
Significance of Training Images and Feature Extraction in Lesion
Classification
Ad
´
el Bajcsi
a
, Anca Andreica
b
and Camelia Chira
c
Babes
,
–Bolyai University, Cluj-Napoca, Cluj, Romania
{firstname.lastname}@ubbcluj.ro
Keywords:
Breast Lesion Classification, Mammogram Analysis, Shape Features, Random Forest, DDSM.
Abstract:
Proper treatment of breast cancer is essential to increase survival rates. Mammography is a widely used, non-
invasive screening method for breast cancer. A challenging task in mammogram analysis is to distinguish
between tumors. In the current study, we address this problem using different feature extraction and classifi-
cation methods. In the literature, numerous feature extraction methods have been presented for breast lesion
classification, such as textural features, shape features, and wavelet features. In the current paper, we propose
the use of shape features. In general, benign lesions have a more regular shape than malignant lesions. How-
ever, there are exceptions and in our experiments, we highlight the importance of a balanced split of these
samples. Decision Tree and Random Forest methods are used for classification due to their simplicity and in-
terpretability. A comparative analysis is conducted to evaluate the effectiveness of the classification methods.
The best results were achieved using the Random Forest classifier with 96.12% accuracy using images from
the Digital Dataset for Screening Mammography – DDSM.
1 INTRODUCTION
Breast cancer is one of the most common cancer types
among women (Chhikara and Parang, 2022). In 2021,
2.26 million women were diagnosed with this cancer
type worldwide. Accurate diagnosis and timely treat-
ment of breast cancer are crucial for the successful
management of this disease.
Mammography is a widely accepted screening
tool for the early detection of breast cancer. In recent
years, several studies have been conducted to develop
mammogram analysis and classification systems that
can help radiologists.
Breast cancer classification based on mammo-
gram analysis consists in (1) detection of possible le-
sions, and (2)classification of those lesions as malig-
nant or benign. In the current study, we focus on con-
structing a classification system for the second step,
to distinguish the different tumors. The difficulty in
mammogram analysis lies in proper preprocessing of
the images, as mammograms have complex and di-
verse characteristics (such as brightness, contrast, and
resolution). In these systems, it is crucial to select an
a
https://orcid.org/0009-0007-9620-8584
b
https://orcid.org/0000-0003-2363-5757
c
https://orcid.org/0000-0002-1949-1298
appropriate dataset with sufficient variability in tumor
shapes and sizes for effective training of the system.
Numerous feature extraction methods have been
presented in the literature for the classification of
breast lesions, including textural features, shape fea-
tures, and wavelet features. In the present study, our
proposal focuses on using shape features to enhance
the classification process. Generally, benign lesions
are more circular, whereas malignant lesions are more
spiculated. Therefore, we decided to use shape fea-
tures. Unfortunately, this is not a universal rule, as
is evident from our experiments. When training the
model, we should pay special attention to including
examples of outliers in both the training and test sets.
In this paper, we address a subsection to emphasize
the importance of a proper split of the dataset.
Computer-aided systems based on mammogram
analysis are a frequently researched area in the field
of breast cancer detection and classification;hence,
several methods have been proposed for the accurate
identification of tumors. These methods often utilize
machine learning algorithms, such as Support Vec-
tor Machines, Gaussian Mixture Models, Decision
Tree-based methods, and Artificial Neural Networks
(ANNs), to classify breast lesions as malignant or be-
nign based on various features extracted from mam-
mograms. In recent years, several studies have shown
Bajcsi, A., Andreica, A. and Chira, C.
Significance of Training Images and Feature Extraction in Lesion Classification.
DOI: 10.5220/0012308900003636
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 16th International Conference on Agents and Artificial Intelligence (ICAART 2024) - Volume 3, pages 117-124
ISBN: 978-989-758-680-4; ISSN: 2184-433X
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
117

promising results in the classification of breast lesions
using ANNs. However, one limitation of these meth-
ods is the requirement for a large amount of annotated
data for training, which is often challenging to obtain
in the medical field. Additionally, for a computer-
aided system, it is important to explain the outcome of
the classification process to provide transparency and
enhance trust in the system. To address these chal-
lenges, we decided to focus on Decision Tree-based
models, as they offer transparency and interpretabil-
ity in the classification process. The key contribution
of this work is the development and evaluation of a
Decision Tree-based model for the classification of
breast lesions using shape features extracted from 904
mammograms and achieving a classification accuracy
of 96.12%.
The rest of the paper is structured as follows. Ex-
isting approaches are presented in Section 2. Sec-
tion 3 details the approach investigated using shape
characteristics, followed by a discussion of the results
in Section 4. Finally,Section 5 presents the main con-
clusions and future directions.
