
The Effect of Rhizophora apiculata Bark Ethanol Extract on Burns
Healing of Rattus norvegicus Sprague Dawley Strain
Syazili Mustofa
1a
, Delisa Mutiara Nabila
2
and Evi Kurniawaty
1
1
Department of Biochemistry and Molecular Biology, Medical Faculty, Universitas Lampung,
Prof. Soemantri Brojonegoro Street, Bandar Lampung, Indonesia
2
Medical Faculty, Universitas Lampung, Bandar Lampung, Indonesia
Keywords: Burn Wound, Rhizophora apiculata Bark Extract, Wound Healing.
Abstract: This study aimed to evaluate the ability of an ethanolic extract of Rhizophora apiculata bark to treat burn
wounds in rats. Second-degree burn wounds were induced in five groups of six rats each, and topical treatment
was done daily for 26 days. Group KN and K+ received aqua bidestilata and bioplacenton® (containing
placenta extract 10% and neomycin sulfate 5%) as a control and reference standard, and group P1, P2 and P3
were given ethanolic Rhizophora apiculata bark extract of 20%, 30% and 40% respectively. The observing
burn wounds for ± 26 days were enrolled for evaluating the effects on the burn healing phase, burn area
reduction, and burn healing time. The area of burn wounds in the groups of rats given the extract began to
shrink more quickly on the 15th day of observation. The One-way ANOVA test revealed p=0.001, and the
LSD Post Hoc test revealed p < 0.05, indicating a significant difference in the area of burns on day 15 for all
treatment groups. The healed wounds in extracts-treated rats were faster and had better wound healing time.
Wound contractions were relatively better in extract groups. In conclusion, the Rhizhopra apiculata bark
ethanolic extract positively affected wound burn healing activity.
1 INTRODUCTION
Burns ranks fourth among the world's most common
injury categories. The World Health Organization
(WHO) estimates that 180,000 people die as a result
of burn injuries out of an estimated 11 million people
who suffer from burn wounds annually. Burns are
complex wounds with a high mortality rate that are
challenging to heal, regardless of the underlying
cause (Opriessnig, 2023).
Burn injuries are generally categorized as a type of
wound where the source is one of several factors,
including heat, cold, electricity, chemicals, friction,
or radiation. Conversely, wound healing is a
complicated process, and knowledge of its biological
trend and the variations in how various wounds heal
can often lower the danger and significantly lessen
the likelihood of further harm to the injured tissue and
other organs. Burn wound healing is a dynamic
process involving various interactions of cytokines
and extracellular matrix. This healing process is
a
https://orcid.org/0000-0002-7646-0869
divided into three phases: inflammation,
proliferation, and tissue remodelling. Wound healing
consists of wound healing and contracture, where
regeneration of the epithelial layer occurs and the
wound shrinks (Żwierełło, 2023). Healing burn
wounds is still a challenge faced by the modern
medical era. Only a few drugs can speed up wound
healing. There is still a need to find new drugs to
speed up the healing of burn wounds. Medicines
derived from medicinal plants have great potential to
be developed as alternative treatments (Huang, 2022).
Rhizophora apiculata, commonly known as
Bakau Minyak in Indonesia, is naturally distributed in
Southeast Asia and India. The plant contains saponin,
tannins, flavonoids and terpenoids (Bulan, 2022).
Traditionally, Rhizophora apiculata is employed by
Indonesians as a medicinal plant for gastrointestinal
and skin diseases. Our earlier research revealed that
this plant might have antioxidant (Mustofa, 2023) and
anti-inflammatory properties (Mustofa, 2019). For
these reasons, we were interested in researching the
effect of Rhizophora apiculata on healing burns.
Mustofa, S., Nabila, D. M. and Kurniawaty, E.
The Effect of Rhizophora apiculata Bark Ethanol Extract on Burns Healing of Rattus norvegicus Sprague Dawley Strain.
DOI: 10.5220/0013668300003873
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Medical Science and Health (ICOMESH 2023), pages 223-227
ISBN: 978-989-758-740-5
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
223

2 MATERIALS AND METHODS
2.1 Plant Material
The bark was obtained from the Forest Management
Unit KPH Gunung Balak, East Lampung, Indonesia,
in June 2022. The plant was authenticated by the
Department of Biology, Faculty of Mathematics and
Natural Sciences, Universitas Lampung.
