
SAMMI: Segment Anything Model for Malaria Identification
Luca Zedda, Andrea Loddo and Cecilia Di Ruberto
Department of Mathematics and Computer Science, University of Cagliari, Italy
Keywords:
Computer Vision, Deep Learning, Object Detection, Image Processing, Blood Smear Images, Malaria Parasite
Detection.
Abstract:
Malaria, a life-threatening disease caused by the Plasmodium parasite, is a pressing global health challenge.
Timely detection is critical for effective treatment. This paper introduces a novel computer-aided diagnosis
system for detecting Plasmodium parasites in blood smear images, aiming to enhance automation and ac-
cessibility in comprehensive screening scenarios. Our approach integrates the Segment Anything Model for
precise unsupervised parasite detection. It then employs a deep learning framework, combining Convolutional
Neural Networks and Vision Transformer to accurately classify malaria-infected cells. We rigorously evalu-
ate our system using the IML public dataset and compare its performance against various off-the-shelf object
detectors. The results underscore the efficacy of our method, demonstrating superior accuracy in detecting
and classifying malaria-infected cells. This innovative Computer-aided diagnosis system presents a reliable
and near real-time solution for malaria diagnosis, offering significant potential for widespread implementation
in healthcare settings. By automating the diagnosis process and ensuring high accuracy, our system can con-
tribute to timely interventions, thereby advancing the fight against malaria globally.
1 INTRODUCTION
Malaria is a deadly disease caused by the Plasmodium
parasite, and it continues to pose a significant pub-
lic health challenge worldwide, with a high number
of cases and fatalities. According to recent statistics
from the WHO, there were approximately 247 mil-
lion malaria cases and 619,000 deaths in 2021 (WHO,
2022). Most of these cases and fatalities occurred in
Africa, with young children being the most vulnera-
ble group. The disease is primarily spread through the
bites of infected female Anopheles mosquitoes, and it
affects red blood cells (RBCs) in humans, causing a
range of symptoms and complications.
There are five species of the Plasmodium parasite,
which are Falciparum (Pf ), Vivax (Pv), Ovale (Po),
Malariae (Pm), and Knowlesi (Pk). Among these, Pf
is currently the most lethal for humans and is respon-
sible for causing most malaria-related deaths. On the
other hand, P. Vivax and P. Ovale are less harmful,
but they can remain dormant for months in the liver
and then reactivate, leading to acute respiratory dis-
tress syndrome. Pm can remain inactive in the blood
for several years, whereas Pk has a shorter cycle and
is the least fatal of all the species.
Detecting malaria as early as possible is crucial
for quick treatment and management. Various diag-
nostic techniques can be used to diagnose malaria, in-
cluding microscopical analysis of blood smears, rapid
diagnostic tests, or real-time polymerase chain reac-
tion. However, microscopy remains the most pre-
ferred method for diagnosing malaria due to its sen-
sitivity, affordability, and ability to identify parasite
species and density. Nevertheless, microscopy has
several drawbacks, such as the need for highly experi-
enced microscopists, limited access to this diagnostic
method in some rural health facilities, and misdiagno-
sis due to low parasitemia or mixed infections.
In this context, Computer-aided diagnosis (CAD)
systems can provide a viable solution to these chal-
lenges by assisting pathologists in diagnosing dis-
eases and monitoring therapy.
This paper proposes a reliable and novel CAD
system for detecting Pv parasites in blood smear im-
ages. The proposed system utilizes FastSAM for im-
age segmentation and a deep learning approach based
on convolutional neural networks (CNNs) and vision
transformers (ViTs) for cell classification. The sys-
tem aims to automate malaria diagnosis and improve
accessibility in comprehensive screening scenarios.
Identifying tiny parasites in near real-time enables the
detection of different malaria species and a successive
classification of the various life stages.
The performance of the proposed CAD system
Zedda, L., Loddo, A. and Di Ruberto, C.
SAMMI: Segment Anything Model for Malaria Identification.
DOI: 10.5220/0012325500003660
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 19th International Joint Conference on Computer Vision, Imaging and Computer Graphics Theory and Applications (VISIGRAPP 2024) - Volume 3: VISAPP, pages
367-374
ISBN: 978-989-758-679-8; ISSN: 2184-4321
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
367

has been evaluated using the publicly available IML
dataset and compared with existing object detec-
tors. The results demonstrate the effectiveness of
our approach in accurately detecting and classifying
malaria-infected cells. Our proposed system con-
tributes to improved diagnosis and management of
malaria by leveraging advancements in deep learning
techniques.
The main contributions of our work are listed as
follows:
1. We have designed a novel pipeline for detecting
Pv parasites in blood smear images.
2. We propose a novel technique to exploit the seg-
mentation capabilities over an unseen domain.
3. We propose a classification approach studied for a
high parasite-to-RBC imbalance scenario.
4. We define a classification approach for the para-
sites’ life stages classification.
The rest of this work is structured as follows. Sec-
tion 2 describes the current state of the art for malaria
detection and stage classification, Section 3 summa-
rizes the used materials and methods, Section 4 de-
scribes the experimental setup, evaluation and results.
The paper concludes with Section 5, which overviews
the work and draws conclusions proposing possible
future outcomes.
