
An Event-Driven Closed-Loop Ultrasound Stimulator Composed of a
Micro-Transducer and Multi-Site Electrodes in Vitro
Ryo Furukawa
1a
, Shuichi Murakami
2b
and Takashi Tateno
3c
1
Bioengineering and Bioinformatics, Graduate School of Information Science and Technology, Hokkaido University,
Kita 14, Nishi 9, Kita-ku, Sapporo, Hokkaido 060-0814, Japan
2
Osaka Research Institute of Industrial Science and Technology, 2-7-1, Ayumino, Izumi, Osaka, 594-1157, Japan
3
Bioengineering and Bioinformatics, Faculty of Information Science and Technology, Hokkaido University, Kita 14,
Nishi 9, Kita-ku, Sapporo, Hokkaido 060-0814, Japan
Keywords: Brain Slice, Calcium Imaging, Closed-Loop System, Micromachined Transducer, Ultrasound Stimulation.
Abstract: Ultrasound neuromodulation, in which local and deep brain areas are stimulated, holds promise for clinical
applications. However, the mechanisms of action underlying the stimulation effects are still unknown. In vitro
experiments are helpful for investigating the stimulation mechanisms because they allow easy control of
extracellular conditions. Compared with closed-loop systems, conventional open-loop systems do not permit
monitoring of neural activity, and thus can lead to excessive neural stimulation. In this study, we developed
a piezoelectric micromachined ultrasound transducer (PMUT) combined with monitoring microelectrodes.
To examine the potential of our device as a neuromodulation tool, we measured the cellular responses to
generated ultrasound stimulation. Subsequently, we constructed a closed-loop system that combined our
PMUT with monitoring electrodes, and applied event-related ultrasound stimulation to brain slices in vitro.
We discuss future applications of a closed-loop ultrasound stimulation system.
1 INTRODUCTION
Ultrasound stimulation enables non-invasive
stimulation of local and deep areas of the brain, which
are difficult to achieve using conventional
electromagnetic stimulation methods (Tufail et al.,
2010; Wagner et al., 2007). However, the cellular
mechanisms of ultrasound-induced neural activation
are unclear. Furthermore, little is known about how
cellular responses to ultrasound stimulation influence
localized neural networks in vivo. For example,
indirect neural activity in the auditory pathway could
affect neuromodulation, complicating investigations
of the mechanism of action (Sato et al., 2018).
Most current brain stimulation strategies are
limited in terms of their ability to control patterns of
brain activity. This is because they generally involve
unidirectional stimulation processes, and thus do not
capture sufficient information about the neural
activity surrounding the stimulator. Flexible
a
https://orcid.org/0000-0001-8920-1025
b
https://orcid.org/0000-0002-8862-8446
c
https://orcid.org/0000-0001-9429-9880
regulation of neural activity could be achieved via
closed-loop approaches to brain stimulation,
including ultrasound stimulation. Accordingly,
researchers have prioritized the creation of
microelectrodes and small-scale stimulators within
the same device, along with the development of
bidirectional technological approaches that enable
simultaneous stimulation and recording of neural
activity.
When examining the cellular mechanisms
underlying the effects of ultrasonic stimulation, in
vitro experimental systems enable easy control of the
extracellular conditions around neurons. Lee et al.
developed a piezoelectric micromachined ultrasound
transducer (PMUT) that was helpful in elucidating the
cellular mechanisms of neuromodulation in
dissociated cultured neurons in vitro (Lee et al., 2019).
However, in their experiments, which used
dissociated cultured cells, the original neural
networks were rebuilt in networks with random
Furukawa, R., Murakami, S. and Tateno, T.
An Event-Driven Closed-Loop Ultrasound Stimulator Composed of a Micro-Transducer and Multi-Site Electrodes in Vitro.
DOI: 10.5220/0012307900003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 95-102
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
95

connections. Furthermore, conventional open-loop
stimulators can induce excessive neural activity,
something which could be addressed by closed-loop
stimulation (Takeuchi & Berényi, 2020). Several
reports have described methods for closed-loop
ultrasound stimulation (Jo et al., 2022; Xie et al.,
2022). However, in these studies, the stimulator and
monitoring electrodes were not packaged together as
one instrument. Considering future applications for
chronic conditions, systems using an integrated
device are optimal.
