
Breast Cancer Detection Using Smart Wearable Devices with Thermal
Sensors
Raniya Ketfi
1 a
, Zeina Al Masry
1 b
, Noureddine Zerhouni
1 c
, Catherine Gay
3
and Christine Devalland
2 d
1
SUPMICROTECH, CNRS, Institut FEMTO-ST, 24 rue Alain Savary, Besanc¸on, F-25000, France
2
Service d’Anatomie et Cytologie Pathologiques, H
ˆ
opital Nord Franche-Comt
´
e, 100 Route de Moval, 90400 Tr
´
evenans,
France
3
Service de Gyn
´
ecologie Obst
´
etrique, H
ˆ
opital Nord Franche-Comt
´
e, 100 Route de Moval,
90400 Tr
´
evenans, France
fi
Keywords:
Breast Cancer, Breast Thermography, Breast Cancer Detection, Wearable Devices, Thermal Sensors, Smart
Bra, Early Detection.
Abstract:
Breast cancer is the most frequent cause of cancer-related mortalities among women worldwide. Early detec-
tion of breast cancer is one of the best approaches to prevent this disease. In some developed countries, the
5-year relative survival rate of breast cancer patients is above 90% due to early prevention. Many early detec-
tion tools have been developed and used such as mammography, ultrasounds, and magnetic resonance imaging
(MRI). Still, these tools are not always the best in terms of cost, effectiveness, and risk-free. Developing a
more effective, risk-free, and affordable technique for breast cancer detection has always been a necessity to
increase survivability. Authors have found the potential of non-radiative and non-invasive thermography for
anomaly breast detection. This systematic review aims to provide an introduction and guide for smart wear-
able devices for breast cancer detection using thermal sensors by discussing the advantages of these devices
as well as the challenges of developing and implementing them. A total of 6 relevant works drawn from 286
papers on the subject were carefully analyzed, and the information was synthesized. The selected papers were
synthesized according to the design of the wearable device, its data collection, and classification methodolo-
gies. Finally, this review tackles the challenges that come with developing such devices and the great promise
and advantages they hold for early breast cancer detection.
1 INTRODUCTION
Breast cancer is a significant health concern world-
wide as it is the most commonly diagnosed can-
cer worldwide (Sung et al., 2021). According to
the World Health Organization, one in eight women
will be diagnosed with breast cancer in their lifetime
(Michaels et al., 2023). Late detection occurs when
the cancerous cells have metastasized and caused dev-
astating results, however, when breast cancer is de-
tected at its early stages, the survival index may go
up to 9O% in high-income countries (Arnold et al.,
2022). Several methods and techniques are used by
a
https://orcid.org/0009-0003-9224-786X
b
https://orcid.org/0000-0002-6673-0140
c
https://orcid.org/0000-0002-8847-3202
d
https://orcid.org/0000-0002-4128-9264
healthcare to detect breast cancer such as mammog-
raphy, Ultrasound, and Magnetic Resonance Imag-
ing (MRI).While breast cancer screening plays a vi-
tal role in early detection, there are certain limitations
to currently used methods. Mammography requires
compression of the breasts and may cause inconve-
niences to the patient, while exposure to ionizing ra-
diation may even increase the health risk to the patient
(Yaffe and Mainprize, 2011). Dense breast tissue ap-
pears white on mammograms, making it more chal-
lenging to detect abnormalities, as cancerous lesions
can also appear white, women with dense breast tis-
sue may require additional screening methods (Thig-
pen et al., 2018). Ultrasound has its limitations too, as
it may miss smaller masses, resulting in the possibil-
ity of both false-positive and false-negative outcomes
(Halim et al., 2021). Additionally, The quality of ul-
Ketfi, R., Al Masry, Z., Zerhouni, N., Gay, C. and Devalland, C.
Breast Cancer Detection Using Smart Wearable Devices with Thermal Sensors.
DOI: 10.5220/0012309400003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 23-33
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
23

trasound images can vary depending on the operator’s
skill and experience (Xiao et al., 2015). MRI’s rela-
tively high cost and restricted accessibility can limit
its utilization as a routine screening method. Also,
its high sensitivity may detect noninvasive conditions
that may not progress, potentially leading to a false
diagnosis (Hylton, 2005).
The 5-year relative survival rate in 12 sub-Saharan
African countries stood at 66% between 2008 and
2015 (Sung et al., 2021). Also, the mortality due
to breast cancer is higher in women from poorer
countries and also from lower socioeconomic status
(Tao et al., 2014). Breast cancer detection in un-
derdeveloped or poor countries faces unique chal-
lenges that can limit its effectiveness and accessibil-
ity. These challenges may manifest as restricted ac-
cess to healthcare services, financial constraints, and
a lack of awareness regarding breast cancer and its
associated risk factors. These limitations accentuate
the need for a more cost-effective and practical tech-
nique for early breast cancer detection. Many wear-
able devices in the form of bras designed for breast
cancer diagnosis are currently in development and are
at the prototype stage. These devices utilize various
technologies to collect the signal, including thermal
sensors, electrical impedance tomography (EIT), or
ultrasound (Al Masry et al., 2021).
This work explores the emerging field of smart
wearable devices designed for breast cancer detec-
tion using thermal sensors. We explore the underly-
ing principles of thermal sensing and its relevance to
breast cancer diagnosis. By highlighting the unique
advantages and challenges of these wearable tech-
nologies, the study aims to provide a comprehen-
sive overview of their potential impact on improv-
ing breast cancer screening methods. This review is
exclusively dedicated to assessing and analyzing sci-
entific devices aimed at research and diagnostic pur-
poses. We do not cover commercially available prod-
ucts, but instead focus on the technical aspects, per-
formance, and applications of these scientific instru-
ments.
This paper is organized as follows: Section 2 de-
scribes the methodology used in the review. In section
3 we go through the fundamental principles underly-
ing thermal sensing and its relevance to breast can-
cer detection. Section 4 presents and discusses the
devices developed in the selected papers. Section 5
underlies the advantages and challenges of the stud-
ied wearable devices. Finally, section 6 concludes the
works with future research direction.
