
Physicochemical Analyses and Antibacterial Potential of Propolis by
Stingless Bee (Homotrigona apicalis) Found in East Kalimantan,
Indonesia
Supomo
1,2 a
, Enos Tangke Arung
1,* b
, Irawan Wijaya Kusuma
3c
and Dewi Sondari
2d
1
Faculty of Forestry, Mulawarman University, Indonesia
2
Department of Pharmacy, Sekolah Tinggi Ilmu Kesehatan Samarinda, Indonesia
3
Research Center for Biomass and Bioproducts, National Research and Innovation Agency, Bogor, Indonesia
Keywords: Propionibacterium acnes, Staphylococcus epidermidis, Propolis, Homotrigona apicalis, Antibacterial
Activity.
Abstract: This study examined the antibacterial potential of Homotrigona apicalis propolis extract against
Staphylococcus epidermidis and Propionibacterium acnes, which are bacteria found on human skin that can
cause opportunistic infections. The extract was obtained through the collection, processing, extraction, and
fractionation of fresh herb samples. The antibacterial activity of the methanol extract, n-hexane fraction,
ethylacetate fraction and residual fraction, at concentrations of 2.5%, 5%, and 10% was evaluated using the
disc diffusion method, with clindamycin 0,1% and DMSO 1% as positive and negative controls respectively.
Statistical analysis was performed using ANOVA. The results revealed that both the methanol extracts and
the ethyl acetate fraction of Homotrigona apicalis propolis showed significant inhibitory effects on bacterial
growth. The greatest antibacterial activity was observed at the concentration ethyl acetate fraction of 10%,
with inhibition zones of 13.27 mm for Staphylococcus epidermidis and 12.86 mm for Propionibacterium
acnes. Importantly, Staphylococcus epidermidis exhibited higher susceptibility to the propolis extract and
fraction compared to Propionibacterium acnes. These findings suggest that Homotrigona apicalis propolis
extract has the potential to be used as an active ingredient in cosmetics targeting acne-causing bacteria,
specifically Staphylococcus epidermidis and Propionibacterium acnes. The extract demonstrated efficacy
against these pathogens suggests its potential as an alternative to conventional antibiotics.
1 INTRODUCTION
Staphylococcus epidermidis and Propionibacterium
acnes are known as commensal bacteria in human
skin which can change into opportunistic (Nakase et
al. 2014; Chessa et al. 2015). Staphylococcus
epidermidis covered various parts of the skin, while
P. acnes resides mainly at pilosebaceous skin
follicles. This microbial interplay mediated through
molecules involved in intercellular competition or
communication, may have an impact on a fine
balanced skin ecosystem. Disturbed balance or
dysbiosis significantly impacts skin health and might
initiate or contribute to events that lead to skin
a
https://orcid.org/0000-0002-3707-9635
b
https://orcid.org/0000-0002-1979-6892
c
https://orcid.org/0000-0002-0177-6615
d
https://orcid.org/0000-0002-4923-2027
disorders. One such disorder is acne vulgaris, a
multifactorial disease of pilosebaceous units of the
skin that affects adolescents (Christensen et al. 2016).
Propionibacterium acnes related significantly to
the initial stage of acne causes increasing lipogenesis
originating in sebaceous glands. It induces
inflammation and pustules on the skin (Neves et al.
2015; Blaskovich et al. 2019). Meanwhile, S.
epidermidis could act as opportunistic when it enters
the bloodstream (Nakase et al. 2014; Tabri 2019).
Skin clinic acne treatment usually uses antibiotics that
could overcome inflammation and kill such bacteria
as tetracycline, erythromycin, doxycycline, and
clindamycin (Nakatsuji et al. 2009; Doǧ an et al.
Supomo, , Arung, E. T., Kusuma, I. W. and Sondari, D.
Physicochemical Analyses and Antibacterial Potential of Propolis by Stingless Bee (Homotrigona apicalis) Found in East Kalimantan, Indonesia.
DOI: 10.5220/0013667100003873
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Medical Science and Health (ICOMESH 2023), pages 143-150
ISBN: 978-989-758-740-5
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
143

2017). However, these drugs' side effects are
identified as irritation and allergy and long-term
consumption of antibiotics causes resistance, organ
damage, and immune hypersensitivity (Adawiyah et
al. 2010; Tan et al. 2018; Dikicier 2019). The entire
side effect leads researchers to discover and develop
new sources for antimicrobial agents of natural
products, e.g. medicinal plants (Abdallah 2011).
Sadeek & Abdallah (2019), some phytochemical
compounds extracted from medicinal plants showed
effective antibacterial potential against multi-drug-
resistant pathogens and these compounds could be
exploited as antibacterial drugs.
