
Characterization of sEMG Spectral Properties During Lower Limb
Muscle Activation
Costa-Garcia Alvaro
1
a
and Shimoda Shingo
2
b
1
National Institute of Advanced Industrial Science and Technology (AIST) Kashiwa II Campus, University of Tokyo,
6-2-3 Kashiwanoha, Kashiwa, Chiba 277-0882, Japan
2
Nagoya University Graduate School of Medicine 64 Tsutumai, Showa-ku, Nagoya 466-8550, Japan
Keywords: Electromyography, Muscle Contraction Types, Artifacts.
Abstract: The analysis of biological data is an effective way to extract implicit information about the human
physiological condition, representing the performance of daily tasks. The use of this information as feedback
for robotic systems can contribute to a smoother transition into societies with a higher level of human-robot
collaboration. Superficial electromyography (sEMG) could be a powerful ally in this field, as muscle activity
serves as a window into our neural system and can be measured non-invasively with relative ease. In this
work, our objective is to extract spectral features that enable the classification between isometric and isotonic
muscle contractions. The switching between these types of contractions during human motion has been widely
linked to various physical conditions, such as muscle pain, fall prediction, postural imbalances, and stress. To
achieve this goal, we recorded muscle activity during both isometric and isotonic contractions under various
conditions. We conducted a time-frequency analysis on the data collected from five lower limb muscles of
four healthy subjects to extract significantly relevant features containing the necessary information to
discriminate between these two types of muscle activations. Our results suggest that this discrimination can
be achieved through the analysis of two spectral features: the median frequency and the power contained in
the frequency range between 11 and 32 Hz. Furthermore, the inclusion of the peak frequency as a third feature
also enables the detection of low-frequency motion artifacts.
1 INTRODUCTION
The development of increasingly efficient and
intuitive systems has enabled the progressive
adoption of new technologies by large population
groups. This phenomenon has opened the doors to an
era in which the integration between human and
technological systems will occur at a fast pace
(Ritchie, 2017; Besley, 1993). Since the COVID-19
pandemic and the burden it placed on medical centers
around the world, governments and health institutions
have been promoting the so-called 'digital health era,'
encouraging people to embrace new digital media
technologies for health self-monitoring. In this
direction, the integration of robotic solutions that
facilitate the self-monitoring of health conditions for
a wider population has been widely proposed by the
scientific community (Ahmed, 2021; Yang, 2019).
Furthermore, over the last few decades, there has
a
https://orcid.org/0000-0003-0097-2793
b
https://orcid.org/0000-0002-7759-7541
been an explosive increase in the development of
robotic systems for human support and augmentation
(Green, 2008; Shimoda, 2022). Given the
fundamental differences between the evolution-based
appearance of humans and the engineering-based
development of robotic systems, one of the biggest
challenges in this field is finding appropriate methods
for a smooth integration between these two natures.
To achieve harmonious collaboration, it is necessary
to establish communication channels between human
and robotic systems that allow for a certain level of
mutual understanding. Current scientific efforts in
this direction involve the use of biological signals as
control commands, feedback, and indicators for
robotic systems (Bainbridge, 2021). A notable
example of this approach is the Smart Wearable
Robot with Bioinspired Sensory-Motor Skill
(BioMot) project (Bacek, 2017; Costa, 2016), a
European project developed between 2013 and 2016.
Alvaro, C. and Shingo, S.
Characterization of sEMG Spectral Properties During Lower Limb Muscle Activation.
DOI: 10.5220/0012305000003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 705-712
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
705

This project aimed to develop a lower limb
exoskeleton that uses kinetic data, muscle activity,
and real-time brain signals recorded from spinal cord
injury patients to adapt their rehabilitation therapies
to their current physical and cognitive state.
The use of muscle activity recorded from
superficial electromyography (sEMG) has shown
high performance when compared to other human
recorded data (Li, 2020). Among the different bio-
electrical signals, sEMG data contains more
information about human behavior (both motor and
neural data) than electrocardiogram (ECG) or
electrooculography (EoG), and its measurement can
be done with increasingly affordable and easy-to-set-
up systems compared to brain signal recordings like
electroencephalography (EEG). sEMG signals also
exhibit a higher signal-to-noise ratio when compared
to the latter systems. However, the challenge of
artifact coupling on sEMG recordings during motion
still needs to be addressed before their effective
integration into robotic systems aimed at daily human
support (Lienhard, 2015). In this regard, the current
paper focuses on characterizing sEMG spectral
properties under different lower limb motions to
establish a ground truth that can be used in future
research when these signals are employed as
biofeedback communication channels for robotic
devices (Lünenburger, 2007).
