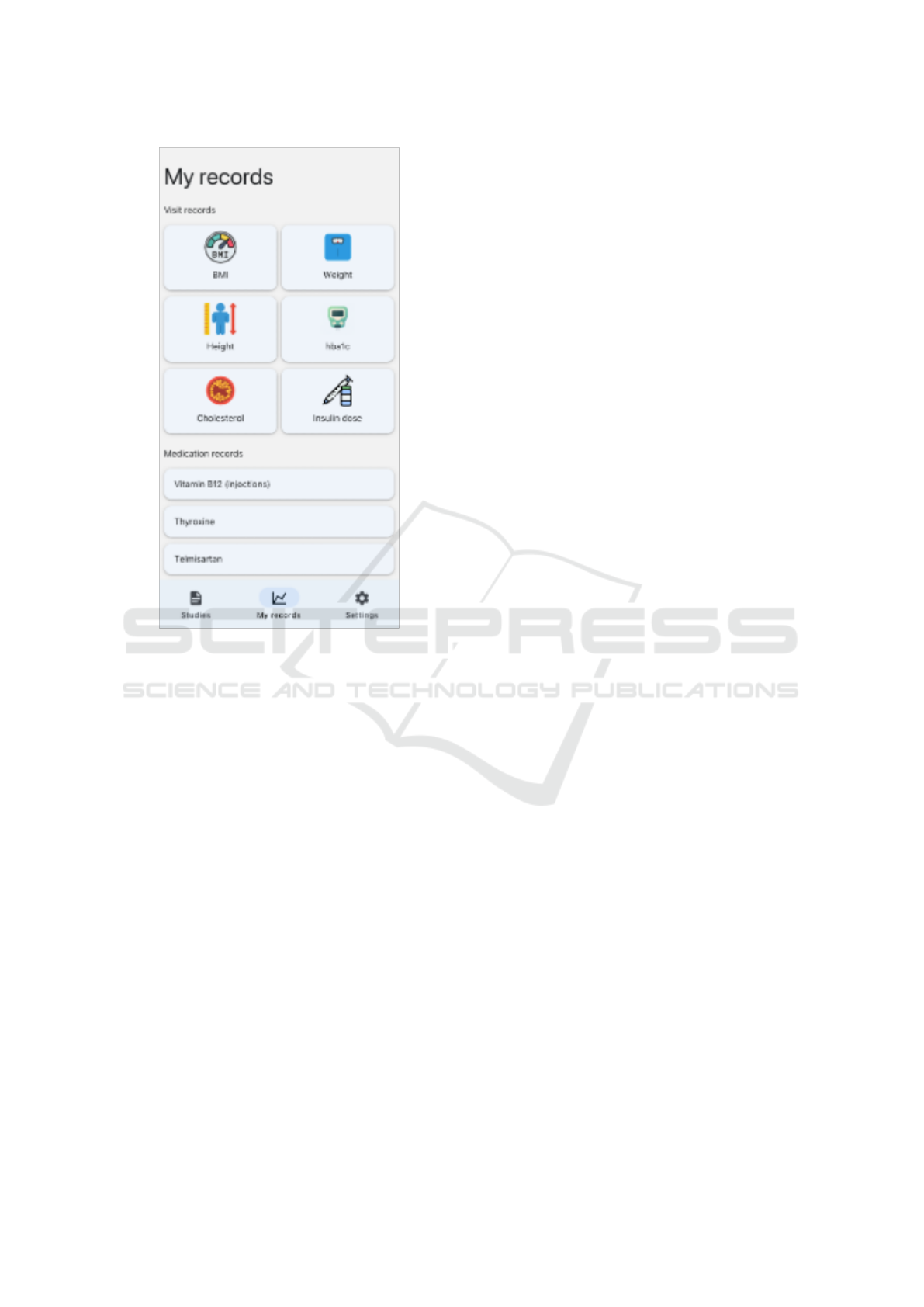
Figure 9: Augmented e-Consent workflow.
condition of GDPR consent, - for those patients who
may opt against using the app - they can still re-
quest their data via traditional means through their
clinicians. Furthermore, for those who use the app
but decide not to provide specific consents to spe-
cific research project requests to use their data, the
”My Records” dashboard remains accessible, ensur-
ing they aren’t disadvantaged or penalised for their
choices.
