
A POCT to Rapid Detect GBS with Highly Sensitivity
Yang Chen
1,2,3
, Zhi-Rui Xie
1,2,3
and Yao-Gen Shu
1,2,3
1
Wenzhou Institute, University of Chinese Academy of Sciences, Wenzhou, Zhejiang 325000, P.R. China
2
Oujiang Laboratory (Zhejiang Lab for Regenerative Medicine, Vision and Brain Health),
Wenzhou, Zhejiang 325024, China
3
Research Center of Quantitative Detection Technology, NMPA Key Laboratory for Quality Monitoring
and Evaluation of Vaccines and Biological Products, Wenzhou, Zhejiang 325024, China
Keywords:
POCT, GBS, Spectral Absorption, Chromogenic Culture Media.
Abstract:
Group B streptococcus (GBS) i s a leading cause of invasive neonatal infections and a significant pathogen
in immunocompromised adults. Screening of GB S colonization in pregnant women determines the need for
antibiotic prophylaxis in that pregnancy. Therefore, efficient and rapid determination of the GBS colonization
status of pregnant women is crucial. Here, we set up a PO CT with specific spectral absorption of chromogenic
culture media to replace the traditional visual identity of GBS, which greatly improved the sensitivity of GBS
detection, and decreased the time to identify it.
1 INTRODUCTION
Group B streptococcus (GBS) is a Gram-positive en-
capsulated bacterium that belongs to the group of pyo-
genic streptococci, and an asymptomatic colonizer of
the digestive and genitourina ry tracts of healthy hu-
man adu lts. However, it can cause severe invasive in-
fections in neonates and immunocompro mised adult
patients. In 1960 s, GBS was identified into a lead-
ing cause of life-threatening neonatal infections(Hood
et al., 1961; Rosa-Fraile and Spellerberg, 2017).
In neonatology, there are two distinguish a ble clin-
ical syndromes: one is c a lled as early-onset disease
(EOD), in which GBS in fection oc curs within the first
week of life (especially within the fir st 24 h); a n-
other is called as late-onset disease (LOD), in which
GBS infection presents after 7 to 90 days postpartum.
EOD is caused by vertical transmission through either
ascending infection from the genital tract or during
labor and birth. Numerous studies have shown that
up to 30% of pregn ant women worldwide are colo-
nized with GBS, and vertica l transmission occurs for
roughly 50% of colonized m others. About 1% of col-
onized newborns develop EOD, which may be in con-
nection with rup tured mem branes because the infec-
tion of the fetus can happen only it exp osed in GBS.
Bacteremia without a focus is the most c ommon clin-
ical syndrome, followed by pneumonia and meningi-
tis. Even today th e case fatality rate for EOD is es-
timated to be 2 to 10%, and fatal outco mes are more
frequent amon g prematur e neonates(Edwards et al.,
2016).
Most EOD is due to the contact of the neonate
with GBS during delivery, therefore , intrapartum an-
tibiotic prophy la xis (IAP) administere d to GBS carri-
ers prevents vertical transmission in the vast majority
of cases, and its widespread use has resulted in sig-
nificant reductions in the incidence of EOD. LOD,
however, is most likely acquired fr om breast milk,
or from nosocomial or community sources. Prema-
turity is the m ain risk factor for developing LOD, and
bacteremia without a focus of infection is the most
common presentation. The mortality rate for LOD
is lower, but meningitis and subsequent sequelae are
more frequently associate d with LOD(Verani et al.,
2010).
GBS also causes significant maternal morbid ity,
including endometritis, chorioamnionitis, bacteremia,
and postpartum wound infections. GBS urinar y tract
infections are associate d with miscarriages, preterm
births, and low-birth-weight newborns. Although
GBS seldom causes disease in healthy adults, it is re-
sponsible for seriou s infections in diabetics, e lderly
individuals, reside nts in nursing ho mes, and other-
wise immunocompr omised patients. The successful
administration of IAP and the treatment of severe
GBS infections r e ly on efficient and reliable detec-
tion of GBS in clinical samples(Edwards and Baker,
2005).