Therefore, the spectral characterization presented
in this paper has a dual objective. On the technical
side, the authors aim to highlight spectral features that
will enable future researchers to detect and remove
sEMG data coupled with motion artifacts, preventing
this contamination from affecting the human-robot
interaction stage. On the functional side, the goal is to
provide a set of features that allow a classification
system to distinguish between fundamentally
different human motions, enabling this information to
be easily shared in real-time with external devices.
For this purpose, the current research measures
sEMG signals during both isometric and isotonic
motions under different environmental conditions
where the coupling of motion artifacts is common.
Data are recorded from five different leg muscles
during regular walking tasks and are separated into
different pairs of motor and noise conditions. A time-
frequency analysis based on the Fast-Fourier
Transform was used to extract the spectral
distribution associated with each task. Comparing
spectral distributions between paired tasks allowed
for the extraction of significant differences between
experimental conditions. Finally, the observed
changes in spectral distribution were used to select
those features that would be most helpful in
differentiating between muscle contraction types and
identifying noisy data during daily motions.
In the following section, the authors introduce the
materials and methods used during this research. This
information includes details about the volunteers
participating in the experiment and technical
information about the experimental protocol, data
recording, data processing, feature extraction, and
analysis methodology.
2 MATERIALS AND METHODS
2.1 Participants
Four participants, consisting of 2 men and 2 women,
participated in the experiment. Their ages ranged
from 27 to 46 years old, with an average age of 36.25
± 8.42. All participants were right-footed and had no
history of motor diseases. They were fully informed
about the experimental conditions and provided their
informed consent in accordance with the Declaration
of Helsinki. The study was also approved by the
ethical review board of the RIKEN research institute,
with the ethical approval code: Wako3 28–13.
Figure 1: Experimental Conditions and Queue. A)
Ground walking. B) Treadmill walking. C) Isometric
contraction of gastrocnemius and vaslus lateralis muscle in
the top image, tibialis anterior in the middle image and
peroneus longus in the bottom image. D) Combination of
band and no band fixation condition with the usage or not
of footwear. E) Graphical representation of an experimental
session composed of 3 repetitions of 12 tasks resulting form
the combonantion of motion types, band fixations and
foowear usage.
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
706
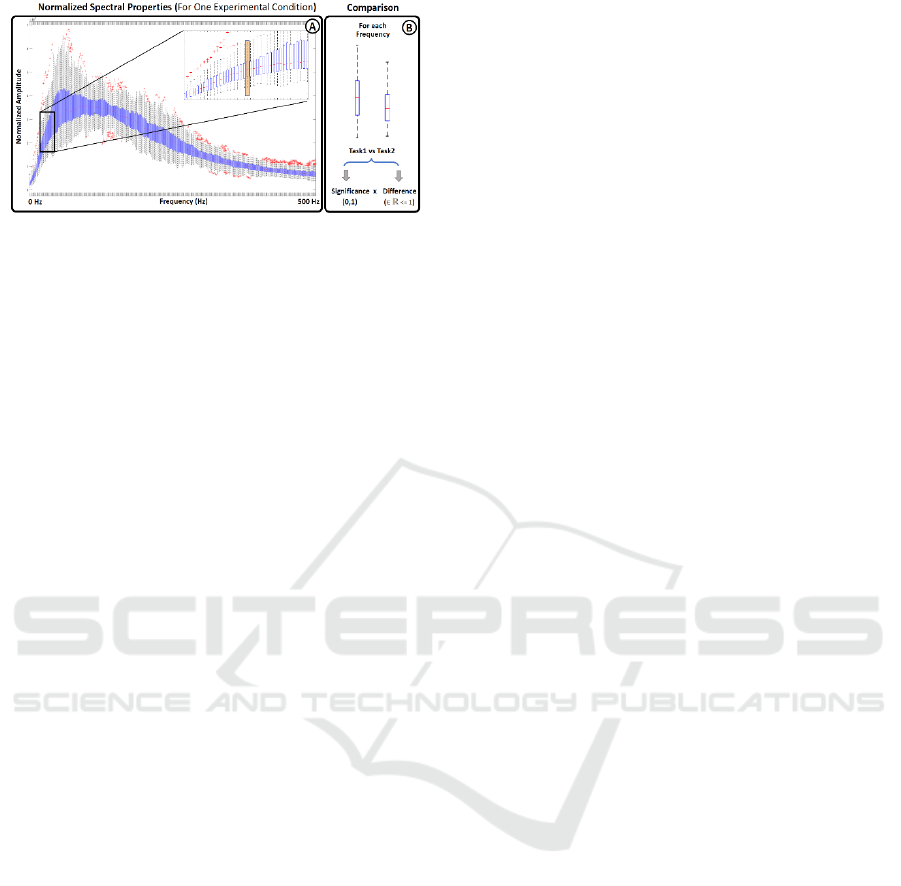
Figure 2: Spectral Representation. Boxplot representation
of the spectral features extracted from a given experimental
condition. The boxplot distribution of paired task were
compared frequency by frequency using a Wilcoxon Sum-
rank Test to find statistical differences between conditions.