2 RELATED WORK
Breast mass classification is a critical task in the
field of breast cancer detection, and various computer-
aided systems have been proposed to accurately iden-
tify tumors. In the following paragraphs, we present
existing approaches from the literature.
An important aspect of mammogram analysis is
the extraction of features. In the literature, there
are two main categories of features commonly used
for breast mass classification: shape-based features,
texture-based features, and intensity-based features.
The shape-based features (used in (Paramkusham
et al., 2021; Gurudas et al., 2022; Singh et al., 2020))
focus on the geometric properties of the lesion, such
as its size, shape and contour characteristics. On
the other hand, texture-based features (used in (Shan-
mugam et al., 2020; George Melekoodappattu et al.,
2022)), capture the spatial arrangement and patterns
of the pixel intensities.
Paramkusham et al. (Paramkusham et al., 2021)
applied Beam angle statistics to extract the shape fea-
tures (1-dimensional signature of the mass). In the ex-
periments, the authors included the K-nearest Neigh-
bor (KNN), Support Vector Machine (SVM), and Ar-
tificial Neural Network (ANN) classification methods
and reported the best accuracy of 88.8% using 147
contours from the Digital Dataset for Screening Mam-
mography (DDSM) (Heath et al., 2001; Heath et al.,
1998) to distinguish benign and malignant lesions.
Gurudas et al. (Gurudas et al., 2022) extracted
18 shape features from images of the Curated Breast
Imaging Subset of DDSM (CBIS-DDSM) (Lee et al.,
2017) including area, bounding box, convex image,
convex hull, length of the minor and major axes, cen-
troid, moments, orientation, aspect ratio, eccentricity,
compactness, solidity, extrema, extent and minimum,
maximum and mean intensities. The authors com-
pared the performance of SVM and ANN to differ-
entiate lesions and concluded that ANN outperforms
SVM, reaching 97. 24% accuracy over 92. 91%
achieved by SVM.
Shanmugam et al. (Shanmugam et al., 2020) com-
pared the performance of different texture features
combined with statistical features to classify tumors
from DDSM (323 benign and 323 malignant images).
The authors fed the computed features to an SVM
classifier and achieved a precision of 79. 7% using
the Gray-Level Cooccurrence Matrix (GLCM), 69%
using the Gray-Level Run-Length Matrix (GLRLM),
91. 5% using the Gray-Level Difference Matrix
(GLDM) and 97. 5% using the Local Binary Pattern
(LBP).
In another experiment, Rani et al. (Rani et al.,
2023) segmented the region of interest, followed by
the extraction of the texture and shape characteristics.
Using images from the DDSM the Adaptive Neuro-
Fuzzy Classifier with Linguistic Hedges (ANFC-LH)
achived 73% while Principal Component Analysis
(PCA) SVM reached 72%.
Kurami et al. (Kumari et al., 2023) proposed a
hybrid feature extraction and hybrid feature selection
(HFSE) framework with ANN classification to dis-
tiguish benign and malign lesion from DDSM. In the
feature extraction, the authors included GLCM, Ga-
bor filter, Tamura and LBP features. For feature se-
lection, first, the correlated features are defined by Ex-
tremely randomized trees classifier-based feature se-
lector, and then the final selection is performed with
the ANOVA F-value test. Kurami et al. (Kumari et al.,
2023) reported 94.57% accuracy.
Convolutional Neural Networks (CNNs) have the
advantage of fuzing the feature extraction and clas-
sification steps. Therefore, they have gained signifi-
cant attention in the field of breast mass classification.
Salama and Aly utilized Convolutional Neural Net-
works (CNNs) in their work (Salama and Aly, 2021).
The authors first applied U-Net to segment the lesion,
which was later classified by Inception V3. To distin-
guish between benign and malignant lesions 98.87%
accuracy was achieved. Felcon
´
ı et al. (Falcon
´
ı et al.,
2020) conducted experiments on popular CNNs such
as VGG, ResNet, DenseNet, and Inception with trans-
fer learning. The authors highlighted the importance
ICAART 2024 - 16th International Conference on Agents and Artificial Intelligence
118

of fine-tuning, which can increase performance by
20%, resulting in 84.4% accuracy on DDSM. Singh
et al. (Singh et al., 2020) presented a CNN for the ex-
traction of shape characteristics and classification of
lesions from DDSM in four classes (irregular, lobu-
lar, oval, and round) and achieved 80% accuracy.
Sajid et al. (Sajid et al., 2023) proposed a com-
bination of high- and low-level characteristics. The
authors simplified the original VGG model to cre-
ate the compact VGG (cVGG) in response to a small
number of classes. The deep (high-level) features ex-
tracted by cVGG were concatenated with low-level
features extracted from Histogram of oriented gradi-
ents (HOG) and LBP. Using the resulting characteris-
tics, they achieved 75%, 85% and 91. 5% accuracy,
respectively, using RF, KNN and Extreme Gradient
Boosting (XGBoost) classification methods. The in-
put images were used from CBIS-DDSM. Sajid et al.