Antibiotic resistance is the changing sensitivity of
microorganisms due to antibiotics, therefore, higher
concentrations are needed to inhibit the growth of
resistant bacteria compared to susceptible strains
(Galderm, 2014). A significant factor that contributes
to increasing resistance is the irrational consumption
of antibiotics and antibiotics (Walshet al, 2016).
Related to this fact other alternatives are sought to
treat infections using natural ingredients (Utamiet al,
2021). The natural ingredient mostly found as an
antibacterial is Propolis from the stingless bee
Homotrigona apicalis.
The bee Homotrigona apicalis is a species of
stingless honey-producing bee part of the
Meliponidae family and it has no sting and is small in
size (Francoy et al., 2019). These insects make nests
in tree holes, wall cracks, and bamboo cavities, a
source of food for bees in the form of pollen, nectar,
and resin, naturally. Bees are herbivorous animals
(Achyani and Wicandra, 2019) and Bee hive
homotrigone useful benefit for the health of the
human body and can produce honey, royal jelly, bee
pollen, and propolis (Syafrizalet al, 2016). This
natural bee product is known to have antibacterial,
antifungal, anticancer, anti-inflammatory, and anti-
asthmatic benefits (Campos et al., 2015; Lopez et al.,
2019; Farias et al., 2014).
It is described that propolis is one of the
substances produced by bees and consists of a
mixture of bee saliva and plant exudates that they
collect (Mardiah, 2017). Propolis is trusted as a
natural ingredient, empirically and relatively safe
completed plenty of benefits (Lutpiatina, 2015). The
common benefits of propolis are as a medicine or
supplement, mouthwash, anti-inflammatory, disease
therapy, and accelerating wound healing. Based on
Rosyidi et al (2018), it has plenty of chemical
compounds and varies depending on the environment
surrounding the bee farm, therefore, plenty of
differences in compounds in propolis in Indonesia are
found. Propolis consists of amino acids, terpenoids,
and polyphenols (phenolic acids, esters, and
flavonoids), in general (Pujirahayu et al, 2014) and
Flavonoids are one of the significantly important
ingredients in propolis which have antioxidant,
anticancer, anti-inflammatory, anti-allergic, antiviral
and antibacterial effects (Rismawati et al, 2017;
Alexandra, 2018; Hermalinda, 2019).
Lutpiatina (2015) found that propolis has
antibacterial properties, the inhibitory zone of the
ethanol extract of kelutut bee propolis (Trigona sp)
originating from the South Kalimantan area at
concentrations of 20%, 40%, 60%, 80% and 100%
against Staphylococcus aureus is 6.4 mm; 10mm;
12.6 mm; 14.4mm; 16.4mm, meanwhile,
Gusmawarni et al, (2021) concluded that the ethanol
extract of bee propolis (Trigona sp) originating from
Pekanbaru area has inhibitory zone activity against
bacterial growth Streptococcus mutans at
concentration of 40%, 60% and 80% respectively 8
mm, 9.3 mm and 11 mm. Methanol extract of propolis
shows the highest inhibitory rate at concentrations of
750 μg/mL (6 mm) and 1000 μg/mL (10 mm) against
E. Coli and Staphylococcus aureus were compared by
hexane and ethyl acetate extracts (Yusop et al, 2018).
Empirical research by Mayangsari (2013) found that
Lawang propolis extract against Fusobacterium
nucleatum obtained MIC (Minimum Inhibitory
Concentration) of 1.48% and MBC (Minimum
Bactericidal Concentration) of 1.54%.
Based on the significant benefits of propolis and
easy bee farm Homotrigona apicalis, this study
implementing bee propolis Homotrigona apicalis by
breeders at Palaran District, City of Samarinda, East
Kalimantan to identify antibacterial characteristics of
propolis methanol extract through bacteria
Propionibacterium acnes and Staphylococcus
epidermidis.
2 METHOD
2.1 Instruments and Materials
This study implemented glassware (pyrex), blender,
vortex, hotplate (Ceran®), petri dishes, spirit lamps,
autoclaves, incubators, magnetic ironer,
micropipette, analytical balance, caliper, water bath,
laminator airflow cabinet (LAF), maserator, rotary
evaporator, spectrophotometer, knife, cutting board.
Meanwhile, materials contributes for this research are
methanol, n-hexane, ethyl-acetate, bee propolis
Homotrigona apicalis, filter paper, sterile cotton,
distilled water, Muller Hinton Agar (MHA) media,
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
144

Nutrient Agar media, clindamycin 0.1%, DMSO,
NaCl 0.9%.
