
Synthesis, Characterization, and Antiproliferation Activity Test of
Dibutyltin(IV)di-2-Hydroxybenzoate and
Dibutyltin(IV)di-3-Hydroxybenzoate Against Cervical Cancer Line
Hela
Sutopo Hadi
1
a
, Hendig Winarno
2
b
, Ermin Katrin Winarno
2
c
, Susanto
2
d
,
Mitha Nurmaya Angely
1
e
, Amelia Mareta
1
f
, Khairun Nisa Berawi
3
g
and Tati Suhartati
1
h
1
Department of Chemistry, University of Lampung Bandar Lampung 35145 Indonesia
2
Research Center for Radiation Process Technology, Research Organization for Nuclear Energy (BATAN) - National
Research and Innovation Agency (NRIA), Gedung 90, KST B.J. Habibie, Jl. Puspiptek, Muncul, Kec. Setu, Tangerang
Selatan, Banten 15314, Indonesia
3
Medical Faculty, University of Lampung Bandar Lampung 35145 Indonesia
Keywords: Antiproliferation Test, Dibutyltin (IV) hydroxybenzoate, HeLa cells, IC
50
.
Abstract: Due to the various side effects of conventional cancer treatment therapy, efforts to find anticancer agents with
minimum side effects are in great demand. One of them is the synthesis of organotin (IV) hydroxybenzoate
derivatives. Two organotin (IV) compounds, namely dibutyltin (IV) 2-hydroxybenzoate and dibutyltin (IV)
di-3-hydroxybenzoate, have been successfully synthesized. The products were obtained by reacting the
dibutyltin (IV) oxide with 2-hydroxybenzoic acid and 3-hydroxybenzoic acid. The compounds synthesized
were fully characterized by UV-Vis, FT-IR, and NMR spectroscopies and microelemental analyzer to see the
purity of the compounds. The antiproliferative activity of compounds have been tested against the HeLa
cancer cell line. The results of the antiproliferation test showed that the compound dibutyltin (IV) 2-
hydroxybenzoate had better antiproliferation activity than the compound dibutyltin (IV) 3-hydroxybenzoate
and showed high selectivity.
1 INTRODUCTION
The interest in the bioactivity of organotin (IV)
derivative compounds is not without reasons. Apart
from the chemical structure of organotin (IV)
compounds are interesting to study, they show good
potential in various biological tests (Annisa et al.,
2017; Hadi et al., 2018; Hadi et al., 2023a; Hadi et
al., 2023b; Roner et al., 2011; Samsuar et al., 2021;
Sirajuddin et al., 2021). The bioactivity of organotin
(IV) derivative compounds itself is determined by the
a
https://orcid.org/0000-0001-6464-7215
b
https://orcid.org/0000-0001-8530-5986
c
https://orcid.org/0000-0001-7920-5911
d
https://orcid.org/0000-0001-9234-8036
e
https://orcid.org/0009-0003-5225-4934
f
https://orcid.org/0009-0001-2261-7293
g
https://orcid.org/0000-0002-9398-1965
h
https://orcid.org/0000-0001-5707-464X
chemical properties and number of organic groups
bound to the central atom of Sn (Pellerito and Nagy,
2002). Meanwhile, the bound anion only plays a role
as a secondary determinant of the bioactivity of
organotin (IV) compounds (Pellerito and Nagy, 2002;
Hadi et al., 2021a; Hadi et al., 2022.
Many bioactivity studies on organotin (IV)
compounds as alternative materials have been widely
carried out and are still interesting to continue
considering the large potential in these compounds.
Organotin(IV) compounds have been tested to have
228
Hadi, S., Winarno, H., Winarno, E. K., Susanto, , Angely, M. N., Mareta, A., Berawi, K. N. and Suhartati, T.
Synthesis, Characterization, and Antiproliferation Activity Test of Dibutyltin(IV)di-2-Hydroxybenzoate and Dibutyltin(IV)di-3- Hydroxybenzoate Against Cervical Cancer Line Hela.
DOI: 10.5220/0013668400003873
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Medical Science and Health (ICOMESH 2023), pages 228-233
ISBN: 978-989-758-740-5
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.