(Sajid et al., 2023) concluded that complex CNNs are
not always robust enough for lesion classification and
the positive effect of the combination of features in
this classification problem.
In recent years, there has been a growing inter-
est in ensemble approaches, where different machine
learning methods are combined. Melekoodappattu et
al. (George Melekoodappattu et al., 2022) proposed
a combination of CNN and a “traditional” model.
The second model was a KNN using as input texture
feature extraction (GLRLM) with Maximum Vari-
ance Unfolding (MVU) feature selection to distin-
guish breast lesions. The proposed ensemble model
achieved 95.2% accuracy.
In these diagnostics systems, it is important the
extraction of features that are used in the classifica-
tion. These studies highlight the advantages of shape
features in mammogram classification systems.
3 PROPOSED APPROACH
The objective of the current study is to differentiate le-
sions from mammograms. In our approach, a simple
computer-aided system is constructed using features
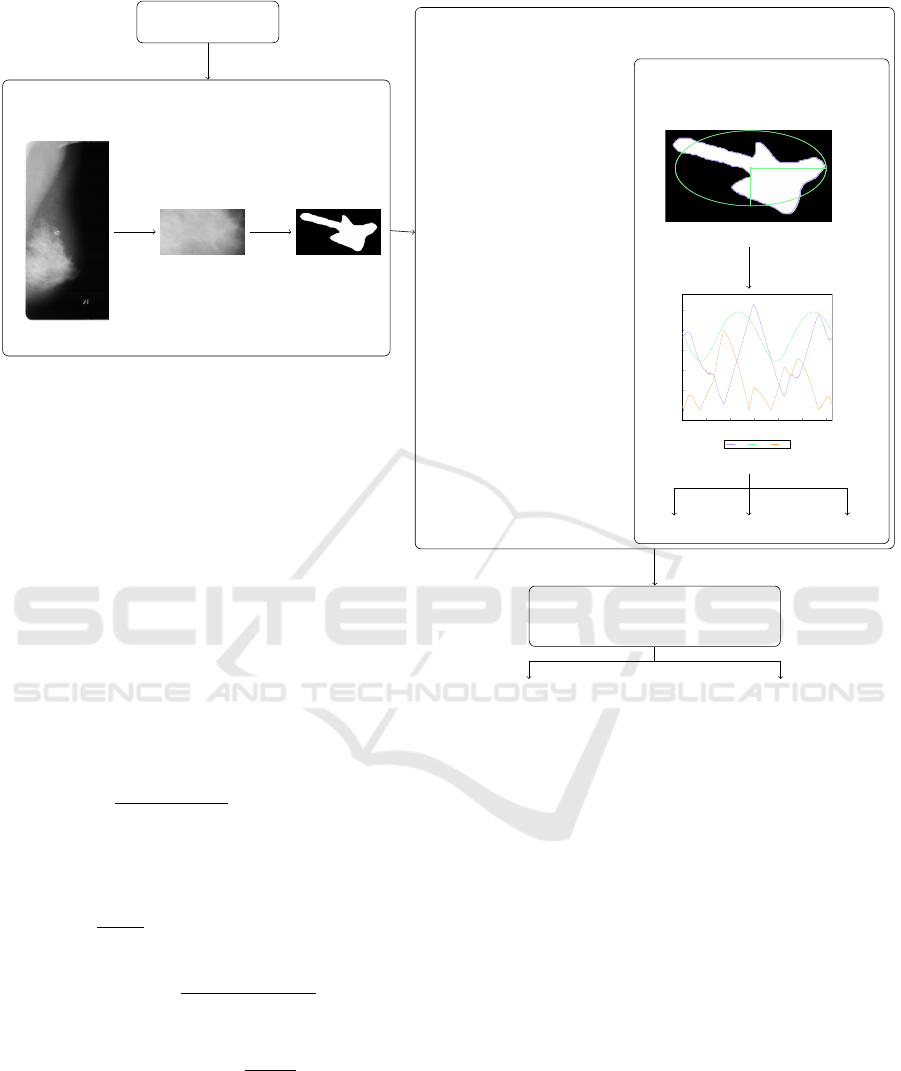
that describe the shape of the tumor. Fig. 1 shows
the flow diagram of our proposed system. In the fol-
lowing paragraphs, the components of this system are
detailed.
3.1 Preprocessing
Mammograms are high-resolution images of breast
tissue. An example is presented in Fig. 1a. In or-
der to extract shape features, the image needs to be
processed by cropping the area of interest (containing
the lesion), as shown in Fig. 1b. The shape charac-
teristics are not influenced by the pixel information;
thus, a binary mask of the tumor (shown in Fig. 1c) is
generated and used in subsequent steps.