2 LITERATURE SEARCH
METHOD
The methodology employed in this study aims to
comprehensively review and synthesize the existing
literature on smart wearable devices for breast can-
cer detection using thermal sensors. Through a sys-
tematic approach, we collected, evaluated, and or-
ganized relevant research articles, conference papers,
and technical reports that contribute to the advance-
ment of this field. To identify pertinent sources, we
conducted a rigorous literature search across various
academic databases. A combination of keywords was
employed to ensure the search’s specificity to our in-
terest: Breast cancer, Breast abnormalities, thermal
sensors, and wearable devices. These keywords are
combined with a search query to get relevant articles
only: (”Breast cancer” OR ”breast anomalies” OR
”breast cancer detection”) AND (”Thermal sensors”
OR (”wearable device” AND ”thermography”)). The
review was conducted on the Google Scholar database
as it includes articles from other specific databases
such as PUBMED, IEEE, and SCOPUS. The first
query returned 286 articles. Excluding the thermal
imaging and thermotherapy keywords with a new
query narrowed it down to 231 articles.
To ensure the selection of high-quality and rele-
vant sources, we established clear inclusion and ex-
clusion criteria:
• Inclusion Criteria: Research papers, conference
papers, and technical reports focusing on wear-
able devices for breast cancer detection using ther-
mal sensors; studies involving thermal sensing
principles, device design, experimental evalua-
tions, and clinical applications were included.
• Exclusion Criteria: Papers published before 2000,
review papers, wearable devices combining other
techniques than thermal sensing, works using
thermal imaging instead of thermal sensing, pa-
pers that do not specifically address breast can-
cer detection (such as thermotherapy), or non-
wearable systems.
Papers resulting from the query are screened
based on their title, abstract, and keywords, irrelevant
papers are excluded based on the exclusion criteria
cited above. Those screened papers are then read in
full text by the reviewers to assess their contribution
to the topic of interest. A total of 6 papers from 2007
to 2023 were selected to be the most relevant to this
study.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
24

3 THERMAL SENSING FOR
BREAST CANCER DETECTION
Breast thermography is a non-invasive technique that
uses infrared cameras or sensors to measure and map
the heat patterns emitted by the breasts (Singh and
Singh, 2020). The underlying principle of breast ther-
mography is based on the fact that abnormal cells,
such as cancer cells, generate more metabolic heat
and alter blood flow patterns in the breast tissue.
Breast thermography is a passive, fast, painless,
moderate, and risk-free imaging technique. It has
been documented that thermography when used with
well-defined protocols, can detect early signs of can-
cer 8 to 10 years earlier than mammography (Singh
and Singh, 2020). Thermography was approved as
an adjunct imaging modality to mammography by the
FDA (Food and Drug Administration) in 1982.
Most of the works found in the literature use ther-
mography with infrared cameras to acquire breast
thermal images. Despite being mostly used, thermal
data acquired by infrared cameras can have many lim-
itations. Some limitations are due to external factors
such as ambient temperature, clothing, or contact with
external heat sources that can impact the surface tem-
perature of the breast and introduce noise or artifacts
in the thermal images. These factors need to be care-
fully controlled or accounted for during data acquisi-
tion to ensure accurate and reliable results. Also, the
quality of thermal data acquired using infrared cam-
eras can be influenced by the operator’s skill and tech-
nique. Factors such as camera positioning, calibra-
tion, and image capture settings can affect the consis-
tency and reliability of the acquired data.
To take advantage of thermography’s potential to
detect breast cancer without these limitations, some
works propose to acquire the breast thermal matrix
with highly sensitive thermal sensors put in contact
with the skin. Digital thermal sensors may be con-
sidered advantageous for medical applications due to
their ability to provide reliable temperature measure-
ments across a broad range from -55°C to 150°C
(Meijer et al., 2018). Their exceptional accuracy,
which can achieve a precision of 0.01°C within the
human body temperature range, makes them particu-
larly suitable for medical devices. Furthermore, their
compact size, as small as 1mm x 1mm, facilitates in-
tegration into a wide array of medical equipment and
wearable health devices. Additionally, these sensors
are budget-friendly, typically priced at around 4 to
5 US dollars per sensor, enhancing their accessibil-
ity for various healthcare applications. Sensors can
be placed in direct contact with the breast tissue, al-
lowing for more accurate temperature measurements.
This direct contact ensures better thermal coupling
and reduces the potential interference with external
factors.
Sensor-based breast thermography systems may
be more cost-effective compared to infrared cameras
as sensors are generally smaller, more portable, and
less expensive than infrared cameras, making them
more accessible for healthcare facilities or clinics
with limited resources. Combining sensors’ potential
to detect temperature variation very sensitively with a
wearable device such as a brassiere, bra, or wearable
textiles can create a very promising, non-invasive,
cost-effective, and portable detection tool for breast
cancer.
The next section reviews the papers found in the
literature about wearable devices using thermal sen-
sors for breast cancer detection.
4 A COMPARATIVE STUDY
The papers found in the literature are different in
terms of device maturity, some of them are just a
proposition for a wearable bra for breast cancer detec-
tion supported by numerical simulation with COM-
SOL or simulated breast phantoms and others are in
the clinical trial phase for validation.
This comparative study employs a two-step ap-
proach. First, it comprehensively evaluates each
wearable device featured in the selected papers by
a classification based on wearable device type, the
number and type of thermal sensors employed, and
the duration of data collection during experiments.
Second, the devices are systematically classified and
compared with a focus on their respective detection
methodologies. This analysis includes data types,
the size of the testing population, data preprocess-
ing steps, data analysis methodologies, and the per-
formance of the used methodology.
4.1 Wearable Device Design
The selected papers from 2007 to 2023 used very dif-
ferent wearable devices. These devices exhibited dis-
tinct designs, including lightweight wearable patches
integrated with miniature sensors and conventional
textile brassieres with fixed thermal sensors. The
number of sensors employed in these devices varied
significantly, ranging from 8 to 28 sensors per breast.
Additionally, the papers employed different types of
temperature sensors with varying accuracies (from
0,75°C to 0.01°C). The collection time also varies
from 30 seconds to 24 hours. Table 1 provides a sum-
mary of the reviewed breast cancer detection devices,
Breast Cancer Detection Using Smart Wearable Devices with Thermal Sensors
25

offering insights into their diverse design features, the
number and type of sensors used, as well as the test-
ing duration. Additionally, pictures of the devices are
included alongside the table for more clarity and ref-
erence.