2 RELATED WORK
Malaria detection remains a challenging task, espe-
cially in resource-limited settings. Microscopic ex-
amination is the gold standard for malaria diagno-
sis. Still, it is subjective and prone to errors as it
involves trained microscopists manually inspecting
blood smears to identify and classify malaria para-
sites based on their morphology. In recent years,
computer-vision-based methods have achieved state-
of-the-art results on malaria detection tasks.
In recent years, computer-vision-based methods
have become popular for automated malaria detec-
tion. For example, CNNs are powerful image analysis
tools that can classify malaria-infected cells by learn-
ing features from raw images (Zedda et al., 2022).
Object detection models, such as You Only Look
Once (YOLO) and Faster R-CNN (FRCNN), have
been employed for precise localization and identifica-
tion of malaria parasites within blood smear images.
These models can detect multiple parasites simultane-
ously and provide bounding box annotations, aiding
in accurate diagnosis (Sultani et al., 2022).
Morphology and texture analysis techniques,
rooted in traditional image processing, have also been
utilized for malaria detection. These methods extract
relevant features from images and employ machine
learning algorithms for classification. By capturing
distinctive morphological and textural characteristics
of malaria parasites, these techniques contribute to ac-
curate detection (Loddo et al., 2018).
Transfer learning has proven to be an effective
strategy for malaria detection. Transfer learning en-
ables high accuracy and efficiency in malaria detec-
tion tasks by leveraging pre-trained models on large-
scale datasets, such as ImageNet, and fine-tuning
them on malaria-specific datasets. This approach ben-
efits from the learned representations of general im-
age features and adapts them to the specific context
of malaria detection (Loh et al., 2021).
Several recent studies have utilized the IML
dataset for segmenting and classifying malaria-
infected cells. For instance, Arshad et al. (Arshad
et al., 2022) employed the ResNet50v2, achieving
an accuracy of 95.63%, while Sengan et al. (Sengar
et al., 2022) utilized Vision Transformer, achieving
90.03% for malaria detection. In addition, Mukher-
jee et al. (Mukherjee et al., 2021) achieved a Dice
score of 95.0% for segmenting malaria-infected cells
with a CNN-based approach. These works demon-
strated the effectiveness of deep learning-based mod-
els in malaria parasite analysis on the IML dataset.
3 MATERIALS AND METHODS
3.1 Dataset
The IML dataset (Arshad et al., 2022) consists of
345 images of blood samples taken from individu-
als infected with P. Vivax malaria in Pakistan’s Pun-
jab province. Each image contains approximately 111
blood cells and includes accurate labels indicating the
life stages and red blood cells. Figure 1 provides var-
ious examples of these full-size samples. The dataset
encompasses four parasite life stages: ring (164 sam-
ples), trophozoite (77 samples), schizont (27 sam-
ples), and gametocyte (261 samples). A visual rep-
resentation of these stages can be seen in Figure 2.
The images have a resolution of 1280×960 pixels
and a 24-bit color depth, captured using a microscope-
attached camera magnified at 100x.
3.2 Convolutional Neural Networks
CNNs excel in image classification and object detec-
tion by learning spatial features and handling large
datasets. They comprise multiple layers, including
convolutional, pooling, and fully connected layers.
VISAPP 2024 - 19th International Conference on Computer Vision Theory and Applications
368
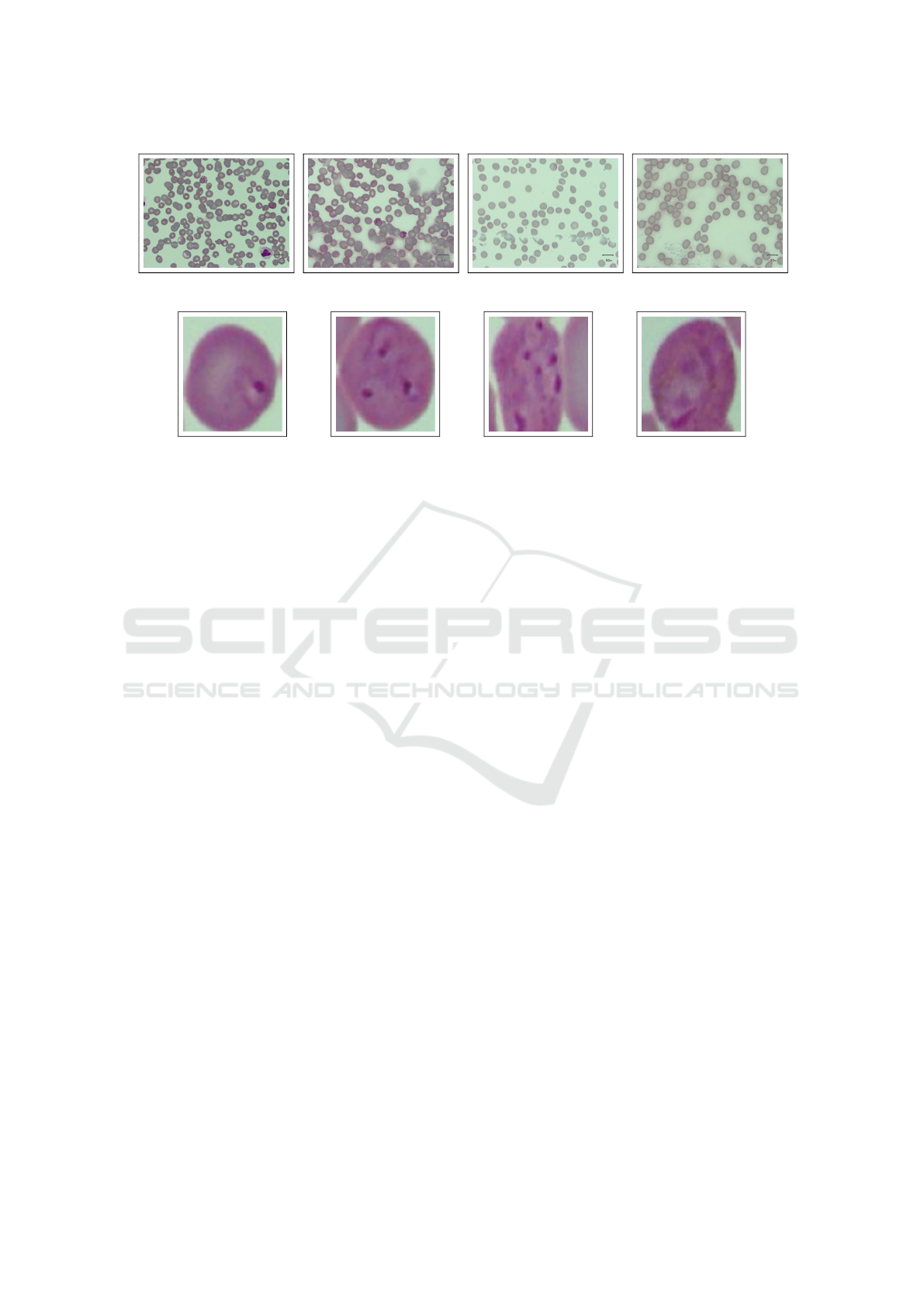
Figure 1: Samples of the full-size images contained in IML.
(a) Ring. (b) Trophozoite. (c) Schizont. (d) Gametocyte.
Figure 2: Life stages of malaria parasites contained in IML. From left to right: ring, trophozoite, schizont, and gametocyte