2.2 Preparation of Extracts
We have carried out the process of making ethanol
extract in two steps, namely maceration and
evaporation. Maceration is the process of soaking
simplicia using a solvent for several days at room
temperature and protection from light. After air
drying, the bark was ground into a fine powder with
a blender. We weighed 100 grams of Rhizophora
apiculata stem bark powder and put it in a 2-litre
Erlenmeyer glass. Next, soaking was done with 2000
ml of 95% ethanol solvent and stirred for
approximately 30 minutes until the powder was
dissolved entirely. Finally, this solution was left to
settle for 18 hours. After this process, the second
stage is carried out, namely evaporation. This process
uses a rotatory evaporator and water bath
(temperature 90˚C). The ethanol solution and active
substance in the flask are allowed to separate until the
ethanol flow stops dripping into the collection flask
(Mustofa, 2020).
2.3 Animals Model
For this experiment, male rats weighing 200–250 g
were employed. They were housed in a central animal
house with a temperature of 23 ± 1o C and a 12-hour
light/dark cycle. They were fed adequately and
appropriately and had unlimited access to water
(Kurniawaty, 2022). Before beginning this
experiment, the medical faculty of Universitas
Lampung approved the ethical clearance for the
research by number
4479/UN26.18/PP.05.02.00/2022.
2.4 Burn Induced
Thirty rats underwent a back shave, 70% ethanol
disinfection, and xylazine and ketamine injections to
induce anaesthesia. Afterwards, an iron plate with a
20 mm diameter that had been heated over an
electrical heater for 10 seconds was used to create a
circular burn wound (3.14 cm2) on their dorsal
portions. After that, distilled water was applied to the
wound for a minute.
2.5 Experiment Protocol
Burn induced Rats were split into five groups (n =6):
group KN got aqua bidestilata as control, group K+
received reference standard treatment
(Bioplacenton®), groups P1, P2, and P3 received
20%, 30%, and 40% (w/w) of the extract,
respectively. Rats' dorsal back wounds were treated
topically with aqua bidestilata, Bioplacenton®, and
the extract every day at 24-hour intervals. The day of
burn generation was zero, and a 26-day treatment
protocol was initiated 24 hours after the burns were
created. Wound care was given once a day, and the
burn wound was cleaned first with 0.9% NaCl, then
dried and covered using sterile gauze.
Figure 1: Induced wound burns on experiment Rats.
2.6 Macroscopic Wound Analysis
Burn wounds are treated until they heal, characterized
by scarring or scabbing and tightening and closing the
wound. Wound healing was assessed by observing for
26 consecutive days using 0.01 mm scale callipers
(see Figure 1). The area of a burn wound was
calculated by measuring the average wound diameter
from four positions and then putting it into the ¼.π.d²
formula for circle size.
Analytical statistics Mean ± S.D. was used to
express all values. After one-way ANOVA data
analysis, a post hoc test was performed. At p<0.05,
the results were deemed to be statistically different.
3 RESULTS
We have conducted a qualitative test of the extract in
the Medical Biochemistry laboratory of the Faculty of
Medicine, Universitas Lampung. This extract
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
224
contains various active substances, as shown in Table
1.
Table 1: Qualitative phytochemical screening of ethanolic
extracts of Rhizophora apiculata bark
Qualitative active
compound
Result
Saponin Positive
Steroid Ne
g
ative
Ter
p
enoids Positive
Tannins Positive
Alkaloids Negative
Flavonoid Positive
The size of burn wounds on day 4 in all groups of rats
was 3.14 cm2, still the same as the initial day of
treatment. There was no extensive reduction, and
inflammation in the form of redness was obtained.
The area of the burn wound on the 10th day appeared
to be reduced by 15-20% of the original size.
However, the mean area of burn wounds for each
group of rats was not significantly different. On day
15, treatment groups P2 and P3 had quite different
macroscopic healing compared to the control group
(p-value = 0.001). It shows that administering
Rhizophora apiculata extract has a good effect on
wound healing (see Table 2).