The first known paper to use thermal sensors to
detect breast cancer in women was (Ng et al., 2007).
They used 8 contact thermal sensors per breast. The
design had more sensors in the upper outer quadrant
of the breast as a high proportion of malignant and
benign diseases arises in this quadrant (Lee, 2005).
Cyrcadia breast monitor (S et al., 2020) is an im-
proved version of the first device proposed by (Ng
et al., 2007), more enhancements are added to the
initial version to improve the data capture and analy-
sis processes. The new device is a smart breast patch
equipped with 8 thermal sensors per breast. The ther-
mal sensors used in this device are ADT7420 digital
temperature sensors with a ± 0.25 °C accuracy, which
are compliant with medical device uses as mentioned
in the manufacturer datasheet. The Cyrcadia breast
monitor collects thermal data for 24 hours at every
five-minute intervals.
In (Laila Fadhillah et al., 2018), the authors also
used a brassiere equipped with the same number of
sensors as (Ng et al., 2007) and (S et al., 2020) (8
sensors per breast). However, it is not known if the
used sensors (LM35 with a ±0,75 °C accuracy) are
adequate for medical or clinical use as it is men-
tioned in their data sheet that they are for power
supplies and battery applications. The authors mea-
sured the temperature simultaneously for a duration
of only 30 seconds, which represents the shortest test
duration among all the devices. In (Antony et al.,
2020), the authors introduced a bra design equipped
with a higher number of sensors per breast compared
to previous devices, ranging from 12 to 20 sensors
per breast, depending on the bra size. These sen-
sors were Nickel Manganate-based NTC chip thermal
probes, which were developed in-house and detailed
in (Arathy et al., ), with an accuracy of ±0.01°C. The
authors in (Elouerghi et al., 2022) employed a flex-
ible card design shaped as a star that incorporated a
significantly higher number of sensors compared to
previous studies, their device featured a total of 28
contact thermal mini biosensors per breast. The au-
thors mentioned that the sensors are compliant with
the ASTM E1112 standard (Standard Specification
for Electronic Thermometer for Intermittent Determi-
nation of Patient Temperature) and come with an ac-
curacy of 0.1°C. Finally, (Ashreetha et al., 2023) pro-
posed an IOT-based system to collect breast temper-
ature data with a wearable device (a jacket). In this
work, neither the number of sensors nor their type or
the acquisition protocol were specified.
It is worth mentioning that (S et al., 2020) and
(Antony et al., 2020) are the only papers among the
reviewed studies that introduced variable sizes for
their wearable systems. However, these papers did
not provide extensive details regarding the exact sizes
or the specific number of sensors allocated to each
size variation of the wearable device.
4.2 Detection Methodologies
As previously mentioned, the reviewed devices dis-
play varying levels of maturity and can be categorized
into two distinct groups. Some devices are primar-
ily focused on demonstrating the feasibility of breast
anomaly detection using contact thermal sensors just
with numerical simulation and physical phantoms
such as (Laila Fadhillah et al., 2018) and (Elouerghi
et al., 2022), while others have progressed beyond this
stage to clinically validate their proposed devices.
These key steps applied for breast cancer detection
for all the reviewed devices are summarized in table
2.
4.2.1 Devices Based On Physical Simulation
In the first category, the papers consisted of a proof
of concept for the proposed device. Two of the six
works (Laila Fadhillah et al., 2018) and (Elouerghi
et al., 2022) used a physical phantom and heaters to
mimic the human breast and the tumor in order to col-
lect data. The developed phantoms were very simple.
In (Laila Fadhillah et al., 2018), they used a phan-
tom made of just one layer of agar, while (Elouerghi
et al., 2022) used the same layer and added a 1mm
skin layer made of silicone. Both papers did not men-
tion the thermal properties of the phantom materials
nor highlighted their limitations as they offer a sim-
plified representation of real breast tissue, lacking the
full complexity and dynamic properties of living tis-
sue. Additionally, the two devices were tested for a
very short period of time (30 seconds for (Laila Fad-
hillah et al., 2018) and one minute according to the