stage.
CNNs detect simple features like edges in lower lay-
ers and complex features like object parts in higher
layers. Popular architectures include ResNet, In-
ception, and ConvNext. CNNs enable applications
like self-driving cars and medical image analysis.
Ongoing research explores innovations like attention
mechanisms (Woo et al., 2018) and generative mod-
els (Croitoru et al., 2023)..
3.3 Object Detectors and YOLO
Object detection methods rely on CNNs and are
categorized into one-stage and two-stage architec-
tures. Two-stage architectures, such as FRCNN, ex-
tract regions of interest, followed by classification and
bounding box regression. One-stage detectors, such
as RetinaNet and YOLO, directly generate bound-
ing boxes and classes from predetermined anchors.
These detectors are faster and better suited for time-
sensitive applications and devices with computational
constraints (Zou et al., 2023).
The YOLO family employs an end-to-end differ-
entiable network that integrates bounding box estima-
tion and object identification. YOLO divides the input
image into a fixed grid and predicts bounding boxes
and classes for each grid. YOLO is renowned for its
speed and has been utilized for real-time object de-
tection (Wang et al., 2022). in self-driving cars and
surveillance systems (Narejo et al., 2021).
3.4 Vision Transformer
ViT uses a transformer encoder instead of standard
convolutions (Khan et al., 2022; Dosovitskiy et al.,
2021). It performs image classification in two phases:
feature extraction and classification. In the feature ex-
traction phase, the original image is transformed into
a 1D sequence of patches, which undergo a linear
projection and combine 1D position embedding with
patch embeddings. The attention mechanism is a sig-
nificant advancement in computer vision tasks, par-
ticularly with the evolution of transformer architec-
tures and multi-head self-attention (MHSA) (Vaswani
et al., 2017). ViT is more effective than tradi-
tional convolutional neural networks in capturing
long-range dependencies and modeling global im-
age features. However, processing large images with
many patches can be computationally expensive. Sev-
eral techniques have been proposed to address this is-
sue, such as using overlapping patches or hierarchical
patch representations for the SwinTransformer (Liu
et al., 2021; Liu et al., 2022a).
3.5 The Proposed Pipeline
The Segment Anything (SA) project introduces a
novel approach to image segmentation, including a
promptable model called SAM and a large dataset,
SA-1B. SAM is designed to transfer knowledge to
new image distributions and tasks without additional
training. It achieves remarkable zero-shot perfor-
mance, rivaling or surpassing fully supervised mod-
els. The promptable segmentation task focuses on
generating accurate segmentation masks given any
prompt, even in cases of ambiguity. SAM’s archi-
tecture, composed of an image encoder, prompt en-
coder, and mask decoder, enables real-time mask gen-
eration and improves efficiency through prompt reuse
SAMMI: Segment Anything Model for Malaria Identification
369

and cost amortization (Kirillov et al., 2023).
FastSAM (Zhao et al., 2023) proposes a CNN-
based detector and prompt-guided selection in two
stages to tackle the real-time constraint. It achieves
comparable performance to SAM while reducing
computational demands by utilizing the SA-1B
dataset and YOLOv8 appropriately adapted for the
segmentation task, namely YOLOv8-seg, FastSAM.
This paper proposes a novel pipeline for object
segmentation and subsequent classification for para-
site detection on blood smear images. The pipeline
employs FastSAM as the region proposal extractor
and ConvNext-small (Liu et al., 2022b) model for ob-
ject classification. The pipeline diagram is depicted
in Figure 3.