3.2 Feature Extraction
Feature extraction plays a crucial role in classifica-
tion systems. As mentioned in Section 1, the literature
presents several types of characteristics used in mam-
mograms, grouped as texture, shape, and wavelet fea-
tures. Shape characteristics capture geometric prop-
erties related to the boundary of the lesion, such as
size (area), perimeter, compactness, irregularity, and
asymmetry. In particular, compared to alternative
characteristics, shape features are the most robust be-
cause they do not depend on intensity, contrast, or
resolution. When presented to radiology specialists,
the shape features are more straightforward, forming
a more understandable decision-making. Last but not
least, shape features are computationally less expen-
sive due to their dependence on the contour of the
lesion instead of the pixels of the lesion and its sur-
rounding. It is crucial to note that defining the exact
boundaries of the lesions is required in order to com-
pute the shape features.
To distinguish between benign and malignant le-
sions, we decided to use shape features. In gen-
eral, benign lesions have a more regular (circular)
shape, whereas malignant lesions have a more irreg-
ular (spiculated) shape. Therefore, we extracted two
types of shape features from the lesions: (1) geomet-
rical and (2) contour-based (Li et al., 2017).
Geometrical features are simple features that are
used as a baseline, including the perimeter, area, and
compactness of the lesion. The contour-based fea-
tures (Li et al., 2017; Bajcsi and Chira, 2023), on
the other hand, are based on the boundary informa-
tion of the lesion. In the first step, an ellipse is fitted
around the lesion by defining its center (C), minor (b)
and major (a) axis lengths, and a rotation angle (α).
The fitted ellipse to a malignant lesion is presented in
Fig. 1d. Next, the distance between C and each point
at the lesion boundary is calculated (d
l
) as well as the
corresponding point distance from the ellipse (d
e
), re-
sulting in a chart similar to Fig. 1e. We denote by ∆d
the difference between d
l
and d
e
. Finally, the irreg-
ularity of the contour is measured by the root mean
slope (R∆q defined in equation (1)), root mean rough-
ness (Rq defined in equation (2)) and the circularity
(defined in equation (3)) of ∆d, detailed in (Li et al.,
2017). In the experiments carried out, we further
investigated the influence of computing the features
mentioned above in subsegments (s equal segments
Significance of Training Images and Feature Extraction in Lesion Classification
119
MAMMOGRAM IMAGE
PREPROCESSING
(a) Original image
(b) Cropped lesion (c) Mask of
the lesion
FEATURE EXTRACTION
Geometrical features
area
perimeter
compactness
Contour-based features
C
a
b
(d) Fitted ellipse
0 200 400 600 800 1,000 1,200
0
50
100
150
200
250
position on the contour
distance
d
l
d
e
∆ d
(e) Computed distances
R∆ q
Rq
circularity
CLASSIFICATION
Decision Tree
Random Forest
benign malignant
Figure 1: The flow of the system. Illustrations of contour feature extraction are used from (Bajcsi and Chira, 2023).
for s ∈ S , S = {1, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20}).
R∆q =
s
µ
∑
i
β
(i)
2
, where
β
(i)
isthe local tilt aroundpoint i
β
(i)
=
1
60∆d
i
∆d
i−3
− 9∆d
i−2
+ 45∆d
i−1
− 45∆d
i+1
+ 9∆d
i+2
− ∆d
i+3
(1)
Rq =
q
µ(∆d
2
) − µ(∆d)
2
(2)
circularity =
µ(∆d)
σ(∆d)
(3)
3.3 Classification
From a lesion mask, three geometrical features and S
contour-based features are extracted. Due to the rela-
tively small number of features in the present experi-
ment, all of them are fed to the classification model.
To classify the lesion based on the extracted features,
two models are used, namely (1) Decision Tree (DT)
and (2) Random Forest (RF). These models can gen-
eralize from fewer inputs than ANNs because they do
not have hidden parameters, which have to be opti-
mized during the training. Furthermore, tree-based
models offer interpretability due to their structure,
giving them an advantage over ANNs.
DTs are nature-inspired models and can be repre-
sented as a series of if-then-else structures, where
every branch either defines the output class or con-
tains another if statement. RFs make their decisions
by constructing multiple DTs and summarizing their
results.
The disadvantage of these models is their ten-
dency to overfit, failing to generalize the features
given in the training set. To address this problem,
we propose the use of ensemble models (RF). On the
other hand, pre-pruning is also applied.
ICAART 2024 - 16th International Conference on Agents and Artificial Intelligence
120

4 EXPERIMENTS AND RESULTS
The scope of our experiment is to distinguish the char-
acter of lesions based on shape features using the sys-
tem presented in Section 3. In the following subsec-
tion, the selected dataset and the achieved results are
presented.