graphs of (Elouerghi et al., 2022)).
In (Laila Fadhillah et al., 2018), authors mea-
sured the temperatures simultaneously for 30 sec-
onds and changed the position of the heater in dif-
ferent quadrants of the phantom. The temperatures
were in the range of 27.34°C to 29.79°C for a nor-
mal phantom while in a heated phantom, the range
was from 30.27°C to 34.18° and higher measurements
were captured in the heated quadrants compared to
the other quadrants which proved that these thermal
sensors are able to detect changes of heat in the phan-
tom. These results are supported by the infrared cam-
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
26
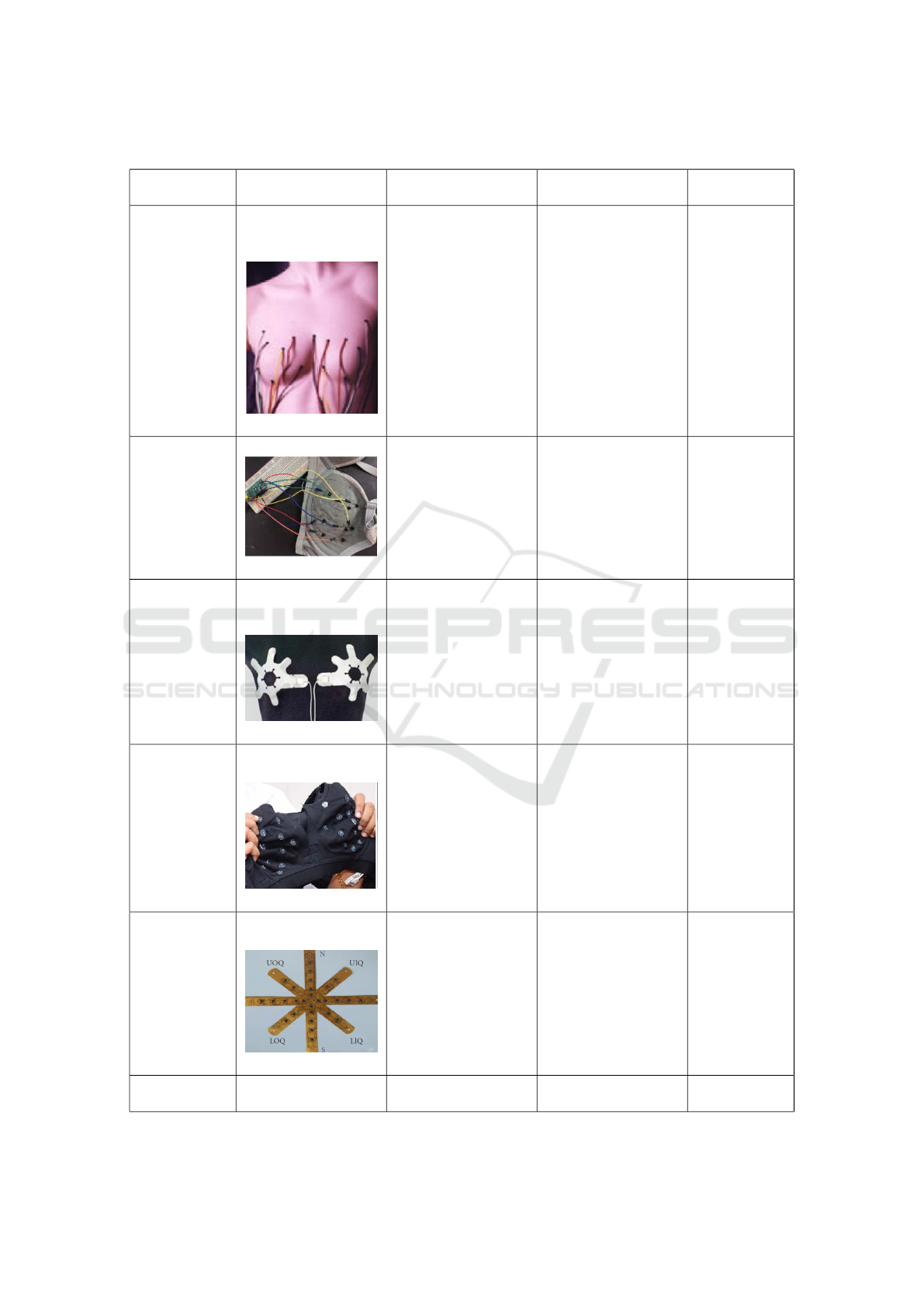
Table 1: A summary of the reviewed smart wearable devices for breast cancer detection using thermal sensors.
Study Device type Number of sensors
per breast
Type of sensors Time of data
collection
(Ng et al.,
2007)
Sensors connected to
a data recording de-
vice with wires.
8 sensors Not mentioned Not men-
tioned
(Laila Fad-
hillah et al.,
2018)
Wearable Brassiere 8 sensors LM35 sensors with
±0,75 °C accuracy
30 seconds
(S et al.,
2020)
Wearable breast
patch in 6 different
sizes
8 sensors ADT7420 sensors
with ±0.25°C accu-
racy
24 hours
(Antony et al.,
2020)
Stretchable bra with
different sizes
From 12 to 20 sen-
sors depending on
the size of the wear-
able device
Nickel Manganate
based thermal sensor
with ±0.01 °C accu-
racy
30 minutes
(Elouerghi
et al., 2022)
Flexible star shaped
card
28 miniature biosen-
sors
Micro biosensors
with 0.1°C accuracy
Not men-
tioned
(Ashreetha
et al., 2023)
Wearable jacket Not mentioned Not mentioned Not men-
tioned
Breast Cancer Detection Using Smart Wearable Devices with Thermal Sensors
27

era reading, where the difference between the sensor’s
measurement and the camera’s measurement was in
the range of 0.82°C to 1.27°C.
Similarly, the authors of (Elouerghi et al., 2022)
conducted a comparative analysis against a reference
phantom without heat sources, displaying minimal
temperature fluctuations (∆T < 0.1°C). Another ex-
periment scenario of a tumor located at a depth of
15mm is done, eliciting a temperature disparity of
+0.6°C. (Elouerghi et al., 2022) also compared the
phantom collected data with the numerical simulated
data to find a 0.11°C maximal difference. They high-
lighted that their study’s ultimate objective is to ex-
plore alternative sensor options, integrate the gathered
data with artificial intelligence models, and undergo
clinical validation for their proposed devices.
4.3 Devices Based on Clinical Trials
Within the second category of devices, those undergo-
ing clinical validation, a notable consistency emerges
in the testing procedures. The authors initiated the
process by defining a target population and establish-
ing inclusion and exclusion criteria. After that, they
performed a preprocessing phase to clean and prepare
the collected thermal data for further analysis. This
analysis step involved either the application of tradi-
tional statistical techniques or the training of machine
learning models. The results were evaluated using
metrics such as accuracy, specificity, and sensitivity.
Data Collection: First, in the data collection step,
authors designated a testing population with specific
inclusion and exclusion criteria. Inclusion criteria
were similar in all clinical tests, consisting mainly
of age (at least 21 years old), recent breast mammo-
gram availability for healthy subjects, and a biopsy
for patient subjects. Common exclusion criteria were:
pregnant or lactating, previous breast mastectomy or
breast surgery or biopsy within the last 90 days for
healthy subjects. In the study conducted by (Ng et al.,
2007), data was collected from a cohort of 54 indi-
viduals, while (Antony et al., 2020) performed clini-
cal tests involving 60 individuals. Notably, (S et al.,
2020) stood out as the sole study that conducted data
collection at two distinct centers spanning two coun-
tries (Clem Plam Breast Clinic in La Plata, Argentina,
and Ohio State University (OSU) in Ohio, USA).
This multi-center approach to clinical trials holds the
potential to enhance population diversity, ultimately
supporting the validity and generalizability of the pro-
posed device. On the other hand, (Ashreetha et al.,
2023) did not mention any details about the clinical
data collection step, such as the number of partici-
pants or the inclusion and exclusion criteria.