3.6 Metrics
Classification Metrics. To evaluate the models’
performance in classification, we considered several
metrics: accuracy, precision, sensitivity, and F1-
score, which are defined below.
The classification outcome influences the follow-
ing values for a given instance:
• True Negatives (TN): Instances belonging to the
negative class that were correctly predicted.
• False Positives (FP): Instances belonging to the
negative class that were incorrectly predicted.
• False Negatives (FN): Instances belonging to the
positive class that were incorrectly predicted.
• True Positives (TP): Instances belonging to the
positive class that were correctly predicted.
Accuracy (Acc) (see Equation (1)) measures the
overall correctness of the model’s predictions by cal-
culating the ratio of correctly classified samples to the
total number of samples. It is expressed as:
Accuracy =
T P + T F
T P + T F + FP + FN
(1)
Sensitivity (Sen), or Recall (Rec) (see Equa-
tion (2)) measures the ability of the classifier to pre-
dict the positive class against FN:
Sensitivity =
T P
T P + FN
(2)
Precision (Pre) (see Equation (3)) measures the
positive instances correctly classified among all in-
stances classified as positive:
Precision =
T P
T P + FP
(3)
F-Measure (F1) (see Equation (4)) (F1) provides
a balanced evaluation by considering both false posi-
tives and false negatives. The F-Measure is calculated
using the harmonic mean of precision and recall:
F1 = 2 ·
Pre · Rec
Pre + Rec
(4)
Also, we used the macro average as we deal with
an unbalanced dataset, and the number of samples in
different classes varies significantly. It calculates the
metric for each class separately and then takes the av-
erage, providing a balanced assessment of the model’s
performance across all classes.
Detection Metrics. Object detection methods are
commonly assessed using the mean average precision
(AP) metric and its variations (Lin et al., 2015). Preci-
sion relies on the Intersection over Union (IoU) con-
cept to gauge detection accuracy. Specifically, IoU
measures the ratio of the overlap area between the pre-
dicted bounding box and the actual object relative to
the combined total area.
If the IoU surpasses a specific threshold, the detec-
tion is accurate and categorized as a TP. Conversely,
the detection is labeled an FP if the IoU falls below
the threshold. Moreover, if the model fails to detect
an object in the ground truth, it is termed a FN.
As precision for object detection is defined in the
same way as the classification one (see Equation (3)),
the experimental evaluations were conducted consid-
ering five variants of the mAP metric:
• AP is evaluated with 10 different IOUs varying in
a range of 50% to 95% with steps of 5%;
• AP
50
is evaluated with a single values of IOU cor-
responding to 50%;
• AP
s
is the AP determined for small objects (with
area < 32
2
pixels);
• AP
m
is the AP determined for medium objects
(with 32
2
< area < 96
2
pixels);
• AP
L
is the AP determined for large objects (with
area > 96
2
pixels).
average recall (AR) is another widely used met-
ric in object detection, calculating recall values across
various IoU thresholds, akin to AP. For consistency,
we assess AR using identical IoU steps as AP, rang-