2.2 Experimental Protocol
Each participant completed a single experimental
session consisting of three repetitions of the task
sequence depicted in Figure 1. Each task was defined
as a combination of a motion condition (isometric
contraction, ground walking, and treadmill walking -
as shown in Figure 1A-C), electrode fixation system
(using a band for electrode fixation or not - as
depicted in Figure 1D), and footwear usage (with or
without footwear - as illustrated in Figure 1E). This
resulted in a total of 12 tasks per repetition, as
indicated in Figure 1E. During isotonic motions
(treadmill and ground walking conditions),
participants were asked to walk for 10 steps, while
during isometric contraction tasks, they were
instructed to activate their muscles for 10 seconds.
After completing the three repetitions, the sEMG data
included muscle activation data from 30 steps for
each task performed under isotonic motion and 30
seconds of data from tasks recorded during isometric
contractions. The inclusion of different electrode
fixation conditions aimed to assess motion artifacts
related to the vibration of hanging electrodes caused
by the momentum generated in the lower limb during
gait. Additionally, the use of footwear, along with the
choice between a treadmill or regular ground
walking, was considered to account for spectral
changes associated with the coupling of power line
noise originating from the environment or other
external devices.
2.3 sEMG Recordings
Muscle activity was recorded using five wireless
bipolar electrodes (BTS FREEEMG; BTS
Bioengineering Corp., Milan, Italy) placed on the
following muscles: peroneus longus (PL), tibialis
anterior (TA), vastus lateralis (VLAT),
gastrocnemius medialis (GAN), and gastrocnemius
lateralis (GAL). The placement of electrodes
followed the guidelines established by the Surface
Electromyography for the Non-Invasive Assessment
of Muscles (SENIAM) project (Stegeman, 2007).
Data were digitized at a rate of 1000 Hz.
The selection of these muscles was based on
criteria targeting muscles that are highly active during
walking motions (Costa, 2021). These muscles were
chosen because they are located in the lower part of
the legs, where motion momentum is higher, and
therefore, motion artifacts are also expected to be
stronger.
To ensure the correct activation of the five
selected muscles during all tasks, isometric
contraction conditions were recorded under two
different exercises, as shown in Figure 1C. The top
image in Figure 1C illustrates the exercise used for
the isometric activation of the GAN, GAL, and
VLAT muscles, while the lower images in Figure 1C
depict the position and exercise used for the TA and
PL isometric contraction.
2.4 Data Processing and Feature
Extraction
In this study, one of the primary objectives was to
evaluate the effects of artifacts on the spectral
properties of sEMG data. To ensure that the impact of
motion artifacts was included in the analysis, the
recorded signals did not undergo rectification or
filtering.
Before performing spectral computations, each
task was segmented into 10 epochs. The segmentation
techniques applied to isotonic and isometric tasks
differed due to their fundamental nature. For
isometric contractions (where 10 seconds of data
were extracted for each task), epochs were obtained
as consecutive one-second segments. In the case of
isotonic motion tasks, each epoch corresponded to the
muscle activation produced by a single step. The
starting and ending values of each segment were
determined independently for each muscle using a
methodology for periodic motion segmentation
previously introduced in (Costa, 2020). This method
identifies minima and maxima in the sEMG envelope
that best align with the expected number of
activations (10 steps per task in this study). It uses an
iterative low-pass filtering process to adjust the cutoff
frequency until the envelope synchronizes with the
number of steps. The extracted points serve as
segmentation markers for epoching the sEMG raw
segmentation markers for epoching the sEMG raw
segment data.