Data Processing: Wearable devices continuously
capture a stream of data, and this data may contain
errors or anomalies due to various reasons, includ-
ing sensor inaccuracies, signal noise, or device mal-
functions. For these reasons, the authors performed
data preprocessing before analyzing or driving con-
clusions from the collected data. In (Ng et al., 2007)
and (S et al., 2020), the authors removed missing data
and outliers while (Ashreetha et al., 2023) replaced
irrelevant and missing data with the mean tempera-
ture which may appear as a better way in order to not
lose valuable information from the collected data. Ac-
knowledging the diversity in individual temperature
profiles, authors in (Ng et al., 2007) proceeded to nor-
malize the dataset to a standardized ratio ranging from
0 to 1. Additionally, the authors of (S et al., 2020) and
(Ashreetha et al., 2023) did not use raw temperature
data to detect breast anomalies. (S et al., 2020) used a
wrapper feature selection technique to rank features,
which resulted in using the best 13 features. On the
other hand, (Ashreetha et al., 2023) calculated statisti-
cal features such as Mean, mode, median, range, vari-
ance, and standard deviation in order to use them in a
detection algorithm.
Data Analysis: For analyzing the preprocessed data
in order to detect breast anomalies, the authors used
various detection methodologies. First, (Ng et al.,
2007) used a backpropagation (BPA) neural network
with an input layer, output layer, and 2 hidden lay-
ers and compared it to an RBF-based (Radial Basis
Function) neural network. The RBF model had more
specific, accurate, and sensitive results compared to
BPA yielding 100% classification efficiency for nor-
mal and cancer cases, 92% for benign cases, and 90%
for cancer patients. Second, (S et al., 2020) used
the best-extracted features to train several classifiers
(Decision Trees, Support Vector Machines, Random
Forest, and Back Propagation Neural Networks. . . ).
The classifier and Best Features combination that pre-
sented the best prediction accuracy are chosen as the
final predictive models. It is pertinent to note that
the detailed composition of the extracted features and
the classifier remained undisclosed due to ongoing
patent proceedings. This methodology yielded a pre-
dictive model of considerable performances. This
model demonstrated an accuracy of 78%, sensitivity
of 83.6%, and specificity of 71.5% under a 10-fold
cross-validation. On the other hand, (Antony et al.,
2020) used a different methodology. (Antony et al.,
2020) author’s work consisted of estimating the tu-
mor’s size and depth and reconstructing a 3D ther-
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
28
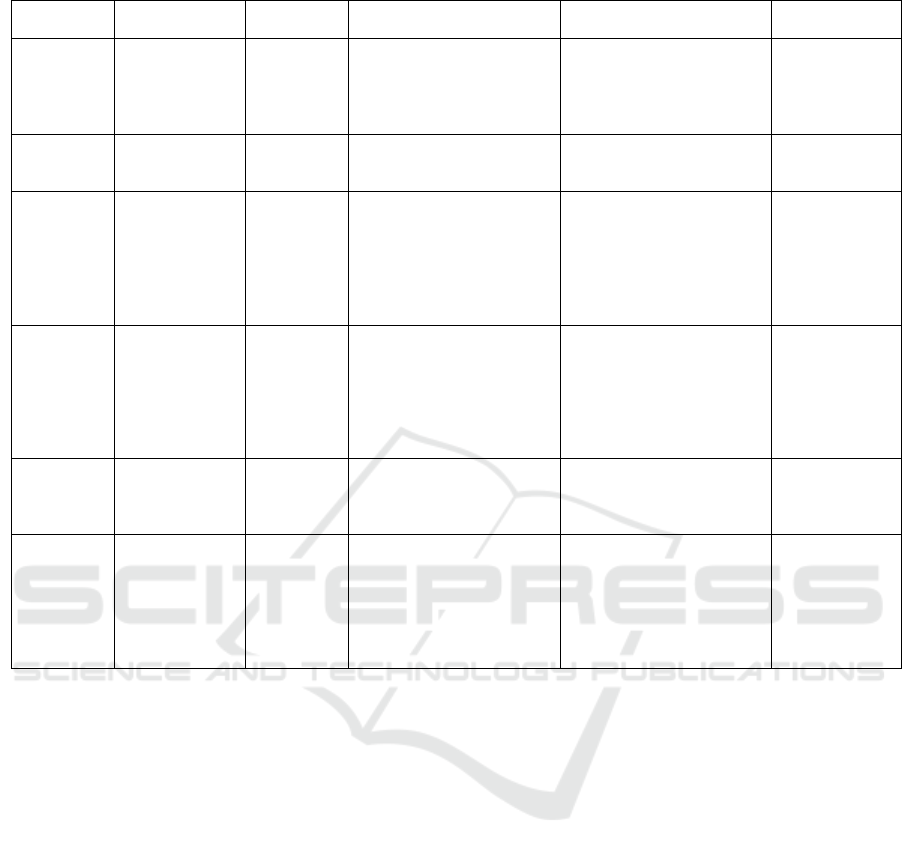
Table 2: Breast cancer detection methodologies.
Study Data Collec-
tion
Population
size
Data preprocessing Data Analysis Results
(Ng et al.,
2007)
Clinical trial 54 individ-
uals
-Removing temperature
from defectuous sensors
and outside the normal
range. - Data normaliza-
tion.
Backpropagation neural
network and Radial basis
function (RBF) classifier
Sensitivity
=91.67%,
Specificity
=100%, Accu-
racy =92%
(Laila Fad-
hillah et al.,
2018)
physical simu-
lation
None None Studied the difference be-
tween a phantom with a
heater and no heater ∆T
2.93°C < ∆ T
< 4.39°C
(S et al.,
2020)
Clinical trial 93 benign
cases and
108 malig-
nant
-Removing missing values
and outliers. -Best Feature
ranking and selection.
Decision Tree, Support
Vector Machines, Random
Forest, and Back Prop-
agation Neural Network
including bagging and
boosting ensemble tech-
niques.
Sensitivity
=83.6%, Speci-
ficity =71.5%,
Accuracy
=78%
(Antony
et al., 2020)
Numerical and
physical simu-
lation, Clinical
trial.
60 females
(29 patients
and 31
healthy)
None Tumor parameter esti-
mation (location, blood
perfusion, diameter, and
metabolic heat generation)
with FEM and genetic
algorithm. 3D thermal
image construction.
Sensitivity=
82.78%, Speci-
ficity= 87.09%,
Accuracy=85%
(Elouerghi
et al., 2022)
Numerical and
physical simu-
lation
None None Compared between phan-
toms temperatures with and
without heaters
T= 0.1°C with
no heater and
T= 0.6°C with a
heater
(Ashreetha
et al., 2023)
Clinical trials 150 obser-
vations
-Null or irrelevant data is
replaced by the mean tem-
perature. -Mean, mode,
median, range, variance,
and standard deviation of
the breast temperature are
calculated.