Table 2. Results of measuring the average area of burn
wounds per day.
Experiment
day
Average burn area (cm
2
)
KN K+ P1 P2 P3
4
th
3.14 3.14 3.14 3.14 3.14
10
th
2.7 2.8 2.9 2.7 2.4
15
th
1.4 1.3 1.4 0.8* 0.7*
21
th
0.17 0.12 0.64 0* 0*
(* significantly difference compared to control group)
Figure 2: the average area of burns on each day of the
experiment.
The reduction in the area of burn wounds occurred
more quickly in the group of mice given the extract.
Burns were no longer visible on day 18 in group P2
and day 19 in group P3. Meanwhile, burns were no
longer visible on day 21 in the control group (see
Figure 2).
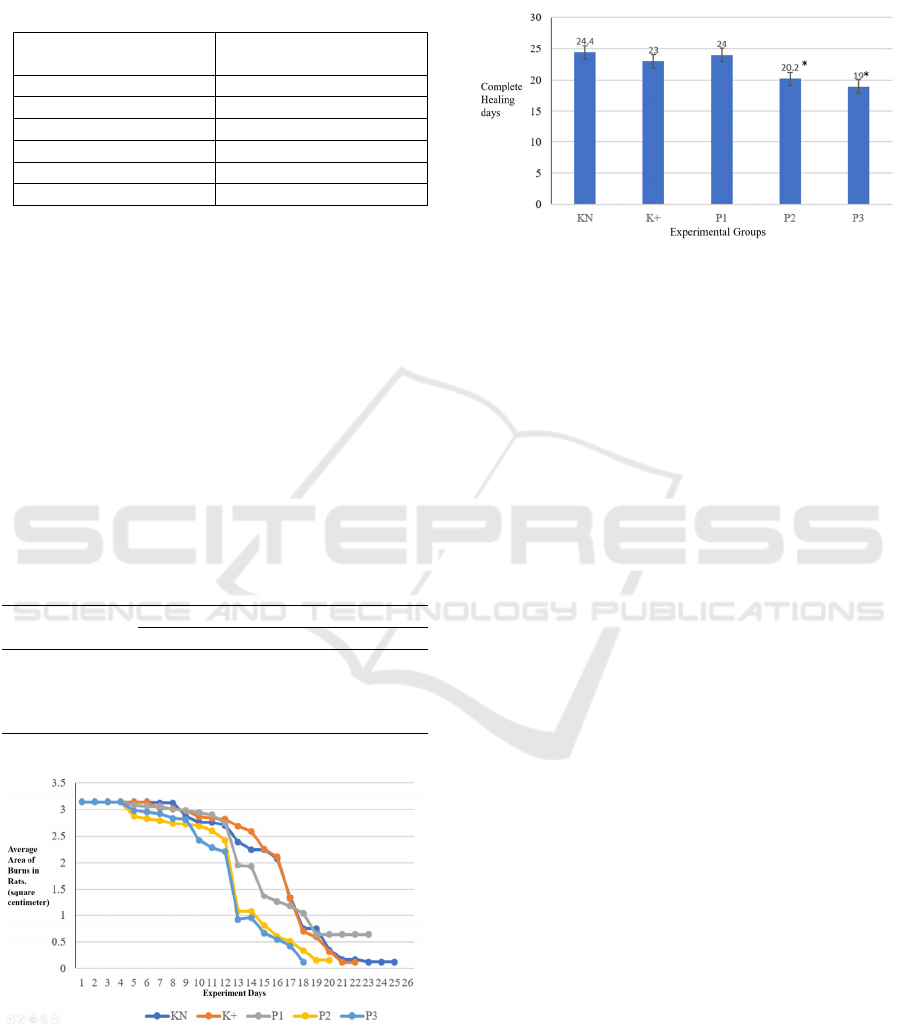
Figure 3: The averages complete burn wound healing time.
The results of macroscopic observations found that
the groups given the extract, namely P2 and P3,
experienced faster wound healing, namely on day 19
and day 20. Meanwhile, the control group only
experienced the healing of burn wounds on days 23
and 24 (see Figure 3).