Characterization of sEMG Spectral Properties During Lower Limb Muscle Activation
707
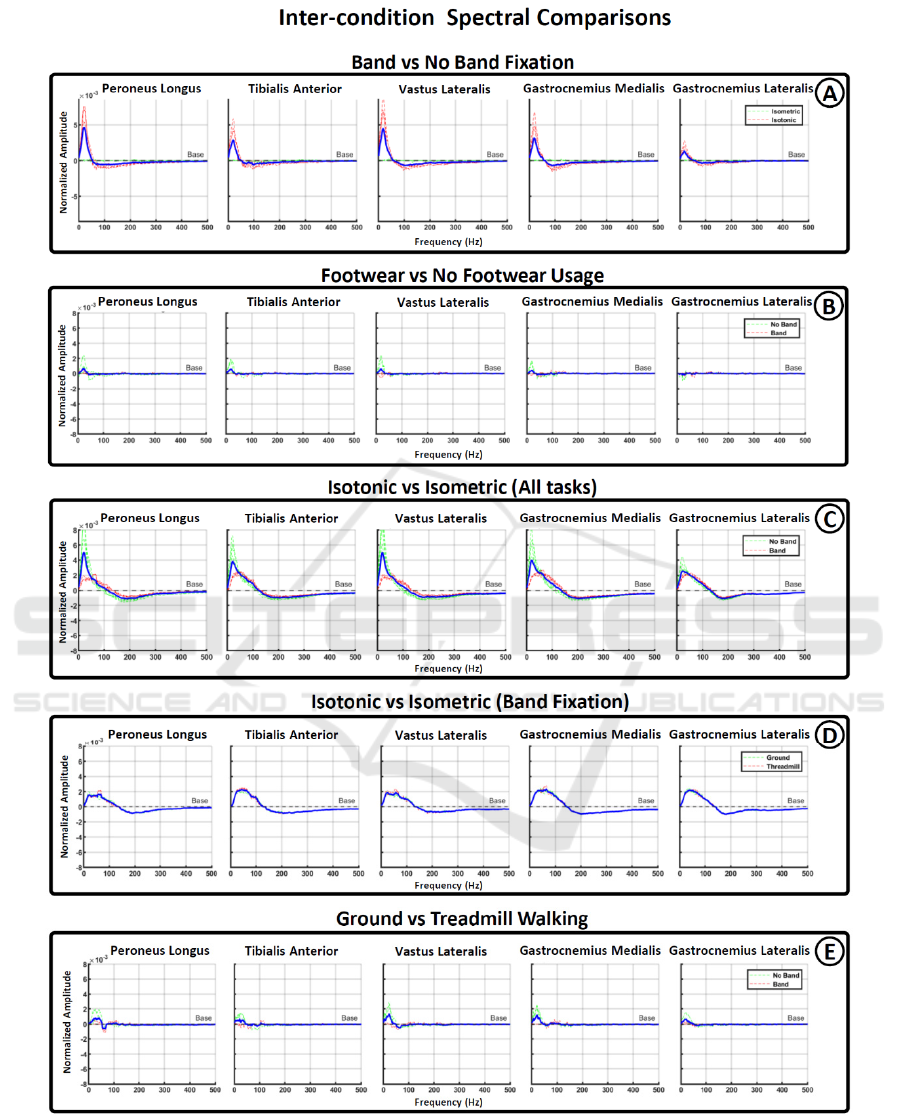
Figure 3: Spectral differences across conditions. Figures from A to E show the frequency range in which statistical
significance was found among different pair of condition. Vertically arranged graph show the results for each one of the five
muscle recorded during the experiment. Red and green lines were used to show subdivision among the conditions compared
and blue lines represent average values.
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
708
Next, a Fast Fourier Transformation (FFT) was
applied to each raw epoch to extract their spectral
features. Additionally, the power at each frequency
was divided by the total power within the range of 1-
500 Hz, respecting the Nyquist criteria for not
aliasing frequencies (Robinson, 1991). This
normalization scales the total power of the spectrum
to 1, highlighting how power is distributed within the
spectrum by removing amplitude variations. This
normalization allows for better comparisons between
tasks and subjects in terms of spectral distribution.
Due to the observed spectral differences between
the various conditions of the analyzed data (for more
details, refer to the Results section), three distinct
features were extracted from each normalized
spectrum: a) peak frequency: this represents the
single frequency value containing the highest
amplitude in the spectrum; b) median frequency: This
indicates the frequency value that divides the
spectrum into two areas with equal power; and c)
power within the range of 11 to 32 Hz: This measures
the power contained within this specific frequency
range.