Statistical features compar-
ison
Not mentioned
mal image of the breast based on the discrete mea-
sured temperature of the surface of the breast. The pa-
rameter estimation methodology consisted of 3 parts:
forward heat transfer problem, inverse heat transfer
problem, and 3D thermal imaging. The forward heat
transform problem is the breast surface temperature
estimation by a breast numerical model using Penne’s
bioheat equation on the software COMSOL. The in-
verse heat transfer problem aimed to minimize the dif-
ference between experimental and simulation results
using an evolutionary optimization algorithm. The
obtained parameter for these experiments is within an
error of 10% (0.005 W.cm
−3
) for heat generation and
15% (0.3 cm) for tumor size. Also, the proposed esti-
mation methodology yielded a sensitivity of 82.78%
and a specificity of 87.09% on the clinical data. In
order to differentiate between normal and abnormal
breasts, (Ashreetha et al., 2023) used a conventional
rule-based algorithm to compare the calculated statis-
tical features to show that the asymmetry analysis of
the left and right breasts could differentiate between
abnormal and normal breasts.
4.4 Evaluation Based on Device
Development Process
The reviewed devices yielded very good perfor-
mances in clinical tests, although neither of the stud-
ies tackled an acceptance study before the clinical
tests. An acceptance study is a phase before the clin-
ical trials where the device is evaluated for its ac-
ceptability, feasibility, and practicality among poten-
tial participants and healthcare providers in order to
improve its efficiency and integration in the current
healthcare process.
Also, the authors of the reviewed papers presented
the performances of their detection methodologies
without interpretation. The papers mentioned that the
advantage of these wearable devices embedded with
thermal sensors is being able to detect breast abnor-
malities better in mammography, especially in dense
breasts and younger women, but no interpretation of
the used detection models was presented based on the
proportion of dense breasts or age in the studied pop-
ulation. Analyzing the detection efficiency based on
Breast Cancer Detection Using Smart Wearable Devices with Thermal Sensors
29

different categories of breast, age, and ethnicity... can
widely support the validation and the utility of the de-
vice.
The papers did not mention a follow-up clini-
cal investigation to assess the long-term performance,
safety, and clinical utility of the device. A follow-up
study is crucial before validation of this type of clin-
ical device, it helps to explore patient-reported out-
comes, including quality of test, comfort, and satis-
faction with the wearable technology.
Cost-effectiveness is one of the major advantages
that these wearable devices can offer, that’s why as-
sessing the cost of the proposed wearable devices
for breast cancer detection is vital for the effective
integration of this technology into healthcare sys-
tems. Unfortunately, this cost analysis was not tack-
led in any of the reviewed devices, despite its impor-
tance. Understanding the cost structure aids in set-
ting fair pricing strategies and ensuring that patients
have access to these potentially life-saving technolo-
gies. Transparent cost assessments promote account-
ability and help optimize the utilization of healthcare
resources, ultimately facilitating the successful inte-
gration of such innovative devices into clinical prac-
tice while ensuring economic feasibility and patient
accessibility.
In order to compare and evaluate the devices pre-
sented in this section, table 3 summarizes this eval-
uation by checking what has been tackled by the re-
viewed papers and their limitations.
5 DISCUSSION
The studies presented about wearable devices embed-
ded with thermal sensors show the potential of this
new technique for detecting breast cancer in an early
stage. Thermal sensors are proven to be capable of de-
tecting specific temperature variations that are able to
indicate the presence of breast abnormalities. These
devices hold great promise in the field of non-invasive
and cost-effective breast cancer detection. In this sec-
tion, we will present the advantages and challenges
of the mentioned studies. Table 4 summarizes the ad-
vantages, challenges, and areas of improvement for
developing wearable devices embedded with thermal
sensors for breast cancer detection.
One of the most significant advantages of these
devices is their ability to detect breast abnormalities at
an early stage. According to (Ng, 2001), it has been
recorded that, with the implementation of carefully
established protocols, thermography has the potential
to identify early signs of cancer approximately 8 to
10 years prior to the detection capabilities of mam-
mography. These devices provide a significant benefit
of being non-irradiative as they don’t expose patients
to any radiations of X-rays and are non-invasive by
measuring the temperature only on the breast surface.
Devices embedded with thermal sensors can be exclu-
sively beneficial for breast abnormalities detection in
dense breasts since conventional methods have prob-
lems of false diagnosis in dense breasts, especially
mammography.
These devices can provide a cost-effective breast
cancer detection method. They eliminate the need
for expensive imaging equipment and reduce the fi-
nancial burden on both healthcare systems and pa-
tients. This affordability can make breast cancer
screening more accessible to a broader population,
including those with limited financial resources. In
many third-world countries, healthcare resources are
concentrated in urban areas, leaving rural regions un-
derserved. Portable wearable devices can be taken
to remote and rural locations, ensuring that women
in these areas have access to breast cancer screening
without the need for long and costly journeys to urban
centers.
In some communities, discussing breast health
or undergoing breast screening may carry stigma or
taboos. Portable wearable devices can help destigma-
tize these topics by offering a discreet and less inva-
sive way to monitor breast health, potentially encour-
aging more women to participate in screening pro-
grams. While the field of wearable devices employing
thermal sensors for breast cancer detection holds im-
mense promise, it is not devoid of challenges. The
pursuit of accurate and reliable detection through this
innovative approach demands a critical examination
of the obstacles that lie ahead. In this discussion, we
unravel the intricacies of these challenges and their
potential impact on the implementation of this trans-
formative technology.
The integration of these devices, with other tech-
nologies holds the potential for advancing breast can-
cer detection in the future. By combining sensors with
artificial intelligence, machine learning, and cloud
computing techniques, we can improve the accuracy
and effectiveness of diagnosing breast cancer. These
advanced technologies have the capability to analyze
data, identify patterns, and offer valuable insights to
healthcare professionals. Moreover, integrating wear-
able devices with telemedicine platforms can enable
monitoring and consultation, thus increasing access
to breast cancer detection, in underserved regions.