In this study, we firstly describe the design and
fabrication of a PMUT combined with monitoring
microelectrodes. We used microelectromechanical
systems (MEMS) technology for microfabrication.
Next, we conducted intracellular calcium imaging in
vitro to examine whether the device could be used for
ultrasound neuromodulation. Finally, we constructed
a closed-loop system including our PMUT and
microelectrodes, and conducted event-related
ultrasound stimulation.
2 METHODS
2.1 Design, Microfabrication, and
Packaging
To locally stimulate a brain slice, we aimed to
develop a PMUT that satisfied three numerical
conditions: (i) a diaphragm resonant frequency of 500
kHz, (ii) a stimulation ultrasound pressure greater
than 65 kPa, and (iii) a diaphragm radius smaller than
600 µm (Lee et al., 2019; Oh et al., 2019).
Our PMUT consisted of the following five
components: a piezoelectric film, a silicon (Si) layer,
a SiO
2
membrane, top and bottom Pt/Ti electrodes,
and a Si supporting layer (Fig. 1). To convert electric
(voltage) signals into ultrasound pressures, we used a
thin film of a piezoelectric material as the transducer.
Prior to microfabrication, we conducted a
simulation using general-purpose physics simulation
software (COMSOL Multiphysics, Ver. 5.5,
COMSOL AB, Sweden) on a supercomputer system
(PRIMERGY CX 400/CX2550, FUJITSU, Japan) at
the Hokkaido University Computer Center. We
calculated the resonant frequency (500 kHz) and
determined the potential size range of the PMUT.
For microfabrication, the sizes of the circular
diaphragms were set according to the simulation
results. We created eight microelectrodes (200 × 200
µm
2
), patterned to monitor the neural activity of a
brain slice (Fig. 1A). The fabricated PMUT was
packaged with the printed circuit board (Fig. 1B,C).
2.2 Physical Properties
To characterize the resonant frequency of each
diaphragm, we applied a sinusoidal voltage input
from a multifunction generator (WF1947, NF Co.,
Japan) and measured the acoustic pressure using a
needle hydrophone (HY05N, Toray Engineering Co.,
Japan). Subsequently, we assessed the electrical chara-
cteristics of the microelectrodes via electrochemical
impedance spectroscopy. To characterize the
electrochemical properties of the microelectrodes on
the PMUT device, we used a potentiostat (Electro-
chemical Analyzer ALS720E, BAS Inc., Japan) with a
built-in frequency analyzer (Takahashi et al., 2019).
2.3 Preparation of Brain Slices
All animal experiments described below were
approved by the Institutional Animal Care and Use
Committee of Hokkaido University and carried out in
accordance with the National Institutes of Health
Guidelines for the Care and Use of Laboratory
Animals. Here, we used four C57BL/6J mice (two
male and two female mice, 7–10 weeks old, Japan
SLC Inc., Hamamatsu, Japan). Each mouse was
deeply anesthetized with isoflurane and decapitated.
The brains were rapidly removed and transferred into
ice-cold artificial cerebral spinal fluid (119 NaCl, 2.5
KCl, 2.5 CaCl
2
, 1.3 MgSO
4
, 1.0 NaH
2
PO
4
, and 11.0
D-glucose, in mM, pH = 7.4). We prepared 400-µm
brain slices including the auditory cortex (Furukawa
et al., 2022).