ing from 50% to 95% with 5% increments, ensuring
clear and coherent evaluation.
4 EXPERIMENTAL EVALUATION
4.1 Experimental Setup
The experiments were conducted on a desktop PC
equipped with the following hardware specifications:
VISAPP 2024 - 19th International Conference on Computer Vision Theory and Applications
370
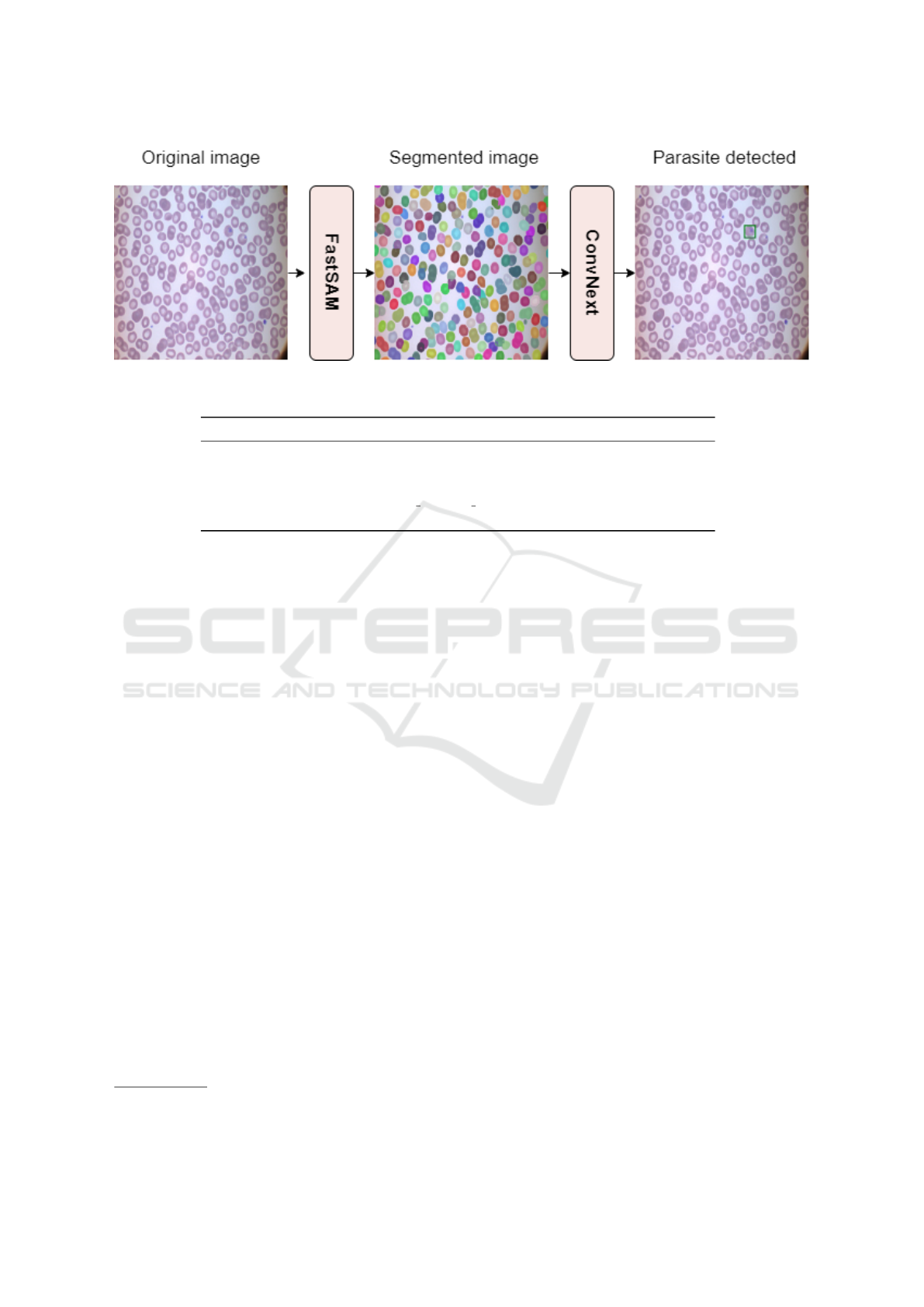
Figure 3: Pipeline visualization using ConvNext as the parasite discriminator.
Table 1: Image augmentation setup.
Augmentation Parameters Probability (%)
Horizontal Flip - 50
Vertical Flip - 50
Random Rotate 90 - 50
Random Resized Crop Size: (img size, img size), Scale: (0.5, 1.0) 50
Grid Distortion Num Steps: 5, Distort Limit: 0.3 50
an Intel(R) Core(TM) i5-9400f CPU operating at
4.1GHz, 32GB RAM, and an NVIDIA RTX 3060 GPU
with 12GB memory.
Training Details. We opted for a confidence thresh-
old of 0.1 for FastSAM predictions and an IoU thresh-
old of 0.8 for NMS. Two distinct classification archi-
tectures, namely ViT-base and ConvNext-base, were
utilized. All networks were initialized using pre-
trained weights from the ImageNet dataset (Deng
et al., 2009). For optimization, we employed the
AdamW optimizer with a learning rate of 1e − 4 and
weight decay of 0.001. Each classification model un-
derwent training for 100 epochs, utilizing a batch size
of 32.
The original IML splits were used for parasite
extraction using FastSAM and life stage classifica-
tion. Additionally, we extracted 10% of the orig-
inal training set to create a validation set. The
best-performing model, determined based on cross-
entropy loss using the validation set, was chosen
as the reference for evaluation. We utilized vari-
ous YOLO versions and sizes to assess the pipeline’s
performance, specifically YOLOv5 and YOLOv8
with medium and large-sized models. The detec-
tion models for comparison were trained for 50
epochs with the default YOLOv8 Ultralytics param-
eters
1
https://github.com/ultralytics/ultralytics.
To ensure the stability and significance of our
method, the experiments were repeated five times,
1
Glenn Jocher, Ayush Chaurasia, and Jing Qiu, YOLO
by Ultralytics, version 8.0.0 (accessed on October 9, 2023
with the starting seed changed at each iteration.
Data Selection and Preparation. Considering the
large number of predicted FastSAM structures, in-
cluding red and white blood cells and cell clumps,
we mitigated the imbalance issue by selecting 25 ran-
dom non-parasitized structures as negative examples.
Additionally, we implemented several augmentation
techniques to balance the number of parasites with the
negative examples. Details of the augmentations em-
ployed are provided in Table 1.
4.2 Experimental Results
Results on Malaria Parasites Detection. The out-
comes of the detection experiments are outlined in
Table 2. Despite possessing lower AP values, our
approach yielded superior AP
50
results. Notably,
YOLO-based object detectors were trained explic-
itly for malaria detection, while FastSAM operates
without requiring any training, facilitating an unsu-
pervised structure detection phase within the adopted
domain. Additionally, our classifier training phase
works on reduced image sizes compared to the origi-
nal full-size images, enabling fast fitting and a modu-
lar discriminator.