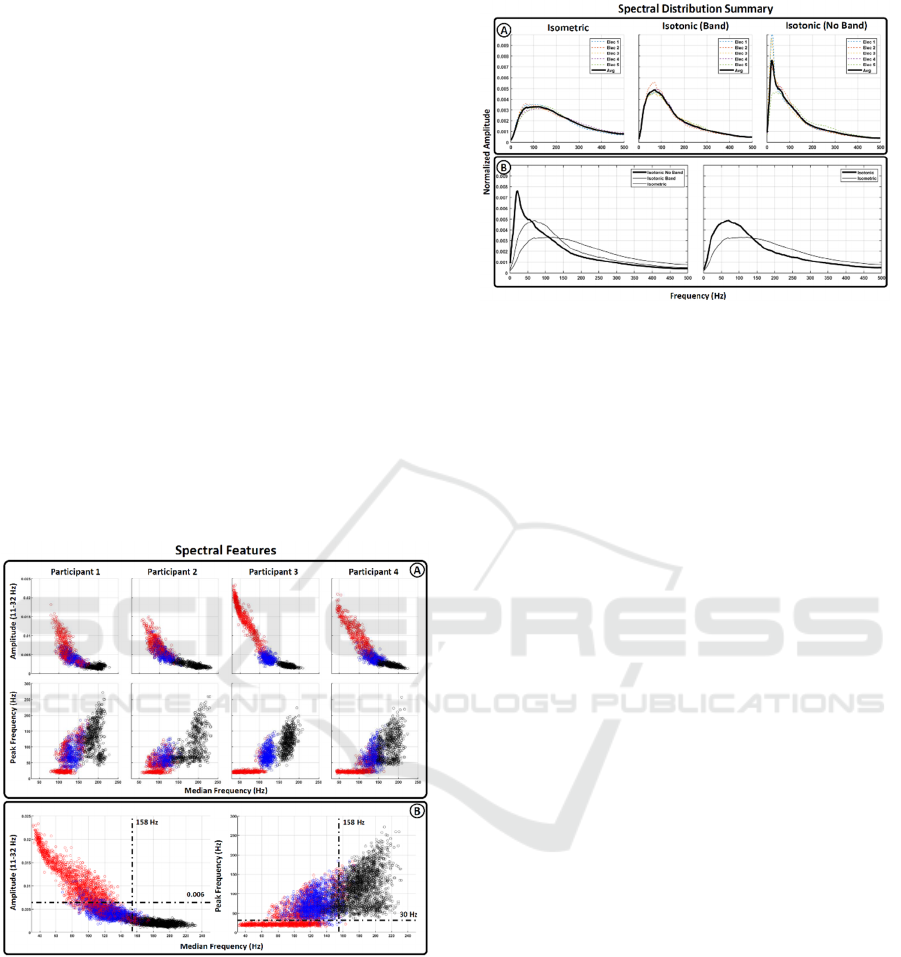
Figure 4: Spectral features. Bidimensional representation
of the 3 spectral features extracted from the main spectral
distributions. X-axis represents median frequency and Y-
axis represents the normalized amplitude in the range
between 11-32 Hz and the frequency recording the peak
amplitude respectively. Feature were painted with three
different colors to differentiate between isometric motions
(black dots), isometric motion without (red dots) and with
(blue) electrode fixation. A) Represent the feature extracted
for each subject independently. B) Shows the average data
for all participants.
Figure 5: Summary of main spectral distribution.
Average values associated to the main spectral distribution
found after sEMG analysis. A) Isometric contraction (left
graph), isotonic contraction during electrode fixation
(middle graph) and isotonic contraction without electrode
fixation (right graph). B) Comparison between main
spectral distributions
2.5 Data Comparison
The various tasks recorded during a single session
were organized into different groups to facilitate
comparisons between different experimental
conditions. These groupings encompassed
comparisons between isometric and isotonic tasks,
ground walking and treadmill walking tasks, tasks
with and without a band for electrode fixation, and
tasks with and without the use of footwear.