Chen, Y., Xie, Z. and Shu, Y.
A POCT to Rapid Detect GBS with Highly Sensitivity.
DOI: 10.5220/0012287600003657
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 17th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2024) - Volume 1, pages 91-94
ISBN: 978-989-758-688-0; ISSN: 2184-4305
Proceedings Copyright © 2024 by SCITEPRESS – Science and Technology Publications, Lda.
91

In recent years, there has been rapid expansion
in the availability of chromogenic media for the de-
tection of GBS. Th ese culture media contain enzyme
substrates linked to ind oxyl chromogens, and the tar-
get microorganisms are characterized by specific en-
zyme systems that metabolize the substrate, result-
ing in release of the chromogen. Su bsequently, the
indigoid dye formed up on oxidation and dimeriza-
tion of indoxyl mo lecules in the p resence of oxy-
gen precipitates within the colonies, leading to typ-
ical brightly contrasting colors(Oren ga et al., 200 9).
Therefore, the amplitude of the contrasting is p osi-
tively to concentration of GBS. However, the tradi-
tional visual identity usua lly need at least 72 h to
determine whether the GBS colonize, thus, a rapid
highly sensitive detection of GBS is urgent.
2 MATERIALS AND METHODS
2.1 A POCT Based on the Specific
Spectral Absorption of Chromogen
We developed a POCT ( a point-o f-care testing(Shu
and Chen, 2022)) based o n chromogenic media to
rapid detect GBS, which meet the c linical nee ds with
highly sensitivity and rapid detection.
Figure 1 shows the mechanism of detection of the
POCT device. A parallel excitation light with a wave-
length of λ
1
is filtrate from filter 1, and then inci-
dent to the sample by perpendicular to the bottom of
tube. As a result, the irradiated sample emits another
light (λ
2
), which is the so-called emission light, how-
ever, partial λ
2
light will be absorbed by indoxyl chro-
mogen, and the rest emission light will b e finally de-
tected by PMT through filter 2. The absolute detected
value of time progress is directly displayed in Fig.2.
The absorbed amount of light λ
2
is proportional
to the concentration of indoxy l chromogen, while the
latter is also proportional to the concentration of GBS.
Thus, the measurable absorbed amount of light λ
2
is
indirectly proportio nal to the concentration of GBS,
that is, the concentratio n of GBS (c) can be quan tita-
tively measured by the absorbed a mount of lig ht λ
2
(Q
0
− Q
i
) as defined in Eq.(1).
2.2 Preparation of Samples and
Measuring
Two tubes of culture media with 1.2 mL were sup-
plied by Wenzhou Beikete Medical Equipment Co.,
Ltd. One contains indoxyl chromogens with higher
concentr ation and is labeled “5”, a nother is without
Figure 1: a: The mechanism of the POCT. LED is t he
source of excited l ight. A parallel excitation light with a
wavelength of λ
1
is filtrate from filter 1, and then incident to
the sample by perpendicular to the bottom of tube. As a re-
sult, the i rradiated sample emits another light (λ
2
), which is
the so-called emission light, however, partial λ
2
light will be
absorbed by indoxyl chromogen, and the rest emission light
will be finally detected by PMT through filter 2. The abso-
lute value of time progress is directly displayed in Fig.2. b:
The sample of POCT i n experiment.