bioactivity such as antimicrobial (Gilles et al., 2011;
Hadi et al., 2021b; Sirajuddin et al., 2021), antifungal
activity (Kovala-Demertzi et al., 2002; Hadi et al.,
2021a), antimalarial (Hadi et al., 2018; Hadi et al.,
2021b), antioxidant (Arraq and Hadi, 2023; Hussain
et al., 2023; Sari et al., 2020; Tyurin et al., 2015) as
well as antitumor and anticancer (Al-Rikabi et al.,
2023; Cepeda et al., 2007; Hadi et al., 2023a; Hadi et
al., 2023b). In previous research (Hadi et al., 2023),
the results of in vitro tests of dibutyltin (IV)
hydroxybenzoate derivatives against leukemia cancer
cells were reported, obtaining an IC
50
value of 24.4
µg/mL. If the IC
50
of this compound is ≤ 50 μg/mL,
then the organotin (IV) 3-hydroxybenzoate
compound has the potential to be an anticancer
compound (Mans et al., 2000). Therefore, it is hoped
that the compounds dibutyltin (IV) di-2-
hydroxybenzoate and dibutyltin (IV) di-3-
hydroxybenzoate, which are benzoic acid derivative
compounds, have anticancer activity and have IC
50
values that are lower than previous studies.
Among various organotin (IV) complexes,
organotin (IV) carboxylates have strong bioactivity
anticancer agent (Al-Rikabi et al., 2023; Cepeda et
al., 2007; Hadi and Rilyanti, 2010; Hadi et al., 2012;
Hadi et al., 2023a; Hadi et al., 2023b). This is also
driven by the increase in cancer sufferers throughout
the world, where according to the latest data from the
Global Cancer Observatory (2021), in 2020 in
Indonesia there were more than 396 thousand cases
of cancer recorded with 234 thousand patients dying,
which is 9.2 % of them are deaths due to cervical
cancer. This makes researchers in this field try to find
potential anticancer agents with minimum side effects
for sufferers.
In this research, we reported the synthesis and
characterization of dibutyltin (IV) di-2-
hydroxybenzoate and dibutyltin (IV)-di-3-
hydroxybenzoate to then test their antiproliferative
activity against the cervical cancer cell line, HeLa.
2 MATERIALS AND METHOD
2.1 Materials
All reagents used were of Analytical Reagent grade.
Dibutyltin(IV) oxide (1), 2-hydroxybenzoic acid, 3-
hydroxybenzoic acid, Dulbecco’s Modified Eagle’s
Medium (DMEM), NaHCO
3
,
fetal bovine serum
(FBS) were obtained from Sigm-Aldrich (MA,
USA). Methanol and dimethylsulfoxide (DMSO)
were obtained from Merck Millipore (MA, USA). All
of these reageants were used as received and without
any further purification. Cervical cancer line Helas
was obtained from Elabscience®, USA.
2.2 Synthesis of Dibutyltin (IV)
hydroxybenzoate
The synthesis procedure for the compounds 2 and 3
was adopted from the procedure carried out (Hadi &
Rilyanti, 2010; Hadi et al., 2012), which was an
adaptation of the procedure used by Szorcsik et al.
(2002).
In the synthesis of dibutyltin(IV) di-2-
hydroxybenzoate (2) the starting materials used as
follows: 1.3579 grams of dibutyltin(IV) oxide (1) was
reacted with 0.9036 grams of 2-hydroxybenzoic acid
in 10 mL of methanol p.a. as a solvent The reaction
mixture was refluxed for 4 hours in a hotplate stirrer
at a temperature of 60-61℃. After the reaction was
complete, the methanol was evaporated and dried in
a vacuum desiccator until dry crystals with constant
weight are obtained. The compound was then
characterized using an UV-Vis, FT-IR,
1
H-NMR and
13
C-NMR spectroscopic analyses, the composition of
the element (hydrogen and carbon) was determined
using a microelemental analyzer, and then tested its
antiproliferative activity against cervical cancer cell
line HeLa. The same procedure was used in the
preparation of compound 3.