However, the proposed methodology pipeline ex-
hibited a recall value of only 0.51, which is inferior
to fully supervised methods. This discrepancy can be
attributed primarily to the presence of clumps formed
by healthy and parasitized red blood cells. Future
investigations should devise effective preprocessing
SAMMI: Segment Anything Model for Malaria Identification
371
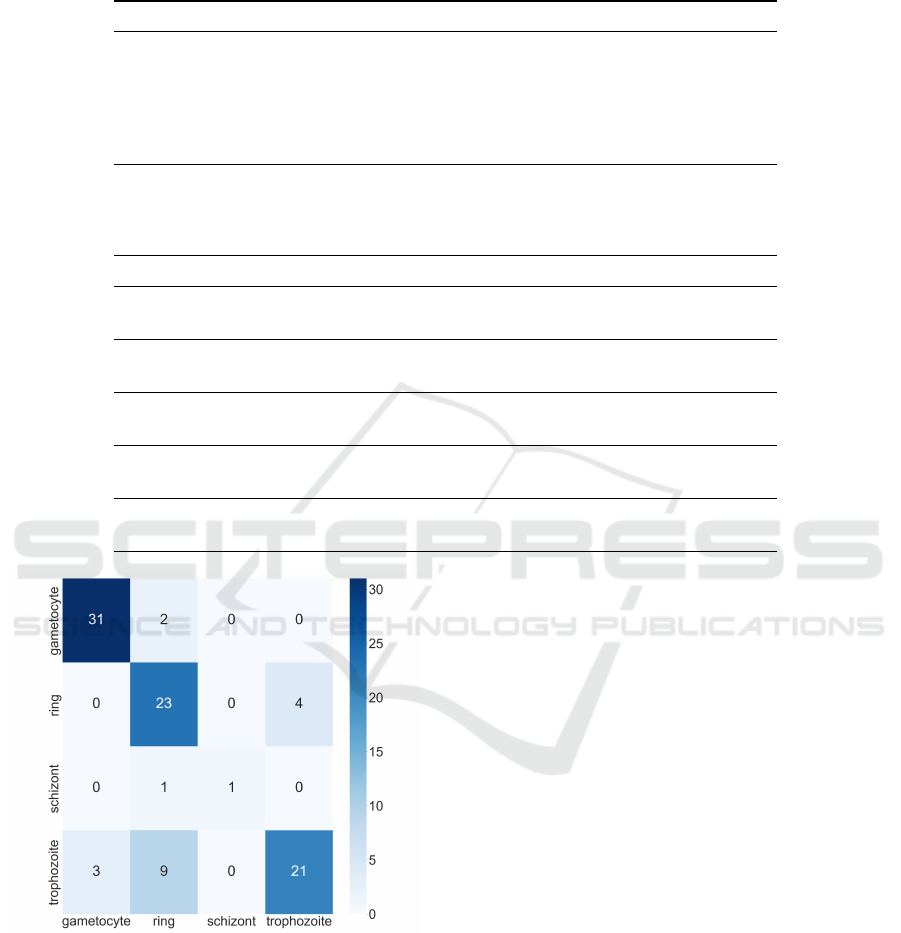
Table 2: Experimental detection results obtained on the IML dataset (Arshad et al., 2022). The reported performance metrics
include AP and AR at different IoU thresholds and AP at different scales. The number of parameters for each model is also
provided. The best results are indicated in bold.
Model AP AP
50
AP
s
AP
m
AP
L
AR Params (M)
YOLOv5m 0.52 0.62 - 0.38 0.56 0.66 21
YOLOv8m 0.54 0.60 - 0.46 0.57 0.67 26
YOLOv5l 0.49 0.56 - 0.35 0.54 0.65 47
YOLOv8l 0.52 0.60 - 0.43 0.56 0.66 44
Our method (w. ConvNext) 0.40 0.85 - 0.31 0.43 0.51 27
Our method (w. ViT) 0.39 0.81 - 0.29 0.41 0.52 86
Table 3: Experimental results are presented for the stage classification task on the IML dataset (Arshad et al., 2022). Original
crops are utilized as training samples, and the evaluation is performed on detected test set crops. The best results for every
type of architecture are indicated in bold.
Model Accuracy F1-score Precision Sensitivity Params (M)
internimage-t 0.65 0.56 0.56 0.62 29
internimage-s 0.30 0.21 0.25 0.21 50
dino-vits16 0.54 0.42 0.44 0.41 21
dino-vitb16 0.76 0.57 0.56 0.58 85
convnextv2-tiny 0.71 0.54 0.56 0.54 27
convnextv2-base 0.75 0.56 0.56 0.57 87
swinv2-tiny 0.74 0.56 0.55 0.56 27
swinv2-base 0.75 0.56 0.59 0.58 86
vit-base 0.76 0.73 0.81 0.69 86
vit-large 0.80 0.76 0.85 0.73 303
Figure 4: Confusion matrix illustrating the results of
malaria parasite life stage classification. Rows represent the
actual life stages (ring, trophozoite, schizont, gametocyte),
while columns indicate the predicted stages.
strategies tailored to address this challenge.
In terms of training time, YOLO-based architec-
tures demand 10 GPU/hours for medium-sized mod-
els and 20 GPU/hours for large-sized ones. In con-
trast, our discriminator necessitates only 1 GPU/hour
of training, making it significantly faster and more
lightweight. These timescales accelerate the train-
ing pace, facilitate rapid experimentation, and enable
further studies, even on computationally limited ma-
chines.
Results on the Life Stage Classification. For fair-
ness, multiple classification models were trained us-
ing the detected parasites, allowing for classification
across various parasite stages. The best-performing
model was evaluated on the validation set, employing
methodologies consistent with those used for the par-
asite discriminator. More precisely, based on its supe-
rior AP, the classification analysis was conducted on
the crops extracted from the top-performing detection
model, specifically YOLOv8m. The comprehensive
experimental results are summarized in Table 3.