2.4 Calcium Imaging
Before attempting closed-loop ultrasound stimulation,
we examined whether our PMUT could activate cells
in a brain slice. To observe cellular activities, we used
Fura-2 AM dye to image changes in the fluorescence
of intracellular Ca
2+
concentrations. This enabled us
to avoid ultrasound vibration artifacts (Qiu et al.,
2019). Fura-2 AM is excitable at light wavelengths of
either 340 or 380 nm and emits fluorescence at 510
nm. We alternately measured fluorescence intensities
with excitation at 340 (F
340
) or 380 nm (F
380
) at a
frame rate of 50 Hz (Tateno, 2010). Then, we
estimated the ratiometric fluorescence intensity by
calculating the F
340
/F
380
ratio. In the absence of
stimulation, we first monitored baseline changes in
Ca
2+
transients for a period of 1 s. Subsequently, we
applied ultrasound stimulation (880 kHz, continuous
wave, 65.6 ± 1.8 kPa) for 1 s and imaged the Ca
2+
transients, followed by monitoring of the Ca
2+
transients during the recovery period for 1 s.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
96
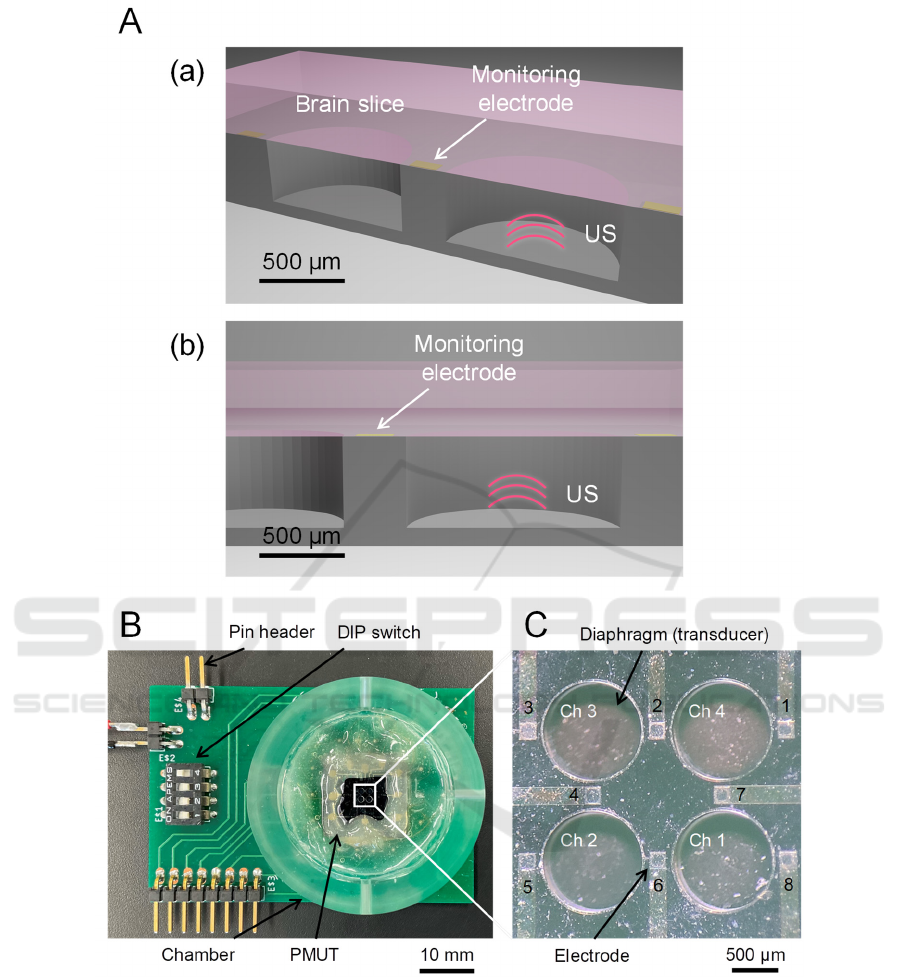
Figure 1: Structure and design of a PMUT device. (A) Schematic of the PMUT device under a brain slice. (a) Cross view. (b)
Front side of the cross section. (B) A packaged substrate combined with the PMUT device. (C) The front side of the PMUT
device with four diaphragms (four channels, chs. 1 to 4) and eight electrodes (1 to 8).