Clinical validation is a critical aspect of the de-
velopment and implementation of wearable medical
devices in healthcare. Conducting rigorous studies
to compare the device’s performance against estab-
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
30
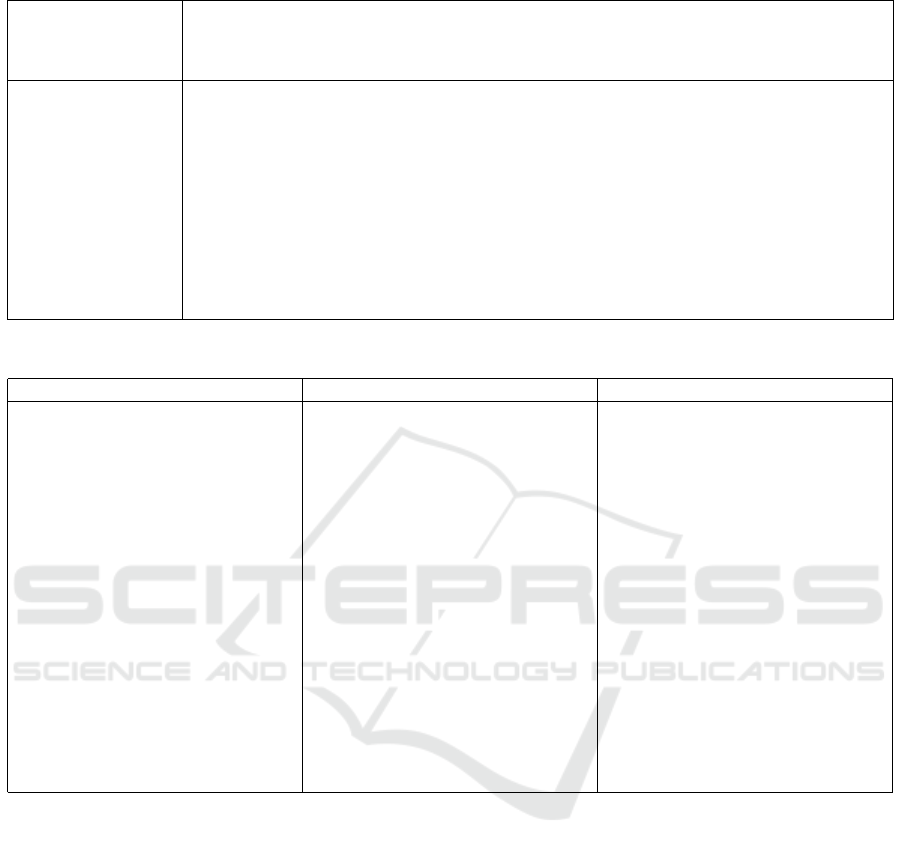
Table 3: Evaluation of the reviewed studies based on a wearable device development process.
Study Phases
Acceptance Numerical Physical Clinical Multi-centric Data follow-up Cost Commercialization
Study Simulation Simulation Tests Clinical tests Cleaning Study Analysis
(Ng et al., 2007) - - - ✓ - ✓ - - -
(Laila Fadhillah et al., 2018) - - ✓ - - - - - -
(S et al., 2020) - - - ✓ ✓ ✓ - - -
(Antony et al., 2020) - ✓ ✓ ✓ - ✓ - - -
(Elouerghi et al., 2022) - ✓ ✓ - - - - - -
(Ashreetha et al., 2023) - - - ✓ - ✓ - - -
CBRA ✓ ✓ ✓ ✓ ✓ ✓ ✓ - -
Table 4: Advantages, challenges, and areas of improvement for developing wearable devices for breast cancer detection.
Advantages Challenges Areas of improvements
• Early detection of breast Abnor-
malities
• Non-irradiative
• Non-invasive
• Effective in dense breasts
• Affordable and cost-effective
• Accessible for women from
low-income countries
• Wearable, painless, and easy to
use
• Physical and numerical simula-
tions complexity
• The size of the testing popula-
tion
• Patient data privacy
• Clinical trials patients recruit-
ment
• User acceptance and usability
of the device
• Integration in current healthcare
workflows
• Enhancing patients recruitment
strategies
• Promoting inclusivity in the
testing population
• Thermal data quality assess-
ment and improvement
• Integrating machine learning
into for analyzing complex ther-
mal patterns to improve the de-
tectio
• Data privacy and security mea-
sures
• Interoapbility with the health-
care system
lished breast cancer diagnostic methods to determine
its sensitivity, specificity, and overall diagnostic ac-
curacy is not very evident. Clinical trials come with
several challenges that need to be carefully addressed
to ensure the reliability of trial results and the safety
of participants.
First, Finding and enrolling a sufficient number
of eligible participants can be challenging. The re-
cruitment step can be very long and challenging for
clinical trials. Second, achieving a diverse participant
population that represents the broader patient popula-
tion can be difficult. In breast cancer detection clin-
ical trials, the target population must include diverse
age categories, breast type, breast cancer types, breast
size, and even underrepresented groups, such as racial
and ethnic minorities. Achieving population diver-
sity in breast cancer detection clinical trials is essen-
tial for ensuring that research findings are relevant,
generalizable, and equitable. Efforts to enhance di-
versity should be integrated into the trial design, re-
cruitment strategies, and participant engagement pro-
cesses, with a focus on addressing barriers to partici-
pation and promoting inclusivity in breast cancer re-
search.
In (Laila Fadhillah et al., 2018) and (Elouerghi
et al., 2022)’s work, authors used only physical or
numerical simulation in tests. While these simula-
tions can help collect and assess the device’s per-
formance, but are not enough to validate its use for
diagnostic purposes. Creating accurate breast tissue
models is challenging. Tissue composition can vary
widely between individuals, and accurately represent-
ing this variability in simulations is complex. Sim-
ulations also should replicate the diversity of breast
Breast Cancer Detection Using Smart Wearable Devices with Thermal Sensors
31

cancer types in size, shape, and location, which is
also very complex due to tumor diversity and inter-
individual variability. Due to these simulation com-
plexities, clinical trials with a sufficient and diverse
population are mandatory to validate wearable de-
vices for breast cancer detection.
Ethical and privacy considerations are of
paramount importance when developing and using
breast wearable devices for breast cancer detection.