The compounds synthesized obtained were as
follows:
Compound 2: white solid; UV λ
max
. (MeOH) nm
(log ε): 242 and 304.8; IR ν
max
. (KBr) cm
-1
: 2928.98-
2870.07 (C-H (-CH
3
) in Bu), 1558.7 (C=O), 1419.6
(CO
2
asym), 1251.74 (Sn-O-C), 1077.62 (Sn–C in
Bu), 753.35 (Sn–O);
1
H NMR (in DMSO-d
6
, 600
MHz) δ (ppm): Hα: 1.6 (t), Hβ:1.4 (m); Hγ: 1.29 (t);
Hδ: 0.93 (t), H in benzoate = 7.35–7.85 (m);
13
C NMR
(in DMSO-d
6
, 150 MHz): δ (ppm): Cα: 26.8, Cβ:
25.5, Cγ: 21.4, Cδ: 13.5, C1: 164.3; C2: 131.5, C3:
132.2, C4: 138.4, C5: 125.1, C6: 128.6, C7: 129.7;
microelemental analysis: found (calculated): C 52.29
(52.07), H 5.48 (5.52).
Compound 3: white-light yellowish solid; UV
λ
max
. (MeOH) nm (log ε): 243 and 299; IR ν
max
. (KBr)
cm
-1
: 2958.78-2872.92 (C-H (-CH
3
) in Bu), 1558.7
(C=O), 1419.6 (CO
2
asym), 1258.73 (Sn-O-C),
1079.10 (Sn–C in Bu), 763.34 (Sn–O);
1
H NMR (in
DMSO-d
6
, 600 MHz) δ (ppm): Hα: 1.6 (t), Hβ:1.4
(m); Hγ: 1.29 (t); Hδ: 0.93 (t), H in benzoate = 7.35–
7.85 (m);
13
C NMR (in DMSO-d
6
, 150 MHz): δ
(ppm): Cα: 26.8, Cβ: 25.5, Cγ: 21.4, Cδ: 13.5, C1:
164.3; C2: 131.5, C3: 132.2, C4: 138.4, C5: 125.1,
C6: 128.6, C7: 129.7; microelemental analysis: found
(calculated): C 52.29 (52.07), H 5.48 (5.52).
Synthesis, Characterization, and Antiproliferation Activity Test of Dibutyltin(IV)di-2-Hydroxybenzoate and Dibutyltin(IV)di-3-
Hydroxybenzoate Against Cervical Cancer Line Hela
229
2.3 Antiproliferation Activity Test
The procedure for testing antiproliferative activity as
an anticancer in this study is part of a series of cancer
cell bioassays adopted from procedures carried out by
Winarno et al., (2009) and Hadi & Rilyanti (2010).
The media was prepared as follows: 10.4 grams
of Dulbecco’s Modified Eagle’s Medium (DMEM)
that is containing L-glutamine was dissolved in 1 L
of aquabiadest and then was added with 2.3 grams of
NaHCO
3
that was dissolved in 1 L of aqubidest in an
Erlenmeyer flask, then the solution mixture was
stirred until homogeneous, and the pH of the solution
was then measured with a pH indicator until a normal
pH was obtained (pH 7-7.5). For cell culture
purposes, 15 mL of 10% fetal bovine serum (FBS)
was added to 85 mL of the prepared media. All works
were carried out in laminar air flow under sterile
conditions.
The activity test was carried out on each sample
dissolved in DMSO. 5 concentration variations were
used, i.e. 0; 0.5; 1; 4; 8 µg/mL which was then
compared with Vero cells with 5 concentration
variations at 0; 16; 32; 64, 128 µg/mL. Media
containing HeLa cell suspension (2 x 10
6
cells/mL)
was placed into a multi-well plate tissue's culture with
24 wells, 1 mL in each well. As a control, 10 µL of
DMSO was used to which 990 µL of HeLa cell
suspension was added and a sample of the compound
tested was added in each concentration. Then, 30 µL
of penicillin as an antibiotic was added. The
experiment was carried out in triplicate, then the cell
suspension filled with the test substance was closed
tightly and wrapped in HVS. Then, incubated for 72
hours at 37°C in a 5% CO
2
incubator.