To identify statistically significant differences in
the spectrum between pairs of conditions, all the
spectra associated with the same condition were
represented using boxplots, as illustrated in Figure
2A. The values used for the boxplot representation at
each frequency were then compared between paired
tasks using a Wilcoxon sum-rank test, followed by a
Bonferroni-Holm correction to account for multiple
comparisons (Rey, 2011; Abdi, 2010). Subsequently,
the frequency values that exhibited statistical
significance were represented as the difference
between the median values of the respective tasks.
3 RESULTS
3.1 Spectral Shape Comparison
Figure 3 presents the results of spectral comparisons
for various paired conditions. In Figure 3A, it
becomes evident that the use of band fixation
significantly reduces low-frequency activation,
typically associated with motion artifacts. This
Characterization of sEMG Spectral Properties During Lower Limb Muscle Activation
709

reduction is particularly pronounced during isotonic
tasks but has minimal impact during isometric
contractions. In Figure 3B, the comparison between
tasks with and without footwear shows less
pronounced spectral differences, primarily affecting
the no-band fixation conditions. This suggests that
proper electrode fixation is more critical than the type
of footwear used.
Figures 3C-D compare isotonic and isometric
tasks. Figure 3C includes both band and no-band
fixation conditions, revealing an increased effect on
lower-frequency activation associated with the no-
band condition. When the no-band condition is
removed (Figure 3D), it becomes apparent that
isotonic motions lead to an expansion in the spectral
range between 20-140 Hz compared to isometric
motions. In this case, there are no notable differences
between ground walking and treadmill walking.
Furthermore, Figure 3E illustrates the spectral
differences between ground and treadmill walking
conditions. It is noticeable that an increase in low
frequencies during ground walking occurs only under
the no-band fixation condition, further supporting the
idea that when electrodes are securely fixed, there are
no significant spectral differences between ground
and treadmill walking.
Lastly, Figure 4A provides a summary of the main
spectral differences observed among the analyzed
conditions. The leftmost graph depicts the spectral
distribution of isometric tasks for the five evaluated
muscles, revealing no significant changes related to
footwear or fixation conditions. The middle graph
displays the spectral distribution of isotonic tasks
during electrode fixation conditions, showing no
significant differences between ground/treadmill
walking or footwear usage. The right graph
demonstrates the effects of not using a band for
electrode fixation in the low frequencies of the
spectrum. Electrodes 4 and 5, located in the
gastrocnemius muscle with less momentum, appear
less affected by lower frequency increases, further
supporting the hypothesis that this activation is a
consequence of motion artifacts. Figure 4B presents a
comparison between the three previous spectra (left
graph) and between isotonic and isometric conditions
when motion artifact conditions are not included
(right graph).
3.2 Feature Representation for
Classification
In Figure 5B, two bidimensional graphs provide a
comparison of the three extracted spectral features
(peak frequency, median frequency, and normalized
amplitude within the 11-32 Hz range) across different
conditions. The right graph illustrates the relationship
between peak and median frequencies, while the left
graph compares median frequency with the power
distribution in the 11-32 Hz range. Red dots represent
features extracted from isotonic motions where
electrodes were not fixed by the band. Blue dots show
the features of isotonic motions with properly fixed
electrodes. Finally, black dots represent features
extracted during isometric motions.
Figure 5A presents the same features, but they are
separated for each participant, which helps
underscore the level of inter-subject variability in the
analyzed data.
4 DISCUSSION
Our results reveal two primary changes in the spectral
distribution of sEMG data under the evaluated
conditions. The first change involves an increase in
the power of low frequencies observed during
isotonic data recordings without electrode fixation.
This change is clearly depicted in Figure 3A (red
dotted line) and Figure 3C (green dotted line).
Importantly, this phenomenon is absent during
isometric contractions (Figure 3C, red dotted line),
suggesting that the rise in lower frequencies is a
consequence of motion artifacts stemming from
electrode vibration during walking tasks. This
frequency alteration affects the range between 11 and
32 Hz, with a peak value at approximately 22 Hz,
aligning with the frequency range traditionally
associated with motion artifacts (Lienhard, 2015;
Fratini, 2009).
The second change involves a more gradual shift
between high and low frequencies when comparing
isometric and isotonic contractions, particularly
under electrode fixation conditions (Figure 3D). This
phenomenon indicates that, for isotonic motions,
there is an increase in the range between 30 and 100
Hz, compensated by a decrease between 200-300 Hz.
Unlike the previous range strongly linked to motion
artifacts, this affected range is much broader and has
minimal overlap. Furthermore, the frequencies
impacted fall within the range at which motor unit
action potentials are generated (Costa, 2022). This
suggests that the spectral differences between
isotonic and isometric contractions arise from
physiological differences rather than noise coupling.