2.5 A Closed-Loop System Using
Ultrasound Stimulation
Figure 2A illustrates a conventional open-loop
ultrasound stimulation system, which evoked local
field potential (LFP) responses (Fig. 2C, left)
(Furukawa et al., 2022). Here, we examined a closed-
loop system with a feedback pathway from the
packaged PMUT (Fig. 2B). In the closed-loop
system, spontaneous brain slice activity was
monitored while ultrasound stimulation was applied
at the time point(s) when the activity exceeded a set
threshold (Fig. 2C, right). We set the threshold at 150
µV. To monitor LFPs, we used a MED amplifier
(MED-A64HS1, MED-A64MD1A, Alpha MED
Scientific, Japan) from a previous MEA-based
An Event-Driven Closed-Loop Ultrasound Stimulator Composed of a Micro-Transducer and Multi-Site Electrodes in Vitro
97
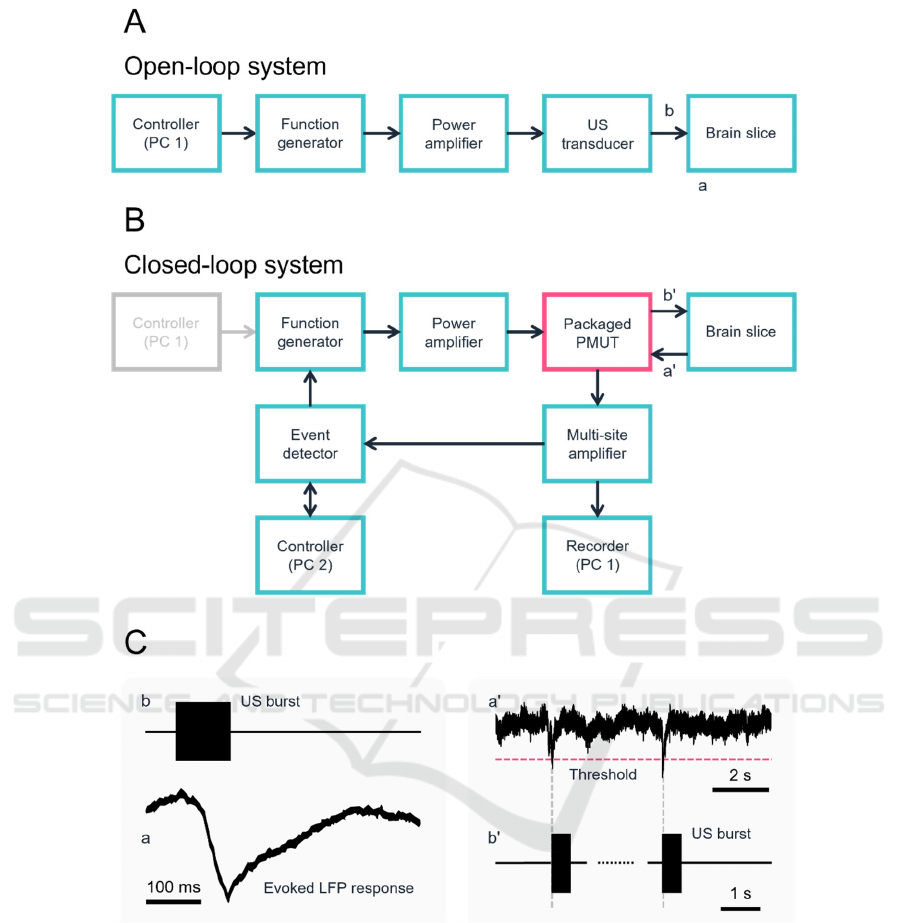
Figure 2: Schematic of ultrasound stimulation systems and related signals. (A) A block diagram of a conventional open-loop
ultrasound stimulation system. (B) Our constructed closed-loop system with the packaged PMUT. (C) Schematic of activity
detected via the extracellular voltage of a brain slice. The event detector sends trigger signals to the multifunction generator
immediately after detecting LFP signals that negatively cross a threshold level. The left panel shows a typical evoked LFP
response to ultrasound stimulation.
recording system (Furukawa et al., 2022). The signals
were recorded with a sampling rate of 20 kHz, and
subjected to filtering within a frequency range from 1
Hz to 10 kHz. We employed a multifunctional I/O
data acquisition system (USB-6343, National
Instruments, USA) for real-time signal control, which
acquired neural signals from the MED amplifier and
fed a trigger signal to a multifunction generator when
a threshold-crossing event was detected. We used a
customized Python program (Python Ver. 3.11.1) for
real-time processing. To detect neural activities, we
used electrode 7 for monitoring. We applied
ultrasound stimulation (540 kHz, continuous wave,
95.5 ± 1.4 kPa) from a diaphragm (ch 4, which was
close to electrode 7). For a single trial, the
measurement period was 1 min.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
98
3 RESULTS
3.1 Microfabricated PMUT
According to the result obtained from our numerical
simulation, we determined the optimal thickness of
the material layers (PZT, 100 µm; Si, 15 µm; SiO
2
, 1
µm) and the radius (580 µm). We estimated that the
diaphragm would oscillate with a resonant frequency
of 540 kHz.