any chromogens and labeled “0”. Fig.2 shows the
samples of chromoge nic media with different concen-
tration of the mixture of chromogens and correspond-
ing measured absolute light intensity, which is pre-
pared and measured simultaneously as follows:
Firstly, We measured the value of the emission
light from tube “0”, and the platform “0” (the first
higher platform from right) in Fig.2 is its time pro -
cess;
Secondly, a 5 µL samples of “5” is injected in tube
“0”, the new sample is labeled “1”, an d the measured
value is shown in platfor m “1” (the second higher
platform from right) in Fig.2;
Thirdly, Another 5 µL samples of “5” is injected
in tube “1”, the new sample is labeled “2”, and the
measured value is shown in platform “2” (the third
higher platform from right) in Fig.2;
Fourthly, A 10 µL samples of “5” is injected in
tube “2”, the new sample is labeled “3”, and the
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
92
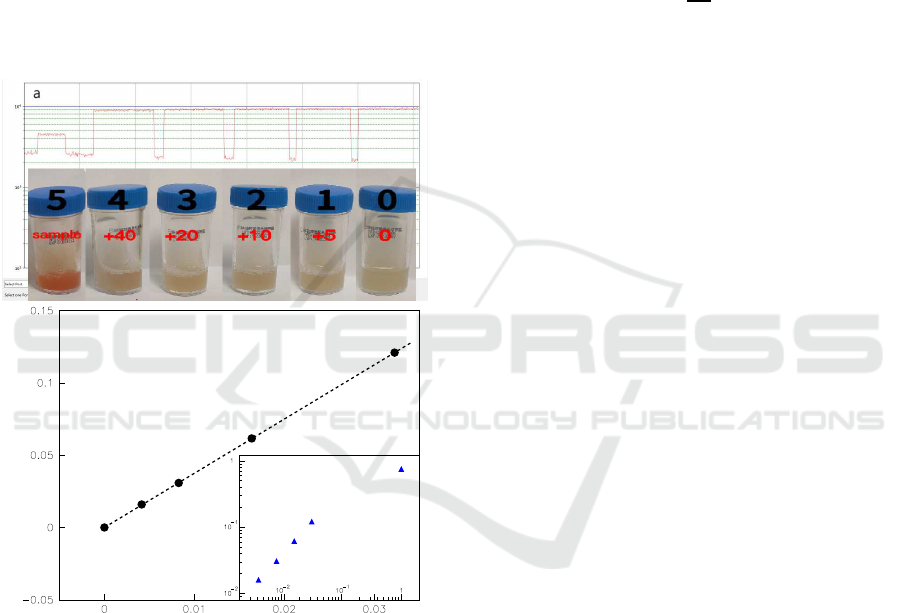
measured value is shown in platform “3” (the fourth
higher platform from right) in Fig.2;
Fifthly, A 20 µL samples of “5” is injecte d in tu be
“3”, the new sample is labeled “4”, an d the measured
value is shown in platform “4” (the fifth higher plat-
form from right) in Fig.2;
Finally, We measured the value o f the emission
light from tube “5”, and the platform “5” (the sixth
higher platform from right) in Fig.2 is its time pro-
cess;
The no rmalized concentrations of chromogens
of sample “0∼5”(with respect to that of the sam-
ple “5”), c, approximate to 0, 4.15 × 10
−3
, 8.26 ×
10
−3
, 1.64 × 10
−2
, 3.23 × 10
−2
, and 1.00 respec-
tively.
relative absorptive amplitude(ρ)
The normalized concentrations (c)
b
ρ=ac, R≈1.0
0
1
2
3
4
1
2
3
4
5
Figure 2: a: The measured absolute light intensity of sam-
ples by our POCT vs time. Each higher platform from right
to left corresponds to the value of sample 0,1,2,3,4 and 5
(The inserted panel) respectively, w hil e the lower platform
is the background value, so that the absolute light intensity
is t he higher one minus the lower one. The relative absorp-
tive amplitude of sample i, ρ
i
, can be defined by Eq.(1),
and shown in bottom. b: The relat ive absorptive amplitude
(ρ) vs normalized concentrations of samples (c), which is
almost linear (ρ = ac, where a(= 3.75) is the fitted con-
stant) at lower concentrations with regression coefficient(R)
of 1.0. The inserted panel displays that the ρ
5
/ρ
1
is nearly
10
2
, which means our POCT is very much more sensitive
than the traditional visual one.
3 RESULTS
The absolute intensity of the measur ed emission light
(Q
i
) equals the value of higher platform minus the
backgr ound noise ( c orresponds th e value of lower
platform). Thus, the absorptive amp litude o f sample
is Q
0
− Q
i
, where Q
0
is supposed the value that there
is not any indoxyl chromogens in chr omogenic media
such as sample “0”.