Cell counting was carried out using an improved
Neubauer hemocytometer. To differentiate between
live cells and dead cells, before counting, 20 µL of
1% tryphan blue solution was added and
homogenized. A mixture of samples that had been
stained with tryphan blue in the amount of 100 µL of
solution was flowed into the Neubauer Improved
Haemocytometer. The cell suspension was inserted
into the chamber where the suspension must be
sufficiently dilute so that cells or other particles do
not overlap in the counting chamber and must be
evenly distributed. After that, the number of living
cells was counted under a microscope with 4000x
magnification. Live cells appear as clear spheres with
a blue spot of cell nucleus in the center of the sphere,
while dead cells appear as dark blue-black spots with
an irregular shape. The percentage of inhibition of the
test substance on the growth of HeLa cancer cells was
calculated as follows:
% inhibition = 1 – 𝐴/𝐵 x 100% (1)
A: the number of living cells in the medium
containing the test substance
B: number of living cells in media that does not
contain the test substance (control).
The inhibition percentage data was plotted into a
probit table to obtain a probit value. Then a graph was
made between log concentration (x) and probit (y) to
obtain the linear regression equation y = a + bx. By
entering the value y = 5 (probit of 50%), the x value
(log concentration) was obtained, the IC
50
value by
converting the log concentration value to anti-log
form. IC
50
is the concentration of the test substance
that can inhibit cell division by 50% after an
incubation period of 72 hours. The activity of a
sample is said to be active as an anti-cancer if the IC
50
value is ≤ 50 µg/mL (Mans et al., 2000).
3 RESULTS AND DISCUSSION
3.1 Synthesis and Characterization of
Dibutyltin (IV) hydroxybenzoate
Compounds 2 and 3 were obtained as white solids
with the yields of 84.45% and 80.88% respectively.
Characterization using UV, FT-IR,
1
H NMR, and
13
C
NMR, provide good spectra, and the values of micro
elemental analysis data are in agreement with those
of the theoretical values, confirming successful
synthesis of the compounds. The reaction scheme for
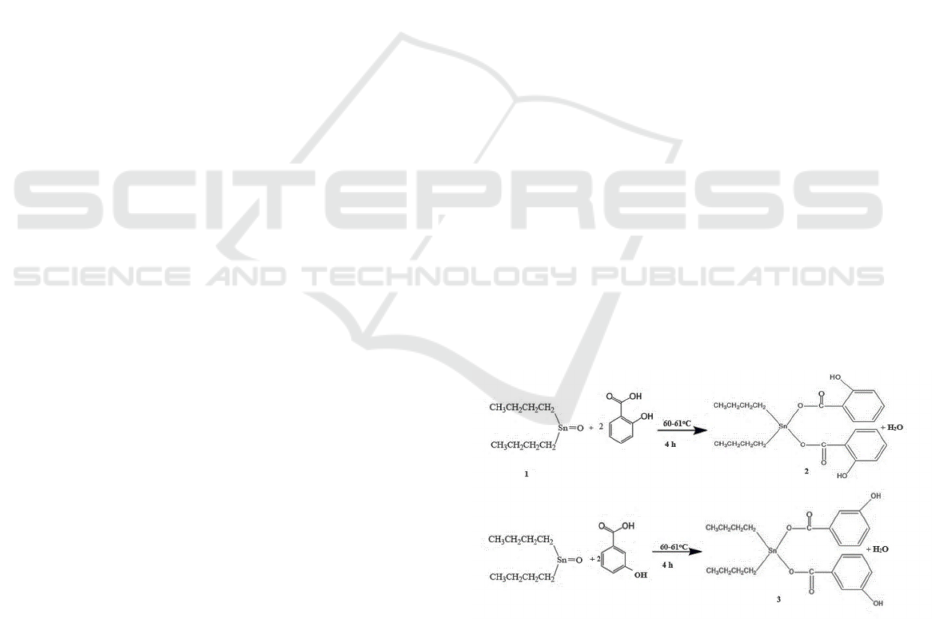
the preparation of 2 and 3 is shown in Figure 1.
Figure 1: The Scheme of the preparation of the compounds
studied
The UV spectroscopy analysis of compounds 2
and 3 produced characteristic absorption values with
λ
max
of the compounds prepared, associated with
transitions of π→π* and n→π*. At the same time, the
starting material 1 gives only one characteristic peak
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
230
at 203 nm resulted from a π→π* transition due to
delocalization of butyl electrons. In Figure 1a, the
reaction of 1 with 2-hydroxybenzoic acid to produce
compound 2, a bathochromic shift, occurs due to the
influence of the chromophore group from the
carbonyl group and addition of the benzene ring. In
compound 2, there is also a n→π* transition at 304
nm due to alone pair electrons at the hydroxide group.