Our acoustic pressure measurement indicated
forced diaphragm oscillations driven by voltage
signals with a wide range of frequencies including
540 kHz, at which the oscillating amplitude had a
positive peak (Fig. 3A).
When we increased the amplitude of the input
voltage signal at a fixed frequency of 540 kHz, the
output acoustic pressure was monotonically increased
(Fig. 3B). The measured electrode impedance on the
PMUT device was 23.4 ± 3.7 kΩ at 1 kHz (eight
electrodes). Thus, the fabricated low impedance micro-
electrodes were able to monitor LFPs from brain slices.
3.2 Calcium Imaging
We next examined the possibility that our PMUT
could activate cells. Figure 3C shows an example of
Ca
2+
imaging (indicator, Fura-2 AM), with the
averaged waveforms of the fluorescence intensity
ratio obtained via ratiometric imaging (19 cells from
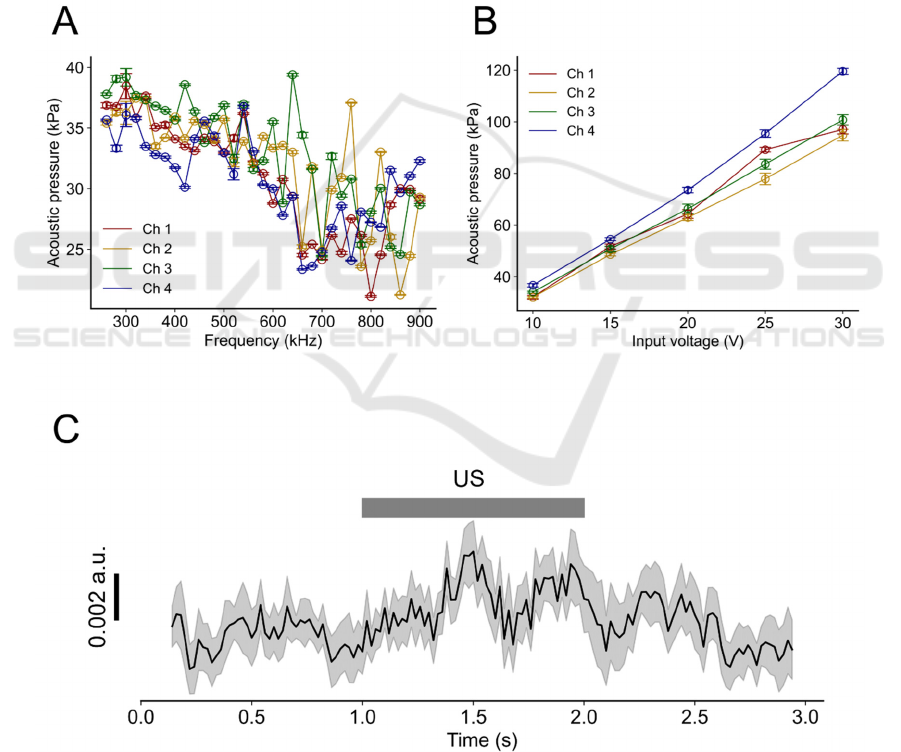
Figure 3: Measured acoustic characteristics of the fabricated PMUT and cellular activation via ultrasound stimulation. (A)
Measured acoustic pressure vs. driving voltage signals with different sinusoidal frequencies (input voltage, 10 V). (B)
Acoustic pressure with different input voltages under a fixed frequency of 540 kHz. The channel numbers correspond to those
shown in Fig. 1D. (C) PMUT-driven average transients of ratiometric imaging (black) data related to intracellular Ca
2+
concentrations for all analysed cells. The edges of the grey band represent the standard error of the mean at each time point
(n = 19 cells from five slices). Grey bar indicates the duration of the ultrasound stimulation (US).