4.1 Data Processing
In the current experiments, images from the Digital
Mammography Screening Dataset (Heath et al., 1998;
Heath et al., 2001) are used from side view (medi-
olateral oblique MLO). The dataset contains for each
mammogram a corresponding mask of the lesion, thus
allowing the extraction of shape features. The DDSM
dataset is made up of 1,440 instances of cancerous
cells that are classified as benign (712 samples) or
malignant (728 samples). In order to have a balanced
dataset, we randomly selected 712 images from the
malignant class, resulting in a dataset containing 1424
images.
After conducting preliminary experiments, pre-
sented in (Bajcsi and Chira, 2023), we decided to fur-
ther analyze the lesions recorded in the dataset. The
analisys revealed that the border (and the calculated
features) of benign and malignant lesions had small
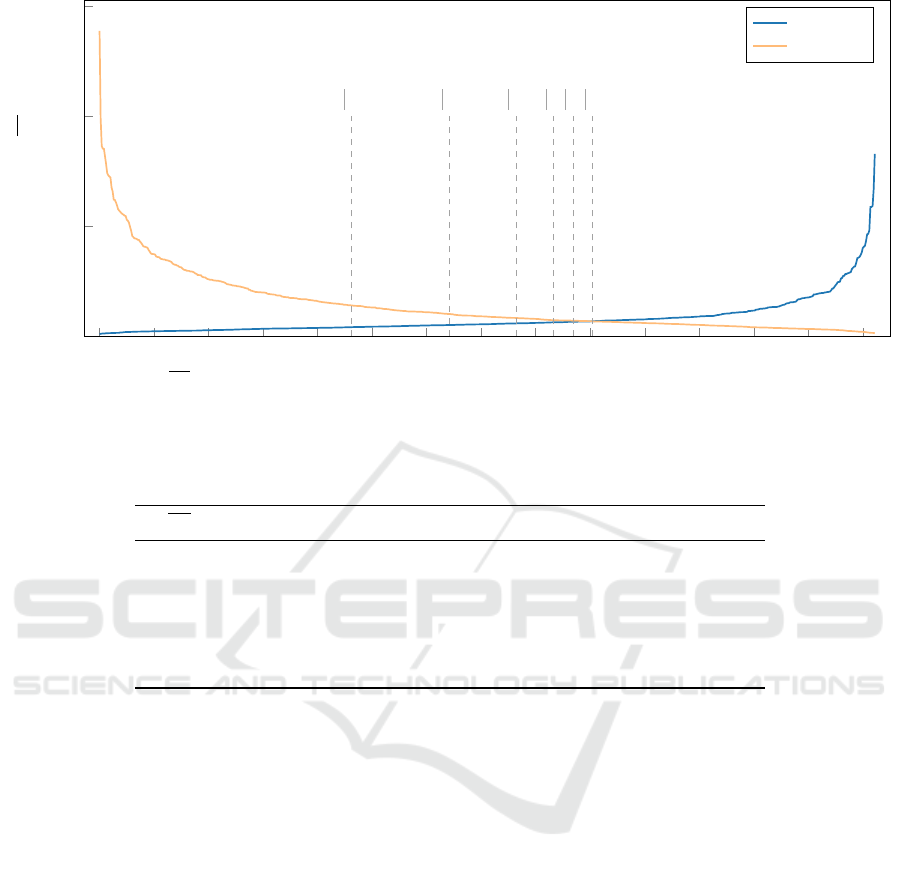
differences. Fig. 2 shows the irregularity of the le-
sions grouped by class. The irregularity is measured
by the average distance (∆d) between the fit ellipse
and the boundary of the tumor. Training input can
greatly influence the construction of the classification
model. If the model is trained using images from the
first part and tested for the second part, then there
will be a massive difference in the training and test-
ing accuracy. In our previous study (Bajcsi and Chira,
2023), we reached 100% training accuracy when only
64.99% test accuracy was achieved using the same
features and classifiers. Therefore, the difference can
be explained by the inadequate split of the images.
To overcome this issue, we decided to extract sub-
sets from the dataset as shown in Fig. 2 where the
minimum difference in the irregularity is fixed. The
lesions corresponding to the specified condition are
selected and randomly split (75% train, 25% test). In
the creation of the new splits, we took special care to
always have the same images in the same set. This ap-
proach is equivalent to a stratified sampling, where in
addition to the type of the lesion, its contour is consid-
ered. This approach resulted in 6 new split files. The
distribution of samples in each partition is presented
in Table 1, which includes the number of images used
for training and testing. In the following, we present
the results using these subsets.