The app itself requires a unique token to be gener-
ated on the server before it can be used. This is used
for several purposes: to activate the mobile applica-
tion; to identify the end user mobile application (and
hence the anonymised patient) so that they can access
and see their data and get notifications of studies re-
lated to the use of their data, and to encrypt the data
sent between the mobile application and the server.
It is important to note that all of the data within the
ADDN data registry has been anonymised at source.
The mobile application also has no uniquely identify-
ing data that is kept. The web and mobile applications
have been developed based on privacy by design prin-
ciples. There is no need to know the specific individ-
ual details. Instead, all patients are associated with a
unique and system-generated identifier on the server.
5 DISCUSSION
Our augmented workflow of e-Consent and the con-
crete example in the ADDN context offers practical
guidance for other biomedical research registries aim-
ing to comply with strict health data access regula-
tions. However, some limitations must be acknowl-
edged. There is no standard implementation for the
consent application due to the multifaceted nature of
biomedical research demands.
Data controllers still need to consider the specific
requirements of their research registry when deter-
mining the most appropriate consent process. Two
key factors to take into account are altruism and the
stage of the research. For instance, the disease reg-
istry RUDY project (Rare UK Diseases of bone, joints
and blood vessels) (Teare et al., 2017) project has
demonstrated that patients with extensive experience
of their disease can become active partners in re-
search. (Garrison et al., 2016)(Spencer and Patel,
2019) also found that altruistic benefits of sharing
health-related data sometimes outweighs the associ-
ated risks, leading participants to prefer broader con-
sent or even full access (Wallace and Miola, 2021) in
their efforts to contribute to society. The maturity of
a given research registry and the level of trust estab-
lished with its participants should also influence the
introduction of new consent mechanisms. As high-
lighted by (Wallace and Miola, 2021) for mature reg-
istries where participants have developed trust in the
project, introducing a new consent system may intro-
duce risks and potentially jeopardize the relationship
with participants instead of providing benefits. There-
fore, the timing and manner of rolling out novel con-
sent procedures need to be considered. Moreover, not
all research registries might have the technological in-
frastructure or expertise to deploy such advanced e-
Consent platforms. Indeed, this is one of the key fac-
tors that controllers should take into account.
Furthermore, data protection regulations are con-
tinuously evolving. In the Australian context, while
our work is based on the latest government response
, the Privacy Act 1988 is still under review. The final
revised version has yet to be released. As such, there
could be further discrepancies between the finalized
version of this Act and GDPR with regards to consent
conditions. Such disparities may necessitate further
adjustments in the future.
6 CONCLUSIONS
In the evolving landscape of biomedical research,
the move towards electronic informed consent (e-
HEALTHINF 2024 - 17th International Conference on Health Informatics
52

Consent) is inevitable. The up-to-date review of e-
consent by (Skelton et al., 2020) presented a work-
flow of the informed consent processes, but this was
more theoretical than practical and did not consider
evolving privacy mandates and the longitudinal na-
ture of research data and evolving research demands.
In this paper we explore the complexities of aligning
e-Consent mechanisms with stringent global data pro-
tection regulations, notably GDPR.
Through the exploration of the biomedical re-
search registry Australasian Diabetes Data Network’s
(ADDN)’s consent app, we illuminate practical im-
plementations that adhere to such regulations while
enhancing the patient experience.
We also presented an improved GDPR-compliant
consent workflow in biomedical research settings.
This provides guidance to other biomedical research
registries attempting to navigate the complexities of
GDPR-compliant e-consent implementations.
We note that the mobile application is undergoing
advanced testing and will be rolled out as part of the
ADDN project in due course. The adoption, use and
feedback of the application will be explored in down-
stream work.
ACKNOWLEDGEMENTS
The authors would like to thank the ADDN project
partners for the ongoing work and support.
REFERENCES
Ausloos, J. (2012). The ’right to be forgotten’ - worth re-
membering? Computer Law and Security Review,
28(2):143–152.
Australian Government (2022). Privacy act review - discus-
sion paper. Webpage. Accessed on 2023-11-29.
Chimonas, S., Lipitz-Snyderman, A., Matsoukas, K., and
Kuperman, G. (2023). Electronic consent in clinical
care: an international scoping review. BMJ Health &
Care Informatics, 30(1).
Cohen, E., Byrom, B., Becher, A., J
¨
ornt
´
en-Karlsson, M.,
and Mackenzie, A. K. (2023). Comparative effective-
ness of econsent: Systematic review. Journal of med-
ical Internet research, 25.