An Event-Driven Closed-Loop Ultrasound Stimulator Composed of a Micro-Transducer and Multi-Site Electrodes in Vitro
99
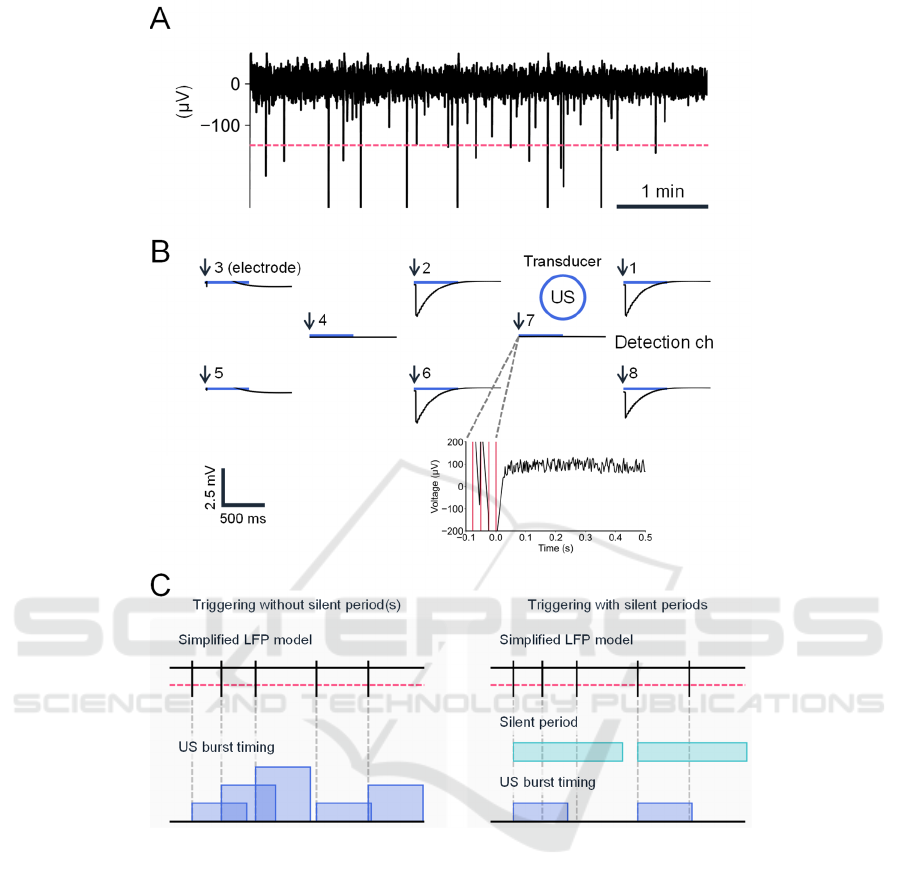
Figure 4: Closed-loop system with real-time detection of spontaneous activity in a brain slice. (A) A typical example of a
spontaneous LFP waveform from a brain slice. The dashed red line indicates the threshold. (B) A typical example of
spatiotemporal LFP recorded at eight microelectrode sites on the PMUT. The horizontal blue bars represent the timings of
ultrasound stimulation (US; duration of each stimulation pulse, 500 ms). The blue circle represents the stimulation site; the
schematic diagram shows only the relative positions of the recording electrodes and the stimulating diaphragms, and the
actual distances between them differ. Electrode 7 was used for detection of spontaneous activity. In the bottom panel, the
timings of threshold crossing are illustrated via vertical red lines. The time point of 0 ms is expressed with arrows in the top
panel. (C) Schematic diagram of event detection and triggering methods. The current triggering method (left) had no
refractory (silent) period(s), whereas our future triggering method will include this feature (right). Vertical bars represent
event timings, and horizontal red lines indicate the threshold level for event detection. In the current system, the stimuli can
overlap according to the timings of events occurring within 500 ms. In our future system, the silent period(s) will ensure that
the US do not temporally overlap.
five slices). The intracellular Ca
2+
concentration of
the cells increased in response to ultrasound
stimulation generated by the PMUT.
3.3 Event-Driven Ultrasound
Stimulation
After confirming that our PMUT could modulate
cellular activity, we next attempted to create a closed-
loop system using ultrasound stimulation. A typical
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
100

example of LFP recording of a brain slice is
illustrated in Fig. 4A. The negative peaks of rapid
voltage changes in LFPs occurred randomly, and the
peak events were detected as negative crossings
below the threshold (−150 µV). The inter-event
intervals were 15.5 ± 1.1 s for the seven channels for
one brain slice.