4.2 Results
The scope of the present study is to verify the impor-
tance of a proper train test split and to evaluate the
performance of shape features in the binary classifi-
cation of lesions. To train the models, the previously
mentioned splits are used. Furthermore, in the train-
ing process, k-fold cross-validation (k = 5) was ap-
plied to avoid overfitting and increase the reliability
of the results. In the current subsection, the results
achieved in the experiments conducted are presented.
As mentioned in Section 3.2, there are three types
of features extracted from the contour of the lesion.
First, we want to select the best contour features.
Therefore, we built separate models for each com-
puted feature and for different numbers of segments,
obtaining 3 features × |S | = 33 RF models. In addi-
tion, we built models using the combination (concate-
nation) of contour features, obtaining |S | = 11 models
corresponding to each number of S . Due to the fact
that with a lower ∆d value the difference in the re-
sult is more emphasized, we present the results ob-
tained on the largest split (∆d > 0) containing a total
of 904 mammograms. The performance of contour-
based features is evaluated and shown in Fig. 3. We
can conclude that from the proposed contour-based
features (root mean roughness, root mean slope, and
circularity), the models that use root mean roughness
(Rq) outperform the models trained using the other
two features, independent of the number of segments.
Moreover, Fig. 3 shows that the combination of the
extracted contour features slightly improves the clas-
sification accuracy. Based on the data presented in
Fig. 3, we can conclude that the Random Forest al-
gorithm is the most effective when using a combina-
tion of contour-based features and s = 10 segments.
The best performing model reached an accuracy of
96.12%.
Fig. 4 compares the performance of different ma-
chine learning algorithms using the most effective
features found in previous experiments (combination
of contour-based features computed from 10 seg-
ments) and our baseline geometric features. First, it
can be observed that with a higher threshold at ∆d we
can increase the accuracy of the classification. The
results then indicate that contour-based features out-
perform baseline geometric features. We can also re-
mark that Random Forest consistently provided the
best classification accuracy across all scenarios tested.
The presented method has the drawback that it re-
quires a precise lesion mask for extracting shape fea-
tures, thus restricting the experiments to datasets with
this information. This could potentially limit its ap-
plicability in real-world scenarios where such detailed
Significance of Training Images and Feature Extraction in Lesion Classification
121
0
50
100
150
200
250
300
350
400
450 500 550 600 650
700
0
50
100
150
∆d > 10
∆d > 5
∆d > 2.5
∆d > 1
∆d > 0.5
∆d > 0
irregularity (∆d)
benign
malignant
Figure 2: Irregularity (∆d) between the ellipse and the lesion boundary for benign and malignant samples. Distances from
the benign class are sorted increasingly, whereas distances from the malignant class are sorted decreasingly, based on the
presumption that benign lesions are usually round, whereas malignant lesions tend to be irregular in shape.
Table 1: Number of selected samples in total after splitting the dataset to have at least the specified difference between the
average distances.
∆d samples per class total samples train samples test samples
10 < 231 462 346 116
5 < 321 642 479 163
2.5 < 382 764 569 195
1 < 416 832 620 212
0.5 < 434 868 647 221
0 < 452 904 674 230
data may not always be available.
4.3 Discussion
In the previous section (Section 4.2), the performance
of the proposed approach is presented. In the current
section, the results obtained are compared with other
methods presented in the literature. Table 2 collects
and compares existing approaches from the literature.
Compared to our previous model (Bajcsi and
Chira, 2023), where we achieved 64.99% due to over-
fitting, the current approach shows a significant im-
provement. Hence, the importance of proper train test
split arises.
Li et al. (Li et al., 2017) reported 99.66% accu-
racy using root mean slope features as input to the
SVM classifier. To train the reported model, the au-
thors used 323 images from the DDSM (from a total
of 14,440). The presented method, using an RF clas-
sifier, outperformed the presented method in (Li et al.,
2017), by reaching 100% test accuracy using 642 im-
ages to train and test the model.
Paramkusham et al. (Paramkusham et al.,
2021) proposed another applied Beam angle statistics
method to extract the shape features. The authors re-
ported the best accuracy of 88.8% using 147 contours
from the DDSM. In our experiment, the best result
achieved is 96.12%.
In the research presented by Falcon
´
ı et al. (Fal-
con
´
ı et al., 2020), different CNNs were compared us-
ing transfer learning on images from CBIS-DDSM
(an updated version of DDSM). The authors reported
the best performance by VGG-16 achieving 64.4%
using the original mammograms (1696). The result
of VGG-16 was increased by employing fine tuning
(generating 60 000 images with augmentation) reach-
ing 84.4%.