The relative absorptive amplitude of sample “i”,
ρ
i
, c an be defined by
ρ
i
≡ 1 −
Q
i
Q
0
, (1)
and shown in Fig.2. The relation between the rela-
tive absorptive amplitude (ρ) and the normalized con-
centrations of samples (c) is almo st linear (ρ = ac,
where a(= 3.75 ) is the fitted constant) at lower con-
centrations with regression coefficient(R) of 1.0. The
value of samples “ 1” ∼ “4” is obviously higher than
“ρ
0
(≡ 0)”, which mea ns that the sensitivity of our
method is much higher than th at of the traditional vi-
sual one as shown in Fig.2. The enormo us qua ntita-
tive difference b etween samples “1” and “5” further-
more displays the advance of the POCT. It is possible
for c hromo genic media to greatly decrease identifica-
tion time if our POCT is loa ded.
4 CONCLUSIONS AND
DISCUSSION
The sensitivity of screening methods b ased on the cul-
ture identification of maternal carriage of GBS de-
pends on the timing of specimen collection, the source
of the specimen, a nd the culture technique used by the
microbiology laboratory. The chromogenic media is
a good alternative for the identification of GBS carrier
status among near-term pregnant women. Decreasing
identification time of GBS as well as improving sen-
sitivity is our motivation to develop the POCT.
It is very difficulty for the traditional visual iden-
tity to de te rmine whether GBS colonized in samples 1
∼ 4 as shown in Fig.2. However, our POCT achieves
a rap id dete c tion of GBS, and reinforced the advance
of chromogenic media. Each platform of time course
displays difference in altitude in Fig.2, which means
our POCT is more sensitive tha n the traditional visual
one.
It has to be pointed that a quantitative measure-
ment of the number of GBS in sample “5 ” should
be done, so that the relation between ρ and number
of GBS can be determined, because it is important
for clinical trial to decid e the “threshold” of posi-
tive/negitive.
A POCT to Rapid Detect GBS with Highly Sensitivity
93

ACKNOWLEDGEMENTS
The authors thank Wenzhou Beikete Medical Equip-
ment Co., Ltd. for their supplying of samples and
chromogenic me dia, and the financial supports by
Wenzhou Institute of UCAS (WIUCASQD2020009
and WIUCASICTP2022), the Discipline Cluster
for Oncology of Wenzhou Medical University (z1-
2023005), and Oujiang Laboratory (Zhejiang Lab
for Regenerative Medicine, Vision and Brain Health)
(OJQDJQ2022001).
REFERENCES
Edwards, M. S. and Baker, C. J. (2005). Group B
streptococcal infections in elderly adults. Clin. In-
fect. Dis., 41:839–847.
Edwards, M. S., Nizet, V., and Baker, C. J. (2016). Group B
streptococcal infections: Remington and Klein’s in-
fectious diseases of the fetus and newborn infant. El-
sevier Saunders, Philadelphia.
Hood, M., Janney, A., and Dameron, G. (1961). Beta
hemolytic streptococcus group B associated with
problems of the perinatal period. Am. J. Obstet. Gy-
necol., 82:809–818.
Orenga, S., James, A. L., Manafi, M., Perry, J. D., and Pin-
cus, D. H. (2009). Enzymatic substrates in microbiol-
ogy. J. Microbiol. Methods, 79:139–155.
Rosa-Fraile, M. and Spellerberg, B. (2017). Reliable detec-
tion of group B streptococcus in the clinical labora-
tory. j. Clin. Microbiol., 55:2590–2598.
Shu, Y. G. and Chen, Y. (2022). A tr ace analysis of GBS
and its POCT. China patent, 202211273343:6.
Verani, J. R., McGee, L., and Schrag, S. J. (2010).
Prevention of perinatal group B streptococcal dis-
ease–revised guidelines from cdc. MMWR Recomm.
Rep., 59(RR-10):1–36.
BIODEVICES 2024 - 17th International Conference on Biomedical Electronics and Devices
94