4 DISCUSSIONS
The complicated process of burn healing causes
dermal and epidermal tissue degradation. It triggers a
few physiological reactions at the injury site, such as
an immediate inflammatory response followed by a
protracted, intense tissue creation phase.
Granulocytes or Polymorphonuclear leukocytes
leucocytes (PMNs) mediate this acute inflammation.
In this stage, the inflammatory site produces free
radicals that have the potential to harm tissues and
hinder the healing process of wounds (Żwierełło,
2023).
Mast cells and macrophages are immune cells
involved in the inflammatory phase. Numerous
growth factors and cytokines are secreted by these
two cells. Nerve Growth Factor (NGF) is a significant
cytokine that contributes to inflammatory responses.
Next, during the proliferation phase, NGF causes
endothelial cells to express Vascular Endothelial
Growth Factor (VEGF) and Fibroblast Growth Factor
(FGF), increasing keratinocyte proliferation and
angiogenesis (El Baassiri, 2023).
Therefore, tissue development, re-epithelization,
and epidermis differentiation can be hastened using
an anti-oxidant to minimize inflammation. Preventing
some of the frequently fatal infections in the wound
area is another critical step in healing. The
antibacterial action can promote wound healing and
The Effect of Rhizophora apiculata Bark Ethanol Extract on Burns Healing of Rattus norvegicus Sprague Dawley Strain
225

partially inhibit the growth of pathogenic microbes on
the skin. Thus, natural ingredients of antibacterial,
anti-inflammatory, and antioxidant properties can be
fundamental in the healing process of burns (Huang,
2023). The extract of Rhizophora apiculata prevented
rat hepatocyte and pancreatic cell necrosis caused by
cigarette smoke in an animal model assessment of its
anti-inflammatory action in rats (Mustofa, 2018).
Moreover, Rhizophora apiculata possesses a broad
antibacterial activity (Acharya, 2023). Thus, the
antibacterial, anti-inflammatory, and anti-
inflammatory properties of Rhizophora apiculata
may partially explain its favourable benefits in burn
healing acceleration and inflammation reduction
(Nisar, 2019). These effects could be attributed to
phytochemical components. Previous research and
our phytochemical analysis indicate that terpenoids,
flavonoids, tannins, and saponins are some of the
significant constituents of Rhizophora apiculata
(Chan, 2022).
One way to think of phenolic phytochemicals as
the main classes of secondary metabolites with
antimicrobial properties is flavonoids and tannins.
Through various mechanisms, including their
astringent, antibacterial, antioxidant, and angiogenic
effects, tannins can hasten the healing of wounds
(Ramya 2023). Terpenoids promote the breakdown of
stored extracellular proteins and prevent
prostaglandin formation, which can reduce tissue
oedema and inflammation. Because of their
antioxidant potential, they can avoid damage caused
by free radicals, just as flavonoids do through other
processes such as direct radical scavenging (Chan,
2022).
Because they regulate inflammation-related cells
and have an antioxidative action, flavonoids have
anti-inflammatory properties in vivo. Additionally,
reducing lipid peroxidation can improve collagen
strength and viability, boost vascularity and
circulation, and stop cell damage and necrosis. These
arguments suggest that aqueous extract's superior
antioxidant and anti-inflammatory properties may
contribute to its comparatively more significant effect
on burn healing, but they are not the only ones. The
results of the qualitative phytochemical screening
indicated that the ethanolic extract contained more
flavonoids. Thus, the higher flavonoid content of the
ethanolic section explains its more decisive
antioxidant action (Nisar, 2019). However, further
quantitative experiments are needed for a more
accurate assessment.
5 CONCLUSIONS
Rhizophora apiculata extracts significantly
accelerated the healing of burn wounds in rats. This
effect may result from several mechanisms, including
a faster rate of vascularization and re-
epithelialization, the inhibition of harmful free
radicals, a decrease in oedema and inflammation, and
the ability of this plant's antioxidant, anti-
inflammatory, and antimicrobial components to
control infection. More research utilizing purified
components is necessary to fully comprehend the
mechanism underlying the burn-healing ability of
Rhizophora apiculata.
ACKNOWLEDGEMENTS
This research was financially supported by HETI
Project Universitas Lampung.