In a study conducted by Salama and Aly (Salama
and Aly, 2021), the performance of CNN was inves-
tigated. The authors cropped the region of the le-
sion, applied data augmentation to achieve a more ro-
bust model, and reported 98.87% accuracy on DDSM
(from 564 images, 1804 were generated with augmen-
tation). Our model is behind the model reported by
ICAART 2024 - 16th International Conference on Agents and Artificial Intelligence
122
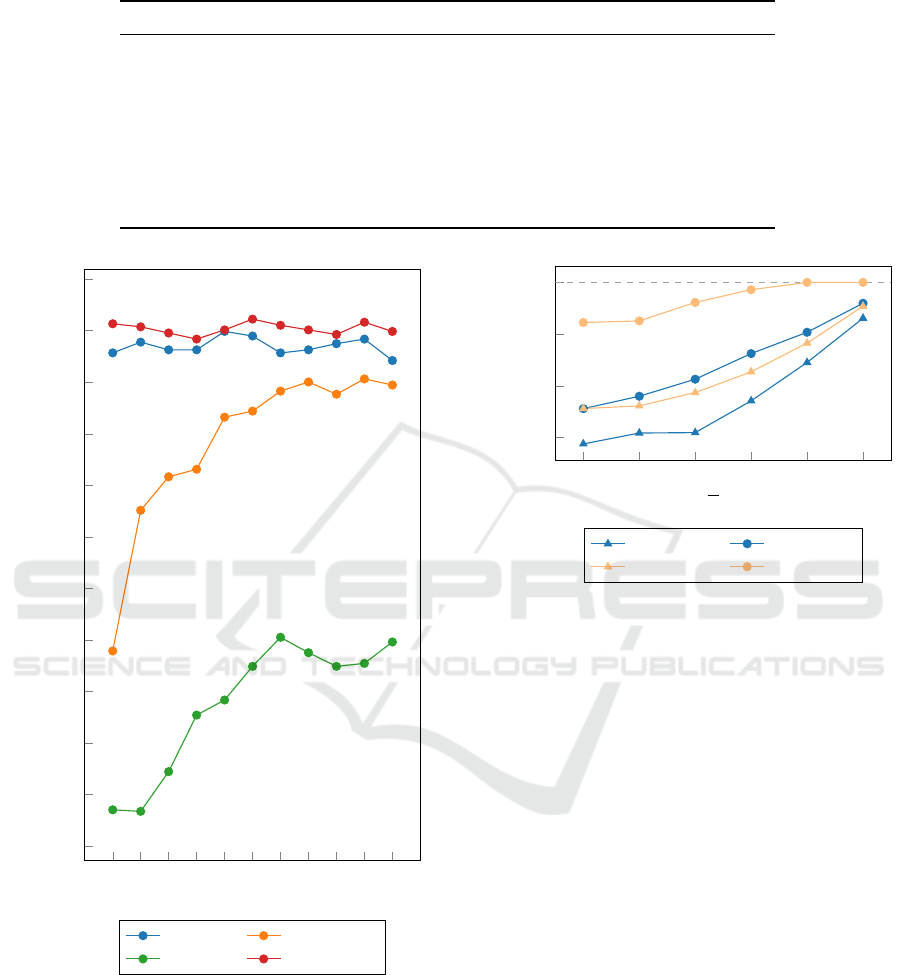
Table 2: Comparison of the current approach to existing solutions from the literature.
Approach Images Model Accuracy
(Rani et al., 2023) 518 ANFC-LH 73%
(Falcon
´
ı et al., 2020) 60 000 VGG-16 84.4%
(Paramkusham et al., 2021) 147 SVM 88.8%
(Kumari et al., 2023) 428 ANN 94.57%
current 904 RF 96.12%
(Salama and Aly, 2021) 1804
U-Net +
Inception V3
98.87%
(Li et al., 2017) 323 SVM 99.66%
1 2 4 6 8 10 12 14 16 18 20
0.45
0.5
0.55
0.6
0.65
0.7
0.75
0.8
0.85
0.9
0.95
1
s
accuracy
Rq
R∆q
circularity combination
Figure 3: Results achieved with the different contour fea-
tures computed for the different number of segments (s),
using RF classifier.
Salama and Aly (Salama and Aly, 2021) by 2.75%.
This difference can be decreased by further investiga-
tion of the model’s parameters.
0 0.5 1 2.5 5 10
0.85
0.9
0.95
1
d >
accuracy
DT geometry DT contour
RF geometry RF contour
Figure 4: Comparison of the geometry- and contour-based
features using different classification model.
5 CONCLUSIONS AND FUTURE
WORK
Mammogram analysis plays a key role in the early
detection of breast cancer. The earlier a lesion is ex-
plored, the higher the chances of recovery. In the pre-
sented paper, an early detection system is proposed
using shape features to distinguish between benign
and malignant tumors. To classify the extracted fea-
tures Random Forest and Decision Tree methods are
used. The best performance achieved was 96.12% ac-
curacy. The results of the experiments conducted have
shown that a proper split between train and test is cru-
cial to achieve accurate classification of the lesions.