Similar shift changes were also observed in the
formation of compounds 3 (Hadi and Rilyanti, 2010;
Hadi et al. 2012; Hadi et al., 2022; 2023a; Hadi et al.,
2023b).
The
1
H and
13
C NMR spectra of compounds 2 and
3 were carefully evaluated. The data for compound 2
were compared with those previously available for
similar compounds (Hadi and Rilyanti, 2010; Hadi et
al. 2012; Hadi et al., 2022; 2023a; Hadi et al., 2023b).
In
1
H NMR, the chemical shifts of the butyl protons
bound to the Sn atom appeared as expected in the
range of 0.836 – 1.673 ppm, while the chemical shifts
of the benzoate protons were in the range of 7.544 –
7.808 ppm.
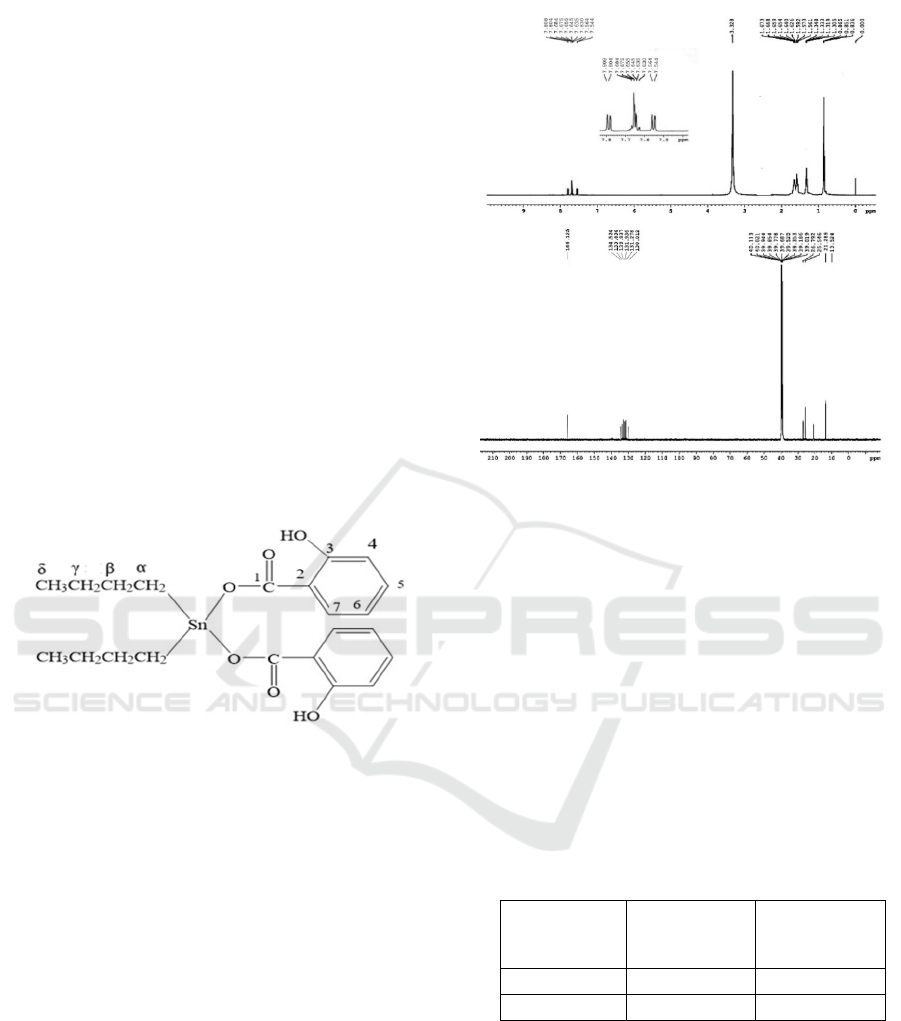
Figure 2. The numbering system of carbon atoms in
compound 2
The
13
C NMR of the butyl bonded to the Sn atom
showed absorption at 130.012 – 166.125 ppm and for
benzoate carbon at 166.125. The expected chemical
shift of carbonyl carbon appears at 166-167 ppm
(Hadi and Rilyanti, 2010; Hadi et al. 2012; Hadi et
al., 2022; 2023a; Hadi et al., 2023b). A similar
pattern was observed for compound 3. The example
of numbering the carbon atom for compound 2 is
shown in Figure 2 and the
1
H and
13
C NMR spectra
of the compound are presented in Figure 3a and 3b.