Under band fixation conditions, no significant
changes were observed between footwear conditions
(Figure 3B, red dotted line) or between ground and
treadmill walking (Figure 3D, red dotted line). This
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
710

indicates that the coupling of external noise sources,
such as power line interference, has minimal
influence on the spectral distribution of sEMG,
making it easier to mitigate during recordings in daily
environments.
These findings enable the classification of sEMG
spectral distributions into three main groups:
isometric contractions, normal isotonic contractions,
and isotonic contractions with coupled motion
artifacts. The spectral features of each group exhibit
sufficient distinctiveness, as illustrated in Figure 5
(median frequency, peak frequency, and normalized
amplitude in the 11-32 Hz range). The black, blue,
and red feature clusters presented in Figure 5 suggest
that differentiation should be feasible with
straightforward classification techniques, enabling
real-time discrimination between isometric/isotonic
contractions and noisy/not noisy trials. Furthermore,
our results indicate low inter-subject variability in the
feature space (Figure 5A), which enhances the
potential for wider generalization of the classification
algorithm.
5 CONCLUSIONS
This study has identified distinct spectral features in
the sEMG spectrum that enable two important
outcomes: a) the discrimination between isotonic and
isometric contractions, and b) the detection of low-
frequency motion artifacts during walking tasks.
The differentiation between isometric and isotonic
contractions has widespread applications in motor
control, offering insights into various cognitive states
such as pre-fall postural instability (Xi, 2017),
motion-related muscle and joint pain (Neblett, 2016),
stress/anxiety-related head pain and migraines
(Bakal, 1977), among others. Consequently,
distinguishing between these fundamental
contractions represents a crucial initial step in
providing robotic systems with valuable information
about the cognitive state of the human. Additionally,
the ability to identify signal segments contaminated
by motion artifacts allows robotic devices to
determine the reliability of received physiological
data. As mentioned in the introduction, the challenge
of motion artifact coupling is pertinent to the
measurement of biosignals during human-robot
interaction. Precisely detecting noisy motion trials
will aid in assessing current integrative solutions and
developing innovative approaches to noise reduction.
Furthermore, comparing the spectral distributions
obtained in this study can facilitate future research in
evaluating changes in sEMG recordings following the
integration of robotic devices into the human control
loop. After further validation through a larger subject
sample, future steps will involve developing a
classification system that enables real-time
discrimination of sEMG segments based on the
extracted features and testing this system in human-
robot collaborative environments.
Lastly, it's important to note that while this study
primarily focuses on physiological signals, there are
also many human-robot interaction solutions based
on the analysis of non-bioelectrical data such as
kinematic or visual information. Although these
signals may not provide as much insight into the
neural processes underlying human behavior, they
offer benefits like easier recording and higher
accuracy in determining motion start and end points.
In general, the existence of such a variety of
approaches is a positive aspect within the scientific
community, and final integrative solutions will likely
emerge from a combination of these diverse
approaches.
ACKNOWLEDGEMENTS
The authors would like to thank the National Institute
of Advanced Industrial Science and Technology
(AIST) and the Japanese government for the
additional financial support provided through
KAKENHI grants (grant number: 18K18431).
REFERENCES
Abdi, H., "Holm’s sequential Bonferroni procedure," in
Encyclopedia of research design vol. 1(8), 2010, pp. 1-
8.
Ahmed, S.N., "Covid, AI, and robotics—A neurologist's
perspective," in Frontiers in Robotics and AI, vol 8,
2021, pp. 617426.
Bacek, T., Moltedo, M., Langlois, K., Prieto, G.A.,
Sanchez-Villamañan, M.C., Gonzalez-Vargas, J.,
Vanderborght, B., Lefeber, D. and Moreno, J.C.,
"BioMot exoskeleton—Towards a smart wearable
robot for symbiotic human-robot interaction," in
International Conference on Rehabilitation Robotics
(ICORR), 2017.
Bainbridge, W.A., Nozawa, S., Ueda, R., Okada, K. and
Inaba, M., "A methodological outline and utility
assessment of sensor-based biosignal measurement in
human-robot interaction," in International Journal of
Social Robotics, vol. 4(3), 2012, pp. 303-316.
Bakal, D.A. and Kaganov, J.A., "Muscle contraction and
migraine headache: psychophysiologic comparison," in
Headache: The Journal of Head and Face Pain, vol.