REFERENCES
Acharya, S., Jali, P., Pradhan, M., Pradhan, C., &
Mohapatra, P. K., 2023. Antimicrobial and Antioxidant
Property of a True Mangrove Rhizophora apiculata Bl.
Chemistry & Biodiversity, 20(9), e202201144.
Bulan, D. E., Nurfadilah, N., Syahrir, M. R., Mismawati,
A., Torambung, A. K., & Rachmawati, M., 2022.
Phytochemical Composition and Antioxidant Activity
of Leaf Extracts from Three Rhizophora Species from
Bontang Waters, Indonesia. Tropical Journal of
Natural Product Research, 6(8).
Chan, E. W. C., Lim, W. Y., Wong, C. W., Ng, Y. K., 2022.
Some notable bioactivities of Rhizophora apiculata and
Sonneratia alba. ISME/GLOMIS Electron J, 20(4), 23-
6.
El Baassiri, M. G., Dosh, L., Haidar, H., Gerges, A.,
Baassiri, S., Leone, A., Jurjus, A., 2023. Nerve growth
factor and burn wound healing: Update of molecular
interactions with skin cells. Burns, 49(5), 989-1002.
Huang, W., Wang, Y., Tian, W., Cui, X., Tu, P., Li, J., ... &
Liu, X., 2022. Biosynthesis investigations of terpenoid,
alkaloid, and flavonoid antimicrobial agents derived
from medicinal plants. Antibiotics, 11(10), 1380.
Kurniawaty, E., Megaputri, S., Mustofa, S., Rahmanisa, S.,
Audah, K. A., & Andriani, S., 2022. Ethanol extract of
Bruguiera gymnorrhiza mangrove leaves and propolis
activity on macroscopic healing of cuts in vivo. Acta
Biochimica Indonesiana, 5(1), 94-94.
Nisar, A., 2019. Identification of Flavonoids from the
Leaves Extract of Mangrove (Rhizophora apiculata).
Recent Adv Biol Med, 5(2019), 9451.
Mustofa, S., Bahagia, W., Kurniawaty, E., Rahmanisa, S.,
& Audah, K. A., 2018. The effect of mangrove
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
226

(Rhizophora apiculata) bark extract ethanol on
histopathology pancreas of male white rats Sprague
Dawley strain exposed to cigarette smoke. Acta
Biochimica Indonesiana, 1(1), 7-13.
Mustofa, S., & Hanif, F., 2019. The protective effect of
Rhizophora apiculata bark extract against testicular
damage induced by cigarette smoke in male rats. Acta
biochimica indonesiana, 2(1), 23-31.
Mustofa, S., Ciptaningrum, I., Zuya, C. S., 2020. Subacute
toxicity test of Rhizophora apiculata bark extract on
liver and pancreas histopathology of rats. Acta
Biochimica Indonesiana, 3(2), 89-97.
Mustofa, S., Tarigan, C. Y., 2023. Efek Protektif Ekstrak
Kulit Batang Bakau Rhizophora apiculata terhadap
Kerusakan Histologi Paru Rattus norvegicus yang
Diinduksi Asap Rokok. Jurnal Kesehatan, 14(2), 241-
250.
Opriessnig, E., Luze, H., Smolle, C., Draschl, A., Zrim, R.,
Giretzlehner, M., ... & Nischwitz, S. P., 2023.
Epidemiology of burn injury and the ideal dressing in
global burn care–Regional differences explored. Burns,
49(1), 1-14.
Ramya, R., Kamoona, S., Hatta, F. A. M., Sulaiman, W. S.
H. W., Latiff, N. H. M., Othman, R., 2023. A Study on
an Active Functional Group and Antimicrobial
Properties From Rhizophora apiculata Extracts Used in
Traditional Malay as Medicine. Malaysian Applied
Biology, 52(4), 153-160.
Żwierełło, W., Piorun, K., Skórka-Majewicz, M.,
Maruszewska, A., Antoniewski, J., & Gutowska, I.,
2023. Burns: classification, pathophysiology, and
treatment: a review. International journal of molecular
sciences, 24(4), 3749.
The Effect of Rhizophora apiculata Bark Ethanol Extract on Burns Healing of Rattus norvegicus Sprague Dawley Strain
227