These devices collect sensitive health data, and
their usage must adhere to strict ethical and privacy
standards. Obtaining informed consent from users is
crucial, users should fully understand the purpose of
the wearable device, how their data will be collected
and used, and any potential risks or benefits. Breast
wearable devices should employ robust encryption
and data protection measures to safeguard user
information from unauthorized access or breaches.
Ensuring data security is particularly important in the
healthcare context, where data can be sensitive and
personally identifiable.
Apart from diagnostic accuracy, clinical valida-
tion should assess the device’s usability in real-world
clinical settings. Factors such as ease of use, inte-
gration into existing healthcare workflows, and user
acceptance are important considerations to take in fu-
ture works.
6 CONCLUSION
In conclusion, the field of smart wearable devices
equipped with thermal sensors represents a promis-
ing frontier in breast cancer detection. These inno-
vative technologies offer a multitude of advantages,
from non-invasiveness and early detection to acces-
sibility and cost-effectiveness. However, as with any
new technology, there are many challenges to over-
come. Clinical validation, population diversity in tri-
als, ethical considerations, and privacy safeguards are
among the critical issues that demand careful atten-
tion.
Through this review, we can say that smart wear-
able devices with thermal sensors for breast cancer
detection projects are not mature enough to be clini-
cally and widely used, but addressing the challenges
can make these devices more effective, accessible,
and user-friendly. These devices hold the promise
of detecting breast cancer at earlier stages, reducing
healthcare disparities, and transforming breast health
awareness. With continued research, validation, and
collaboration between the medical community and
technology developers, they may well become an ac-
curate and validated breast detection method.
REFERENCES
Al Masry, Z., Zerhouni, N., Gay, C., Meraghni, S., Lodi,
and Devalland, C. (2021). D
´
etection du cancer du sein
`
a l’aide de soutiens-gorge connect
´
es en 2021 : analy-
ses et perspectives. 49(12):907–912.
Antony, L., Arathy, K., Sudarsan, N., Muralidharan, M. N.,
and Ansari, S. (2020). Breast tumor parameter estima-
tion and interactive 3d thermal tomography using dis-
crete thermal sensor data. Biomedical Physics &Engi-
neering Express, 7(1):015013.
Arathy, K., Ansari, S., and Malini, K. A. High reliability
thermistor probes for early detection of breast cancer
using skin contact thermometry with thermal imaging.
10(5):620–628.
Arnold, M., Morgan, E., Rumgay, H., Mafra, A., Singh, D.,
Laversanne, M., Vignat, J., Gralow, J. R., Cardoso, F.,
Siesling, S., and Soerjomataram, I. (2022). Current
and future burden of breast cancer: Global statistics
for 2020 and 2040. The Breast, 66:15–23.
Ashreetha, B., V, D. G., Anandaram, H., A, N. B., Gupta,
N., and Verma, B. K. (2023). Iot wearable breast tem-
perature assessment system.
Elouerghi, A., Bellarbi, L., Khomsi, Z., Jbari, A., Errachid,
A., and Yaakoubi, N. (2022). A flexible wearable ther-
mography system based on bioheat microsensors net-
work for early breast cancer detection: IoT technol-
ogy. Journal of Electrical and Computer Engineering,
2022:1–13.
Halim, A. A. A., Andrew, A. M., Yasin, M. N. M., Rahman,
M. A. A., Jusoh, M., Veeraperumal, V., Rahim, H. A.,
Illahi, U., Karim, M. K. A., and Scavino, E. (2021).
Existing and emerging breast cancer detection tech-
nologies and its challenges: A review. Applied Sci-
ences, 11(22):10753.
Hylton, N. (2005). Magnetic resonance imaging of the
breast: Opportunities to improve breast cancer man-
agement. Journal of Clinical Oncology, 23(8):1678–
1684.
Laila Fadhillah, U. D., Nur Afikah, Z. A., Safiee, N. E. N.,
Asnida, A. W., and Rafiq, M. (2018). Development
of a low-cost wearable breast cancer detection device.
pages 41–46.
Lee, A. H. (2005). Why is carcinoma of the breast more
frequent in the upper outer quadrant? a case series
based on needle core biopsy diagnoses. The Breast,
14(2):151–152.
Meijer, G. C., Wang, G., and Heidary, A. (2018). Smart
temperature sensors and temperature sensor systems.
In Smart Sensors and MEMs, pages 57–85. Elsevier.
Michaels, E., Worthington, R. O., and Rusiecki, J. (2023).
Breast cancer. Medical Clinics of North America,
107(2):271–284.
Ng, E., Acharya, U. R., Keith, L. G., and Lockwood, S.
(2007). Detection and differentiation of breast can-
cer using neural classifiers with first warning thermal
sensors. Information Sciences, 177(20):4526–4538.
Ng, E. Y. K. (2001). Statistical analysis of healthy and ma-
lignant breast thermography. Journal of Medical En-
gineering & Technology, 25(6):253–263.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
32

S, V. S., Royea, R., Buckman, K. J., Benardis, Fletcher,
R. L., Eyk, N., and Acharya, R. (2020). An introduc-
tion to the cyrcadia breast monitor: A wearable breast
health monitoring device. 197:105758.
Singh, D. and Singh, A. K. (2020). Role of image thermog-
raphy in early breast cancer detection- past, present
and future. 183:105074.
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjo-
mataram, I., Jemal, A., and Bray, F. (2021). Global
cancer statistics 2020: Globocan estimates of inci-
dence and mortality worldwide for 36 cancers in 185
countries. CA: A Cancer Journal for Clinicians,
71(3):209–249.
Tao, Z., Shi, A., Lu, C., Song, T., Zhang, Z., and Zhao,
J. (2014). Breast cancer: Epidemiology and etiology.
Cell Biochemistry and Biophysics, 72(2):333–338.
Thigpen, D., Kappler, A., and Brem, R. (2018). The role of
ultrasound in screening dense breasts: A review of the
literature and practical solutions for implementation.
Diagnostics, 8(1):20.
Xiao, Y., Zhou, Q., and Chen, Z. (2015). Automated
breast volume scanning versus conventional ultra-
sound in breast cancer screening. Academic Radiol-
ogy, 22(3):387–399.
Yaffe, M. J. and Mainprize, J. G. (2011). Risk of radiation-
induced breast cancer from mammographic screening.
Radiology, 258(1):98–105.
Breast Cancer Detection Using Smart Wearable Devices with Thermal Sensors
33