When each event was detected (Fig. 4B),
ultrasound stimulation was automatically applied to the
brain slice from the PMUT. The stimulation was driven
by an ultrasound signal generated by a functional
generator through a power amplifier (Fig. 2B).
In our closed-loop system (Fig. 2B), peak-event
detection was continuously functioning immediately
after the onset of ultrasound stimulation and during
each stimulus (500 ms). As a result, repetitive stimuli
were triggered without a silent period (Fig. 4B).
Therefore, a longer stimulation duration (> 500 ms)
could occur if the extracellular voltage continued to
exceed the threshold level (Fig. 4C).
For example, Fig. 4B shows how the LFP
responses rapidly decreased after the stimulation
onset and slowly returned to individual baselines at
four chs. (i.e., chs. 1, 2, 6, and 8), which were close
to the ultrasound stimulation site (diaphragm ch. 4 in
the transducer array). Since the LFP at the detection
site (i.e., electrode ch. 7) exceeded the threshold
voltage for a relatively longer period, trigger signals
at multiple timings (four times in Fig. 4B, bottom)
were sent to the multifunction generator, resulting in
a stimulation duration over 500 ms (c.f., Fig. 4C, left).
4 DISCUSSION
In this study, we developed a MEMS-based PMUT
with monitoring microelectrodes for event detection
(i.e., rapid changes in LFP magnitude). We
successfully microfabricated the PMUT with four
circular diaphragms for ultrasound stimulation and
eight microelectrodes for monitoring LFP-peak
events. To demonstrate that our device could perform
ultrasound neuromodulation, we conducted
intracellular calcium imaging. An influx of Ca
2+
into
cells during ultrasound stimulation was successfully
observed in acute brain slices. These intracellular
Ca
2+
transients suggest that our PMUT has potential
for ultrasound neuromodulation applications.
Subsequently, we constructed a closed-loop
system that included the PMUT as part of the
ultrasound stimulator. To the best of our knowledge,
this is the first attempt to combine a PMUT with
electrodes that monitor cellular activity as an
integrated device in a closed-loop system.
In our current system, ultrasound stimulation was
automatically applied to the target when the detected
signals were larger than the voltage threshold.
Therefore, it was unclear whether the detected signals
were truly attributable to spontaneous brain slice
activity or were the result of electrical noise. We are
planning to utilize more robust detection techniques
to detect neural activity in our future work.
Upon improvements to the real-time detector in
our system, our device could be applied to the
detection of abnormal neural activity such as seizure-
like activity (Berényi et al., 2012; Ranjandish &
Schmid, 2020). In our next detector model, we are
planning to include a silent period after each detected
event as a triggering rule (Fig. 4C, right). This
triggering rule could limit excessive stimulation.
Moreover, we plan to test this device in in vivo animal
experiments via chronic ultrasound stimulation.
ACKNOWLEDGEMENTS
R.F. was supported by Grant-in-Aid for JSPS Fellows
[grant number JP23KJ0047]. T.T. was supported by
the Murata Science Foundation, the Suzuken
Memorial Foundation, the Nakatani Foundation for
Advancement of Measuring Technologies in
Biomedical Engineering, a Grant-in-Aid for
Exploratory Research [grant number 18K19794], and
a Grant-in-Aid for Scientific Research (B) [grant
number 19H04178] (Japan).
REFERENCES
Berényi, A., Belluscio, M., Mao, D., & Buzsáki, G. (2012).
Closed-Loop Control of Epilepsy by Transcranial
Electrical Stimulation. Science, 337(6095), 735–737.
https://doi.org/10.1126/science.1223154
Furukawa, R., Kaneta, H., & Tateno, T. (2022). A
Multielectrode Array-Based Recording System for
Analyzing Ultrasound-Driven Neural Responses in
Brain Slices in vitro. Frontiers in Neuroscience,
16(February), 1–19. https://doi.org/10.3389/fnins.20
22.824142
Jo, Y., Lee, S. M., Jung, T., Park, G., Lee, C., Im, G. H.,
Lee, S., Park, J. S., Oh, C., Kook, G., Kim, H., Kim, S.,
Lee, B. C., Suh, G. S. B., Kim, S. G., Kim, J., & Lee,
H. J. (2022). General‐Purpose Ultrasound
Neuromodulation System for Chronic, Closed‐Loop
Preclinical Studies in Freely Behaving Rodents.