Dankar, F. K., Gergely, M., Malin, B., Badji, R., Dankar,
S. K., and Shuaib, K. (2020). Dynamic-informed con-
sent: A potential solution for ethical dilemmas in pop-
ulation sequencing initiatives. Comput Struct Biotech-
nol J, 18:913–921.
de Man, Y., Wieland-Jorna, Y., Torensma, B., de Wit, K.,
Francke, A. L., Oosterveld-Vlug, M. G., and Verheij,
R. A. (2023). Opt-in and opt-out consent procedures
for the reuse of routinely recorded health data in scien-
tific research and their consequences for consent rate
and consent bias: Systematic review. Journal of med-
ical Internet research, 25.
De Sutter, E., Zac¸e, D., Boccia, S., Di Pietro, M. L., Geerts,
D., Borry, P., and Huys, I. (2020). Implementa-
tion of electronic informed consent in biomedical re-
search and stakeholders’ perspectives: systematic re-
view. Journal of medical Internet research, 22(10).
Del Carmen, M. G. and Joffe, S. (2005). Informed consent
for medical treatment and research: a review. The on-
cologist, 10(8):636–641.
Garrison, N. A., Sathe, N. A., Antommaria, A. H. M.,
Holm, I. A., Sanderson, S. C., Smith, M. E.,
McPheeters, M. L., and Clayton, E. W. (2016). A sys-
tematic literature review of individuals’ perspectives
on broad consent and data sharing in the united states.
Genetics in Medicine, 18(7):663–671.
Haas, M. A., Teare, H., Prictor, M., Ceregra, G., Vidgen,
M. E., Bunker, D., Kaye, J., and Boughtwood, T.
(2021). ’ctrl’: an online, dynamic consent and partic-
ipant engagement platform working towards solving
the complexities of consent in genomic research. Eur
J Hum Genet, 29(4):687–698.
Hewitt, R. and Watson, P. (2013). Defining biobank. Biop-
reservation and biobanking, 11(5):309–315.
Holm, S. and Ploug, T. (2017). Big data and health re-
search—the governance challenges in a mixed data
economy. Journal of bioethical inquiry, 14:515–525.
Kaye, J., Whitley, E. A., Lund, D., Morrison, M., Teare, H.,
and Melham, K. (2015). Dynamic consent: a patient
interface for twenty-first-century research networks.
Eur J Hum Genet, 23(2):141–146.
Lee, H. and Lee, U. (2022). Toward dynamic consent
for privacy-aware pervasive health and well-being: A
scoping review and research directions. IEEE Perva-
sive Computing.
Mamo, N., Martin, G. M., Desira, M., Ellul, B., and Ebe-
jer, J. P. (2020). Dwarna: a blockchain solution for
dynamic consent in biobanking. Eur J Hum Genet,
28(5):609–626.
Mascalzoni, D., Melotti, R., Pattaro, C., Pramstaller, P. P.,
G
¨
ogele, M., De Grandi, A., and Biasiotto, R. (2022).
Ten years of dynamic consent in the chris study: in-
formed consent as a dynamic process. Eur J Hum
Genet, 30(12):1391–1397.
Mirza, A. B., Khoja, A. K., Ali, F., El-Sheikh, M., Bibi-
Shahid, A., Trindade, J., Rocos, B., Grahovac, G.,
Bull, J., and Montgomery, A. (2023). The use of e-
consent in surgery and application to neurosurgery:
a systematic review and meta-analysis. Acta Neu-
rochirurgica, pages 1–32.
Ploug, T. and Holm, S. (2015). Meta consent: a flexible
and autonomous way of obtaining informed consent
for secondary research. BMJ, 350:h2146.
Skelton, E., Drey, N., Rutherford, M., Ayers, S., and Mala-
mateniou, C. (2020). Electronic consenting for con-
ducting research remotely: A review of current prac-
tice and key recommendations for using econsent-
e-Consent in Biomedical Research Registries: A GDPR-Compliant Approach Explored in the Context of the Australasian Diabetes Data
Network
53

ing. International Journal of Medical Informatics,
143:104271–104271.
Spencer, A. and Patel, S. (2019). Applying the data pro-
tection act 2018 and general data protection regula-
tion principles in healthcare settings. Nursing Man-
agement, 26(1).
Sugarman, J., McCrory, D. C., and Hubal, R. C. (1998).
Getting meaningful informed consent from older
adults: a structured literature review of empirical re-
search. Journal of the American Geriatrics Society,
46(4):517–524.