3.2 Antiproliferative Activity
The results of the antiproliferative activity test of
compounds 2 and 3 against cervical cancer line HeLa
are shown in Table 1. Based on these data, it was
found that both compounds 2 and 3 have very low
IC
50
values. The relatively small IC
50
value (IC
50
< 20
µg/mL) shows that the activity of the compounds
Figure 3: (a) The
1
H NMR and (b)
13
C NMR spectra of
compound 2
synthesized is categorized to be active as an
anticancer based on the National Cancer Institute
(NCI) Guideline so that further clinical testing can be
carried out for its use as a safe anticancer agent.
Futhermore based on the IC
50
value for Vero cells, the
selectivity index (SI) can be calculated by dividing
the IC
50
of Vero cells by the IC50 of HeLa cells. The
SI numbers of compounds 2 and 3 for Hela cells were
3.94 and 5.82, respectively. A sample is said to have
high selectivity if it has a SI > 3 (López-Lázaro,
2015), therefore, compounds 2 and 3 have high
selectivity.
Table 1: Results of antiproliferative activity test of
compounds against HeLa cancer cells
Compound IC
50
Value
against HeLa
(µg/mL)
IC
50
Value
against Vero
(µg/mL)
2 6.11 24.09
3 16.68 97.10
The reported results are almost similar to the
results obtained by several other researchers such as
Gielen (2003) and Pellerito et al. (2006). More
promising fact is that based on the IC
50
values data in
the literatures (Gielen, 2003; Pellerito et al., 2006),
the results obtained in the research that has been
carried out show that the compound that has been
synthesized actually shows higher anticancer activity
than the
currently available cisplatin, cis-
Synthesis, Characterization, and Antiproliferation Activity Test of Dibutyltin(IV)di-2-Hydroxybenzoate and Dibutyltin(IV)di-3-
Hydroxybenzoate Against Cervical Cancer Line Hela
231

[Pt(NH
3
)
2
Cl
2
]. It is widely used to treat various types
of cancer. The results obtained are certainly very
promising that organotin (IV) carboxylate derivative
compounds are potential candidates for metal-based
anticancer drugs in the future.
4 CONCLUSIONS
The results showed that the two dibutyltin (IV)
hydroxybenzoates synthesized have quite high
anticancer activity when viewed from their
antiproliferative activity. The antiproliferative
activity of the tested compound indicated that the IC
50
value of compound 2 is higher than compound 3 with
an IC
50
value of 6.11 µg/mL against cervical cancer
cell HeLa, although the SI of compound 3 against
Vero cell line is higher than 2. Therefore, these two
compounds are promising to be candidate as
anticancer metal based-drug.
ACKNOWLEDGEMENTS
Thanks must go to Higher Education Technology and
Innovationa (HETI) Universitas Lampung, Republic
of Indonesia for providing the funding through
Domestic Innovation Research and Collaboration
2023 with contract number of
10629/UN26/HK.01.00/2023, 17 October 2023. We
also thank Dr. Huy Hoang of Institute of Molecular
Biosciences (IMB) University of Queensland for
NMR experimentation.
REFERENCES
Al-Rikabi, E.H., Al-Refai, R.A.K., Baqir, S.J., Hadi, A.G.,
Al-Qayyim, A.K., 2023. Synthesis, Structure, and in
vitro Cytotoxic Activity of Two Organotin Complexes
of 2-[(2, 3-Dimethylphenyl) Amino] Benzoic Acid.
Journal of Medicinal and Chemical Sciences, 6(6),
1230–1238
Annissa., Hadi, S., Suhartati, T., and Yandri, 2017.