The outcomes reveal that the vit-large model
achieved the highest F-measure, reaching 76.45%,
despite the considerable imbalance observed in the
distribution of different stages, as expressed in Sec-
tion 3.1. The confusion matrix of the vit-large model
presented in Figure 4 underscores the challenges as-
sociated with accurate classification, particularly con-
cerning the ring and schizont classes. These classes
exhibit morphological similarities, posing significant
difficulties even for domain experts.
VISAPP 2024 - 19th International Conference on Computer Vision Theory and Applications
372

Figure 5: Examples of clumps extracted by FastSAM.
4.3 Discussion
Real-Time Analysis. The real-time capabilities of
the proposed pipelines were evaluated using our des-
ignated test set. For each image within, the Frames
Per Second (FPS) was computed by averaging the
time taken in milliseconds for the complete para-
sitized cells proposal and filtering process. On aver-
age, the pipeline integrating FastSAM and ConvNext
processed each image in 82 milliseconds (equivalent
to 12 FPS), while the ViT-based pipeline required 86
milliseconds per image (equivalent to 11 FPS) due to
its larger parameter count. The processing time in
milliseconds for each image displayed minimal vari-
ance, typically falling within the range of 350 to 400
proposed regions.
Limitations. While our innovative pipeline show-
cased the potential of the FastSAM architecture in
malaria detection, it warrants further investigation.
The experiments revealed challenges arising from
closely juxtaposed red blood cells forming clumps,
leading the models to inaccurately predict them as
singular objects. These challenges, as depicted in Fig-
ure 5, can be attributed to two primary factors: the
utilization of FastSAM without a fine-tuning strategy
and the inherent issue of clumps, which requires spe-
cific post-processing techniques.
5 CONCLUSIONS
Despite the issues in identifying cell clumps, the pro-
posed malaria detection pipeline, utilizing FastSAM
for object proposal extraction and a subsequent life
stage classification phase composed of ConvNext,
demonstrates that the FastSAM architecture is appli-
cable in malaria detection in a semi-supervised con-
text.
It exhibited remarkable versatility. Beyond its
primary purpose in malaria diagnosis, this pipeline
can be tailored for diverse medical imaging tasks,
from identifying and classifying different types of
blood cells, including white blood cells (leucocytes)
and fragmented red blood cells (schistocytes). This
scenario can showcase its adaptability in diagnosing
blood disorders, infections, and conditions.
The first step of future research is to enrich the
analysis of the segmented clumps by estimating the
number of RBCs. Then, our aim is to study the impact
of different preprocessing steps on full-size images to
improve detection results. Also, the stage classifica-
tion results showed impressive results despite the un-
balanced stages. These results may also be increased
by applying preprocessing to the crops to emphasize
the parasitic structures on the inside of the red blood
cells.
Aside from exploring preprocessing techniques on
the data, we will also test our novel pipeline on dif-
ferent datasets to validate our approach. Finally, we
plan to adopt a non-supervised approach for para-
site discrimination, allowing for fully non-supervised
malaria detection.
Funding
We acknowledge financial support under the National
Recovery and Resilience Plan (NRRP), Mission 4
Component 2 Investment 1.5—Call for tender No.
3277 published on December 30, 2021, by the Italian
Ministry of University and Research (MUR) funded
by the European Union—NextGenerationEU. Project
Code ECS0000038—Project Title eINS Ecosystem
of Innovation for Next Generation Sardinia—CUP
F53C22000430001—Grant Assignment Decree No.
1056 adopted on June 23, 2022, by the Italian Min-
istry of University and Research (MUR).
REFERENCES
Arshad, Q. A., Ali, M., Hassan, S., Chen, C., Imran, A., Ra-
sul, G., and Sultani, W. (2022). A dataset and bench-
mark for malaria life-cycle classification in thin blood
smear images. Neural Comput. Appl., 34(6):4473–
4485.
Croitoru, F., Hondru, V., Ionescu, R. T., and Shah, M.
(2023). Diffusion models in vision: A survey.
IEEE Trans. Pattern Anal. Mach. Intell., 45(9):10850–
10869.
SAMMI: Segment Anything Model for Malaria Identification
373

Deng, J., Dong, W., Socher, R., Li, L.-J., Li, K., and Fei-
Fei, L. (2009). Imagenet: A large-scale hierarchical
image database. In 2009 IEEE conference on com-
puter vision and pattern recognition, pages 248–255.
Ieee.
Dosovitskiy, A., Beyer, L., Kolesnikov, A., Weissenborn,
D., Zhai, X., Unterthiner, T., Dehghani, M., Minderer,
M., Heigold, G., Gelly, S., Uszkoreit, J., and Houlsby,
N. (2021). An image is worth 16x16 words: Trans-
formers for image recognition at scale. In 9th Interna-
tional Conference on Learning Representations, ICLR
2021, Virtual Event, Austria, May 3-7, 2021. OpenRe-
view.net.
Khan, S. H., Naseer, M., Hayat, M., Zamir, S. W., Khan,
F. S., and Shah, M. (2022). Transformers in vision: A
survey. ACM Comput. Surv., 54(10s):200:1–200:41.
Kirillov, A., Mintun, E., Ravi, N., Mao, H., Rolland, C.,
Gustafson, L., Xiao, T., Whitehead, S., Berg, A. C.,
Lo, W., Doll
´
ar, P., and Girshick, R. B. (2023). Seg-
ment anything. CoRR, abs/2304.02643.