Teare, H. J. A., Hogg, J., Kaye, J., Luqmani, R., Rush, E.,
Turner, A., Watts, L., Williams, M., and Javaid, M. K.
(2017). The rudy study: using digital technologies
to enable a research partnership. Eur J Hum Genet,
25(7):816–822.
Wallace, S. E. and Miola, J. (2021). Adding dynamic con-
sent to a longitudinal cohort study: A qualitative study
of exceed participant perspectives. BMC Med Ethics,
22(1):12.
Wang, Z., Stell, A., Sinnott, R. O., and Group, A. S. (2022).
The impact of general data protection regulation on
the australasian type-1 diabetes platform. pages 21–
23 July 2022.
Wang, Z., Stell, A., Sinnott, R. O., and The Addn Study, G.
A gdpr-compliant dynamic consent mobile applica-
tion for the australasian type-1 diabetes data network.
Healthcare (Basel), 11(4).
Williams, H., Spencer, K., Sanders, C., Lund, D., Whitley,
E. A., Kaye, J., and Dixon, W. G. (2015). Dynamic
consent: A possible solution to improve patient con-
fidence and trust in how electronic patient records are
used in medical research. JMIR Med Inform, 3(1):e3.
Win, K. T. and Fulcher, J. A. (2007). Consent mechanisms
for electronic health record systems: a simple yet un-
resolved issue. Journal of Medical Systems, 31:91–96.
HEALTHINF 2024 - 17th International Conference on Health Informatics
54

APPENDIX
Table 1: Specific consent requirements associated with the five GDPR conditions based on the augmented e-Consent workflow.
Consent Condition Requirement App Interface Example
Freely Given Consent should be given
without any pressure, en-
suring no imbalance be-
tween the data subject and
controller.
The first screenshot (a) of
the ADDN app shows a
dashboard with a list of
studies. When new stud-
ies are approved by the
ADDN study group, they
are displayed here for tar-
geted participants. Each
study’s entry acts as an in-
vitation, not a command.
The BMI study appears on
the dashboard without any
highlighting or prioritiza-
tion, ensuring users don’t
feel compelled to partici-
pate.
Specific Consent must be collected
for distinct, predefined
purposes.
By selecting a study from
the dashboard, like the
BMI study, users are taken
to (b), which offers de-
tailed information about
the specific study, ensuring
the user knows precisely
what they are consenting
to.
The BMI study informa-
tion clearly outlines the
specific goals and pur-
poses of the study, and
details how the specific
data will be collected and
utilised.
Informed Data subjects should fully
understand the data pro-
cessing activities and any
associated implications.
(b) offers study details and
provides a direct link to the
(c), allowing users to view
the exact data records that
will be used for the study.
For the BMI study, users
can view their BMI data,
ensuring they’re com-
pletely aware of what
information is being used.
Unambiguous & Opt-in It should be crystal clear
whether a user has given
their consent. GDPR de-
mands an explicit opt-in
system.
Within the detailed study
page (b), users have the ex-
plicit choice to ’Consent’
or ’Withdraw’. Only an
active action (like pressing
’Consent’) will register as
the user giving their per-
mission.
If a user decides to con-
sent to the BMI study,
the user has to press the
’Consent’ button; other-
wise, the consent is not
given, and ADDN can’t
use the data.
Ease of Withdrawal Withdrawing consent
should be as simple as giv-
ing it. Upon withdrawal,
their data should be re-
moved from the study.
The ’Withdraw’ option on
the detailed study page (a)
ensures that users can pull
back their consent at any
time, and it will be as easy
as giving the consent.
If a user initially agrees to
the BMI study but later de-
cides against it, they can
simply press ’Withdraw’,
and their BMI data will not
be used, but they can still
consent to other studies.
Right to be Forgotten Users should have the
power to request that all
their data be deleted from
the ADDN platform per-
manently, reflecting the
GDPR’s ”Right to be For-
gotten.”
A ’Delete My Data’ but-
ton (d) lets users remove
all their data from ADDN,
withdrawing from all stud-
ies and permanently eras-
ing their presence on the
platform.
If a user decides to exit the
ADDN platform entirely,
they can press ’Delete
My Data’, wiping out all
their records and simulta-
neously revoking consent
for all studies they had pre-
viously agreed to.
e-Consent in Biomedical Research Registries: A GDPR-Compliant Approach Explored in the Context of the Australasian Diabetes Data
Network
55