Antibacterial Activity of Diphenyltin (IV) and
Triphenyltin(IV) 3-Chlorobenzoat Againts
Pseudomonas aeruginosa and Bacillus subtilis. Oriental
Journal of Chemistry, 33(3), 1133-1139.
Arraq, R.R., Hadi, A.G., 2023. Synthesis, Identification,
and Anti-oxidant Activity of Di-Organotin (IV)-
Cephalexin Complexes. Journal of Medicinal and
Chemical Sciences, 6(2), 392–401.
Cepeda, V., Fuertes, M.A., Castilla, J., Alonso, C.,
Quevedo, C., Perez, J.M., 2007. Biochemical
mechanisms of cisplatin cytotoxicity. Anticancer
Agents in Medicinal Chemistry, 7(3), 3-18.
Cordell, G.A., Kinghorn, D., Pezzuto, J.M., 1993.
Separation, structure elucidation, and bioassay of
cytotoxic natural products, in Colegate, S.M.,
Molyneux, R.J/ (eds): Bioactive natural products. Boca
raton, CRC Press, pp 195–216
Dachriyanus, 2004. Spectroscopic Analysis of the Structure
of Organic Compounds. LPTIK, Universitas Andalas,
Padang, Indonesia 132 p. (in Indonesian)
Gielen, M., 2003. An Overview of Forty Years Organotin
Chemistry Developed at the Free Universities of
Brussels ULB and VUB. Journal of Brazillian
Chemistry Society, 14 (6), 870-877.
Gasser, G., Ott, I., Metzler-Nolte, N., 2011. Organometallic
anticancer compounds. Journal of Medicinal
Chemistry, 54 (1), 3-25
Hadi, S., Rilyanti, M., 2010. Synthesis and in vitro
anticancer activity of some organotin (IV) benzoate
compounds, Oriental Journal of Chemistry, 26 (3),
775-779.
Hadi, S., Rilyanti, M., Suharso, 2012. In vitro activity and
comparative studies of some organotin (iv) benzoate
derivatives against leukemia cancer cell, l-1210.
Indonesian Journal of Chemistry, 12(2), 172-177.
Hadi, S., Noviany, and Rilyanti, M., 2018. In Vitro
Antimalarial Activity of Some Organotin (IV)2-
Nitrobenzoate compounds against Plasmodium
falciparum. Macedonian Journal Chemistry and
Chemical Engineering, 37(2), 185 -191.
Hadi, S., Irawan, B., Yandri, Suhartati, T., 2021a.
Synthesis, characterization and the antifungal activity
test of some organotin (IV) benzoates. Journal of
Physics: Conference Series, 1751(1), 012099
Hadi, S,. Fenska, M.D., Noviany, N., Satria, H.,
Simanjuntak, W., Naseer, M.M., 2021b. Synthesis and
Antimalarial Activity of Some Triphenyltin(IV)
Aminobenzoate Compounds against Plasmodium
falciparum. Main Metal Group Chemistry, 44(1), 256-
60.
Hadi, S., Suhartati, T., Noviany, N., Pandiangan, K.D.,
Yandri, Y., Simanjuntak, W. 2022. Disinfecting
activity of some diphenyltin(IV) benzoate derivative
compounds. Pure and Applied Chemistry, 94(7), 799-
807
Hadi, S., Winarno, E.K., Winarno, H., Berawi, K. N.,
Suhartati, T., Noviany, N., Simanjuntak, W., Yandri,
Y., 2023. Synthesis and in vitro activity investigation of
some dibutyl-, diphenyl-and triphenyltin (IV)
carboxylates against leukemia cancer cell, L-1210.
Pure and Applied Chemistry, 95(7), 823-832.
Hadi, S., Winarno, E.K., Winarno, H., Berawi, K. N.,
Suhartati, T., Yandri, Y., Simanjuntak, W., 2023.
Synthesis, characterization and in vitro activity study of
some organotin (IV) carboxylates against leukemia
cancer cell, L-1210. Sustainable Chemistry Research:
Chemical and Biochemical Aspects, 199 - 206
https://gco.iarc.fr/Global Cancer Observatory, 2021.