Advanced Science, 9(34). https://doi.org/10.1002/
ADVS.202202345
Lee, J., Ko, K., Shin, H., Oh, S.-J., Lee, C. J., Chou, N.,
Choi, N., Tack Oh, M., Chul Lee, B., Chan Jun, S., &
An Event-Driven Closed-Loop Ultrasound Stimulator Composed of a Micro-Transducer and Multi-Site Electrodes in Vitro
101

Cho, I.-J. (2019). A MEMS ultrasound stimulation
system for modulation of neural circuits with high
spatial resolution in vitro. Microsystems &
Nanoengineering, 5(1), Article 1. https://doi.org/
10.1038/s41378-019-0070-5
Oh, S. J., Lee, J. M., Kim, H. B., Lee, J., Han, S., Bae, J.
Y., Hong, G. S., Koh, W., Kwon, J., Hwang, E. S., Woo,
D. H., Youn, I., Cho, I. J., Bae, Y. C., Lee, S., Shim, J.
W., Park, J. H., & Lee, C. J. (2019). Ultrasonic
Neuromodulation via Astrocytic TRPA1. Current
Biology, 29(20), 3386-3401.e8. https://doi.org/
10.1016/j.cub.2019.08.021
Qiu, Z., Guo, J., Kala, S., Zhu, J., Xian, Q., Qiu, W., Li, G.,
Zhu, T., Meng, L., Zhang, R., Chan, H. C., Zheng, H.,
& Sun, L. (2019). The Mechanosensitive Ion Channel
Piezo1 Significantly Mediates In Vitro Ultrasonic
Stimulation of Neurons. iScience, 21, 448–457.
https://doi.org/10.1016/j.isci.2019.10.037
Ranjandish, R., & Schmid, A. (2020). A Review of
Microelectronic Systems and Circuit Techniques for
Electrical Neural Recording Aimed at Closed-Loop
Epilepsy Control. Sensors, 20(19), Article 19.
https://doi.org/10.3390/s20195716
Sato, T., Shapiro, M. G., & Tsao, D. Y. (2018). Ultrasonic
Neuromodulation Causes Widespread Cortical
Activation via an Indirect Auditory Mechanism.
Neuron, 98(5), 1031-1041.e5. https://doi.org/
10.1016/j.neuron.2018.05.009
Takahashi, S., Muramatsu, S., Nishikawa, J., Satoh, K.,
Murakami, S., & Tateno, T. (2019). Laminar responses
in the auditory cortex using a multielectrode array
substrate for simultaneous stimulation and recording.
IEEJ Transactions on Electrical and Electronic
Engineering, 14(2), 303–311. https://doi.org/10.1002
/tee.22810
Takeuchi, Y., & Berényi, A. (2020). Oscillotherapeutics –
Time-targeted interventions in epilepsy and beyond.
Neuroscience Research, 152, 87–107. https://doi.org/
10.1016/j.neures.2020.01.002
Tateno, T. (2010). A small-conductance Ca2+-dependent
K+ current regulates dopamine neuron activity: A
combined approach of dynamic current clamping and
intracellular imaging of calcium signals. NeuroReport,
21(10), 667–674. https://doi.org/10.1097/WNR.0b013e
32833add56
Tufail, Y., Matyushov, A., Baldwin, N., Tauchmann, M. L.,
Georges, J., Yoshihiro, A., Tillery, S. I. H., & Tyler, W.
J. (2010). Transcranial Pulsed Ultrasound Stimulates
Intact Brain Circuits. Neuron, 66(5), 681–694.
https://doi.org/10.1016/j.neuron.2010.05.008
Wagner, T., Valero-Cabre, A., & Pascual-Leone, A. (2007).
Noninvasive human brain stimulation. Annual Review
of Biomedical Engineering,
9, 527–565. https://doi.org/
10.1146/annurev.bioeng.9.061206.133100
Xie, Z., Yan, J., Dong, S., Ji, H., & Yuan, Y. (2022). Phase-
locked closed-loop ultrasound stimulation modulates
theta and gamma rhythms in the mouse hippocampus.
Frontiers in Neuroscience, 16(September), 1–12.
https://doi.org/10.3389/fnins.2022.994570
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
102