Lin, T.-Y., Maire, M., Belongie, S., Bourdev, L., Girshick,
R., Hays, J., Perona, P., Ramanan, D., Zitnick, C. L.,
and Doll
´
ar, P. (2015). Microsoft coco: Common ob-
jects in context.
Liu, Z., Hu, H., Lin, Y., Yao, Z., Xie, Z., Wei, Y., Ning,
J., Cao, Y., Zhang, Z., Dong, L., Wei, F., and Guo,
B. (2022a). Swin transformer V2: scaling up capacity
and resolution. In IEEE/CVF Conference on Com-
puter Vision and Pattern Recognition, CVPR 2022,
New Orleans, LA, USA, June 18-24, 2022, pages
11999–12009. IEEE.
Liu, Z., Lin, Y., Cao, Y., Hu, H., Wei, Y., Zhang, Z., Lin,
S., and Guo, B. (2021). Swin transformer: Hierarchi-
cal vision transformer using shifted windows. In 2021
IEEE/CVF International Conference on Computer Vi-
sion, ICCV 2021, Montreal, QC, Canada, October 10-
17, 2021, pages 9992–10002. IEEE.
Liu, Z., Mao, H., Wu, C., Feichtenhofer, C., Darrell, T.,
and Xie, S. (2022b). A convnet for the 2020s. In
IEEE/CVF Conference on Computer Vision and Pat-
tern Recognition, CVPR 2022, New Orleans, LA,
USA, June 18-24, 2022, pages 11966–11976. IEEE.
Loddo, A., Ruberto, C. D., and Kocher, M. (2018). Recent
advances of malaria parasites detection systems based
on mathematical morphology. Sensors, 18(2):513.
Loh, D. R., Yong, W. X., Yapeter, J., Subburaj, K., and
Chandramohanadas, R. (2021). A deep learning ap-
proach to the screening of malaria infection: Auto-
mated and rapid cell counting, object detection and
instance segmentation using mask R-CNN. Comput.
Medical Imaging Graph., 88:101845.
Mukherjee, S., Chatterjee, S., Bandyopadhyay, O., and
Biswas, A. (2021). Detection of malaria parasites
in thin blood smears using cnn-based approach. In
Mandal, J. K., Mukherjee, I., Bakshi, S., Chatterji, S.,
and Sa, P. K., editors, Computational Intelligence and
Machine Learning, pages 19–27, Singapore. Springer
Singapore.
Narejo, S., Pandey, B., Esenarro Vargas, D., Rodriguez, C.,
and Anjum, M. (2021). Weapon detection using yolo
v3 for smart surveillance system. Mathematical Prob-
lems in Engineering, 2021:1–9.
Sengar, N., Burget, R., and Dutta, M. (2022). A vision
transformer based approach for analysis of plasmod-
ium vivax life cycle for malaria prediction using thin
blood smear microscopic images. Computer Methods
and Programs in Biomedicine, 224:106996.
Sultani, W., Nawaz, W., Javed, S., Danish, M. S., Saadia,
A., and Ali, M. (2022). Towards low-cost and ef-
ficient malaria detection. In IEEE/CVF Conference
on Computer Vision and Pattern Recognition, CVPR
2022, New Orleans, LA, USA, June 18-24, 2022,
pages 20655–20664. IEEE.
Vaswani, A., Shazeer, N., Parmar, N., Uszkoreit, J., Jones,
L., Gomez, A. N., Kaiser, L., and Polosukhin, I.
(2017). Attention is all you need. In Guyon, I., von
Luxburg, U., Bengio, S., Wallach, H. M., Fergus, R.,
Vishwanathan, S. V. N., and Garnett, R., editors, Ad-
vances in Neural Information Processing Systems 30:
Annual Conference on Neural Information Processing
Systems 2017, December 4-9, 2017, Long Beach, CA,
USA, pages 5998–6008.
Wang, C., Bochkovskiy, A., and Liao, H. M. (2022).
Yolov7: Trainable bag-of-freebies sets new state-
of-the-art for real-time object detectors. CoRR,
abs/2207.02696.
WHO, W. H. O. (2022). World Malaria Report 2022.
Woo, S., Park, J., Lee, J., and Kweon, I. S. (2018). CBAM:
convolutional block attention module. In Ferrari, V.,
Hebert, M., Sminchisescu, C., and Weiss, Y., editors,
Computer Vision - ECCV 2018 - 15th European Con-
ference, Munich, Germany, September 8-14, 2018,
Proceedings, Part VII, volume 11211 of Lecture Notes
in Computer Science, pages 3–19. Springer.
Zedda, L., Loddo, A., and Di Ruberto, C. (2022). A deep
learning based framework for malaria diagnosis on
high variation data set. In Image Analysis and Pro-
cessing - ICIAP 2022 - 21st International Conference,
Lecce, Italy, May 23-27, 2022, Proceedings, Part II,
volume 13232 of Lecture Notes in Computer Science,
pages 358–370. Springer.
Zhao, X., Ding, W., An, Y., Du, Y., Yu, T., Li, M., Tang, M.,
and Wang, J. (2023). Fast Segment Anything.
Zou, Z., Chen, K., Shi, Z., Guo, Y., and Ye, J. (2023). Ob-
ject detection in 20 years: A survey. Proc. IEEE,
111(3):257–276.
VISAPP 2024 - 19th International Conference on Computer Vision Theory and Applications
374