Cancer Today. (Accessed on December 25, 2023)
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
232

Kovala-Demertzi, D.K., Dokorou, V., Ciunik, Z.,
Kourkoumelis, N., Demertzis, M.A., 2002. Organotin
mefenamic complexes preparations, spectroscopic
studies and crystal structure of a triphenyltin ester of
mefenamic acid: Novelanti-tuberculosis agents. Appliel
Organometallic Chemistry, 16(7), 360-368.
López-Lázaro, M. 2015. A simple and reliable approach for
assessing anticancer activity in vitro. Current
Medicinal Chemistry, 22(11), 1324–1334.
Mans, D.R.A., da Rocha, A.B., Schwartsmann, G., 2000.
Anti-Cancer Drug Discovery and Development in
Brazil: Targeted Plant Collection as a Rational Strategy
to Acquire Candidate Anti-Cancer Compounds, The
Oncologist, 5 (3), 185-198.
Pellerito, L., Nagy, L., 2002. Organotin (IV)
n+
complexes
formed with biologically active ligands: equilibrium
and structural studies, and some biological aspects,
Coordination Chemistry Reviews, 224, 111 – 150.
Pellerito, C., Nagy, L., Pellerito, L., Szorcsik, A. 2006.
Biological activity studies on organotin (IV)
n+
complexes and parent compounds, Journal of
Organometallic Chemistry, 691, 1733–1747.
Rocha, C.S., de Morais, B.P., Rodrigues, B.L., Donnici,
C.L., de Lima, G.M., Ardisson, J.D., Takahashi, J.A.,
and Bitzer, R.S. 2016. Spectroscopic and X-ray
Structural Characterization of New Organotin
Carboxylates and Their In Vitro Antifungal Activities.
Polyhedron 117, 35‒47.
Roner, M.R., Carraher Jr, C.E., Shahi, K., Barot, K., 2011.
Antiviral Activity of Metal-Containing Polymers-
Organotin and Cisplatin-Like Polymers Materials.
Materials (Basel), 4(6): 91-112.
Samsuar S., Simanjuntak W., Qudus H.I., Yandri Y.,
Herasari H., Hadi S., 2021. In Vitro Antimicrobial
Activity Study of Some Organotin (IV)
Chlorobenzoates against Staphylococcus aureus and
Escherichia coli. Journal of Advanced Pharmacy and
Education Research, 11(2), 17-22.
Sari, W., Qudus, H.I., Hadi, S., 2020.The chemical
reactivity study of organotin (IV) 4-aminobenzoates
using cyclic voltammetry and antioxidant activity test
by the DPPH method. Revista de Chimie, 71(10), 28–
37.
Sirajuddin, M., Ali, S., Tahir, M.N., 2021. Organotin(IV)
Derivatives Based on 2-((2-methoxyphenyl)
carbamoyl) Benzoic Acid: Synthesis, Spectroscopic
Characterization, Assessment of Antibacterial, DNA
Interaction, Anticancer and Antileishmanial Potentials.
Journal of Molecular Structure, 1229: 129600.
Szorcsik, A., Nagy, L., Gadja-Schrantz, K., Pellerito, L.,
Nagy, E., Edelmann, E.T., 2002. Structural studies on
organotin (IV) complexes formed with ligands
containing {S, N, O} donor atoms, Journal of
Radioanalytical and Nuclear Chemistry,252(3), 523–
530.
Tyurin V.Y., Yaouhan W., Prishchenko A.A., Shpakovsky
D.B., Gracheva Y.A., Antonenko T.A., Tafeenko,
V.A., Al´bov, D.V., Aslanov, L.A., Milaeva, E.R, 2015.
Complexes of Organotin Compounds with bis- and
Trisphosphonate Derivatives of 2,6-ditertbutylphenol
Having Antioxidant Activity. Russian Chemistry
Bulletin, 64(6), 1419–1429.
Winarno, E.K., Winarno, H., Susanto. 2009.
Antiproliferative activity of extracts and fractions from
irradiated Curcuma zanthorrhiza rhizomes against
mouse leukemia and human cancer cell lines. Atom
Indonesia, 45(3), 159–164
Synthesis, Characterization, and Antiproliferation Activity Test of Dibutyltin(IV)di-2-Hydroxybenzoate and Dibutyltin(IV)di-3-
Hydroxybenzoate Against Cervical Cancer Line Hela
233