The reported results are comparable with other state-
of-the-art approaches.
In future experiments, we will investigate the pa-
rameters of the classification models used. We will
also compare the performance of the texture features
with the proposed contour-based features on these
new splits. We will also investigate the effect of the
combination of texture- and contour-based features on
the performance of the system. We will consider the
Significance of Training Images and Feature Extraction in Lesion Classification
123

use of other explainable models (e.g. interpretable
ANNs) or representation learning. To increase the
amount of input image, augmentation will be taken
into consideration.
REFERENCES
Bajcsi, A. and Chira, C. (2023). Textural and shape features
for lesion classification in mammogram analysis. In
Hybrid Artificial Intelligent Systems, pages 755–767.
Springer Nature Switzerland.
Chhikara, B. S. and Parang, K. (2022). Global cancer statis-
tics 2022: the trends projection analysis. Chemical
Biology Letters, 10(1):451.
Falcon
´
ı, L. G., P
´
erez, M. H., Aguila, W. G., and Conci,
A. (2020). Transfer learning and fine tuning in breast
mammogram abnormalities classification on CBIS-
DDSM database. Advances in Science, Technology
and Engineering Systems Journal, 5(2):154–165.
George Melekoodappattu, J., Sahaya Dhas, A., Kumar K.,
B., and Adarsh, K. S. (2022). Malignancy detection on
mammograms by integrating modified convolutional
neural network classifier and texture features. Inter-
national Journal of Imaging Systems and Technology,
32(2):564–574.
Gurudas, V. R., Shaila, S. G., and Vadivel, A. (2022). Breast
cancer detection and classification from mammogram
images using multi-model shape features. SN Com-
puter Science, 3(5):404.
Heath, M., Bowyer, K., Kopans, D., Kegelmeyer, P., Moore,
R., Chang, K., and Munishkumaran, S. (1998). Cur-
rent Status of the Digital Database for Screening
Mammography, pages 457–460. Springer Nether-
lands, Dordrecht.
Heath, M., Bowyer, K., Kopans, D., Moore, R., and
Kegelmeyer, P. (2001). The digital database for
screening mammography. In Yaffe, M., editor, Pro-
ceedings of the Fifth International Workshop on Dig-
ital Mammography, pages 212–218. Medical Physics
Publishing.
Kumari, D., Yannam, P. K. R., Gohel, I. N., Naidu, M. V.
S. S., Arora, Y., Rajita, B., Panda, S., and Christo-
pher, J. (2023). Computational model for breast can-
cer diagnosis using hfse framework. Biomedical Sig-
nal Processing and Control, 86:105121.
Lee, R. S., Gimenez, F., Hoogi, A., Miyake, K. K., Gorovoy,
M., and Rubin, D. L. (2017). A curated mammogra-
phy data set for use in computer-aided detection and
diagnosis research. Sci Data, 4:170–177.
Li, H., Meng, X., Wang, T., Tang, Y., and Yin, Y. (2017).
Breast masses in mammography classification with lo-
cal contour features. BioMedical Engineering OnLine,
16(1):44.
Paramkusham, S., Thotempuddi, J., and Rayudu, M. S.
(2021). Breast masses classification using contour
shape descriptors based on beam angle statistics. In
2021 Third International Conference on Inventive Re-
search in Computing Applications (ICIRCA), pages
1340–1345.
Rani, J., Singh, J., and Virmani, J. (2023). Mammographic
mass classification using dl based roi segmentation
and ml based classification. In 2023 International
Conference on Device Intelligence, Computing and
Communication Technologies, (DICCT), pages 302–
306.
Sajid, U., Khan, R. A., Shah, S. M., and Arif, S. (2023).
Breast cancer classification using deep learned fea-
tures boosted with handcrafted features. Biomedical
Signal Processing and Control, 86:105353.
Salama, W. M. and Aly, M. H. (2021). Deep learning
in mammography images segmentation and classifi-
cation: Automated cnn approach. Alexandria Engi-
neering Journal, 60(5):4701–4709.
Shanmugam, S., Shanmugam, A. K., Mayilswamy, B., and
Muthusamy, E. (2020). Analyses of statistical feature
fusion techniques in breast cancer detection. Inter-
national Journal of Applied Science and Engineering,
17:311–317.
Singh, V. K., Rashwan, H. A., Romani, S., Akram, F.,
Pandey, N., Sarker, M. M. K., Saleh, A., Arenas, M.,
Arquez, M., Puig, D., and Torrents-Barrena, J. (2020).
Breast tumor segmentation and shape classification in
mammograms using generative adversarial and con-
volutional neural network. Expert Systems with Ap-
plications, 139:112855.
ICAART 2024 - 16th International Conference on Agents and Artificial Intelligence
124