17(5), 1977, pp. 208-215.
Characterization of sEMG Spectral Properties During Lower Limb Muscle Activation
711

Besley, T., and Case A., "Modeling technology adoption in
developing countries," in The American economic
review, vol. 83 (2), 1993, pp. 396-402.
Costa, Á., Iáñez, E., Úbeda, A., Hortal, E., Del-Ama, A.J.,
Gil-Agudo, A. and Azorín, J.M., "Decoding the
attentional demands of gait through EEG gamma band
features," in PLoS one, vol. 11(4), 2016, pp. e0154136.
Costa-García, Á., Iáñez, E., Sonoo, M., Okajima, S.,
Yamasaki, H., Ueda, S. and Shimoda, S.,
"Segmentation and averaging of sEMG muscle
activations prior to synergy extraction," in IEEE
Robotics and Automation Letters vol. 5(2), 2020, pp.
3106-3112.
Costa‐García, Á., Iáñez, E., Yokoyama, M., Ueda, S.,
Okajima, S. and Shimoda, S., "Quantification of high
and low sEMG spectral components during sustained
isometric contraction," in Physiological Reports vol.
10(10), 2022, pp. e15296.
Costa-García, Á., Úbeda, A. and Shimoda, S., "Effects of
Force Modulation on Large Muscles during Human
Cycling." in Brain Sciences vol. 11(11), 2021, pp. 1537.
Fratini, A., Cesarelli, M., Bifulco, P. and Romano, M.,
"Relevance of motion artifact in electromyography
recordings during vibration treatment," in Journal of
Electromyography and Kinesiology, vol. 19(4), 2009,
pp. 710-718.
Green, S. A., Billinghurst, M., Chen, X., and Chase, J. G,
"Human-robot collaboration: A literature review and
augmented reality approach in design," in International
journal of advanced robotic systems, vol. 5(1), 2008,
pp. 1.
Li, K., Zhang, J., Wang, L., Zhang, M., Li, J. and Bao, S.,
"A review of the key technologies for sEMG-based
human-robot interaction systems," in Biomedical
Signal Processing and Control, vol 62, 2020, pp.
102074.
Lienhard, K., Cabasson, A., Meste, O. and Colson, S.S.,
"sEMG during whole-body vibration contains motion
artifacts and reflex activity," in Journal of sports science
& medicine, vol. 14(1), 2015, pp. 54.
Lünenburger, L., Colombo, G. and Riener, R.,
"Biofeedback for robotic gait rehabilitation," in Journal
of neuroengineering and rehabilitation, vol. 4(1), 2007,
pp. 1-11.
Neblett, R., "Surface electromyographic (SEMG)
biofeedback for chronic low back pain," in Healthcare,
vol. 4(2), 2016, MDPI.
Rey, D. and Neuhäuser, M., "Wilcoxon-signed-rank test,"
in International encyclopedia of statistical science,
2011, pp. 1658-1659.
Ritchie, H., and Roser M., "Technology adoption," in Our
World in Data, 2017.
Robinson, E. and Clark, D., "Sampling and the Nyquist
frequency," in The Leading Edge vol. 10(3), 1991, pp.
51-53.
Shimoda, S., Jamone, L., Ognibene, D., Nagai, T., Sciutti,
A., Costa-Garcia, A., Oseki, Y. and Taniguchi, T.,
"What is the role of the next generation of cognitive
robotics?," in Advanced Robotics, vol. 36(1-2), 2022,
pp. 3-16.
Stegeman, D. and Hermens, H.,"Standards for surface
electromyography: The European project Surface EMG
for noninvasive assessment of muscles (SENIAM)," in
Enschede: Roessingh Research and Development, vol.
10, 2007, pp. 8-12.
Xi, X., Tang, M., Miran, S.M. and Luo, Z., "Evaluation of
feature extraction and recognition for activity
monitoring and fall detection based on wearable sEMG
sensors," in Sensors, vol, 17(6), 2017, pp. 1229.
Yang, J.C., Mun, J., Kwon, S.Y., Park, S., Bao, Z. and Park,
S., "Electronic skin: recent progress and future
prospects for skin-attachable devices for health
monitoring, robotics, and prosthetics," in Advanced
Materials, vol 31(48), 2019, pp. 1904765.
BIOSIGNALS 2024 - 17th International Conference on Bio-inspired Systems and Signal Processing
712