2.2 Procedure
2.2.1 Simplicia Setup
Propolis is taken from Tani Harapan Loa Janan, Kutai
Kartanegara, East Kalimantan, which is then wet
sorted and targeted to separate propolis from the nest
and impurities.
2.2.2 Proceed Extracts
The simplicia obtained was macerated for three days
using methanol. Remaceration is carried out on the
simplicial dregs and it is filtered to obtain macerate,
then evaporated using a rotary evaporator and
evaporated over a water bath until a thick extract is
obtained (14).
2.2.3 Fractionation
Weighed 5 grams of extract and partitioned using
distilled water and n-hexane with a ratio of 1:1 v/v.
The sample was shaken repeatedly in a separating
funnel until homogeneous and left until a layer of
water and a layer of n-hexane were formed. The n-
hexane layer was collected in a different container.
The n-hexane layer was then heated over a water bath
until thick and the n-hexane fraction was obtained.
The water layer was partitioned over using an
ethylacetate completed ratio of 1:1 v/v. the following
phase is shaken in a separating funnel until
homogeneous, left to stand until two layers are
formed, namely the water layer and the ethyl acetate
layer. Each layer is collected into a different
container, heated until thick and the ethyl acetate
fraction and residual fraction are obtained
(Mujipradana, 2018)
2.2.4 Phytochemical Screening
Alkaloid
0.5 g sample was added to 1 mL of 2 N hydrochloric
acid and 9 mL of distilled water heated over a water
bath for two minutes and finally cooled and filtered.
The filtrate obtained was used for alkaloid testing.
Take 3 test tubes, add 0.5 mL of filtrate to each tube.
Add 2 drops of reagent of each test tubemayer,
bouchardat and dragendorf. Alkaloid is positive if
precipitate occurs and at least 2 of the 3 reagents
above are positive then sample is defined to contain
alkaloids, namely the formation of white precipitate
at reagent mayer, brown at reagent Bouchardat and
brick red deposits on the fixer dragendorf
(Handayaniet al, 2019; Nafisah et al, 2014).
Saponin
0.5 g sample is put into a test tube then 10 mL of hot
water is added, cooled briefly after cooling, and
shaken vigorously for 15 minutes, if a stable foam
form for 10 minutes and the foam is 1-10 cm high and
when dripped 1 drop 2 N hydrochloric acid foam is
still present, then the sample contains saponin
compounds (Supomo, et al, 2019).
Flavonoid
1 gram of sample is added to 10 ml of hot water then
boiled for 5 minutes, filtered while it is still hot. 5 mL
of the filtrate obtained was taken then 0.1 g of
magnesium powder, 1 mL of HCl, and 2 mL of amyl
alcohol were added, then shaken and allowed to
separate. Samples contain flavonoids if there is a red
color change at the amyl alcohol layer (Supomo et al,
2019).
Tannin
1 mL sample solution is reacted with 10% iron (III)
chloride solution, if dark blue, blackish blue or
greenish black color occurs, it indicates the presence
of tannins (Supomo et al, 2019).
Phenol
Several dissolved samples were extracted by 20 mL
of 70% ethanol. 1 mL of the resulting solution was
taken and then 2 drops of 5% FeCl
3
were added. A
positive reaction is indicated by the presence of a
green or blue-green color (Anisa et al, 2022).
Quinones
5 mL sample of the experimental solution obtained
from the identification of flavonoids was put into a
test tube, and a few drops of 1 N NaOH solution were
added. The formation of a red color indicates the
presence of quinine group compounds (Lestari and
Andriani, 2021).
2.2.5 Antibacterial Testing
Making positive and negative controls
This study used Clindamycin as a positive control of
0.1% and DMSO as a negative control of 1%.
Preparation of extract and fraction concentration
series
This study used a series concentration of methanol
extract, n-hexane fraction, ethyl acetate fraction and
residual fraction of propolis, namely 2.5%, 5%, and
10% completed calculations (w/v), using 1% DMSO
as a solvent.
Physicochemical Analyses and Antibacterial Potential of Propolis by Stingless Bee (Homotrigona apicalis) Found in East Kalimantan,
Indonesia
145

Sterilization of tools
The first phase is using sterilized glassware for
antimicrobial activity research at an autoclave at 121
ºC for 15 minutes, the tweezers were burned by
burning over a direct flame (Mujipradhana et al,
2018).
Slanted agar media preparation
Weighed 5 g of Nutrient Agar (NA), dissolved in 250
mL of distilled water (20 g/1,000 mL). The
homogenized media was then sterilized in an
autoclave at 121˚C for 15 minutes. Pour 5 ml of NA
media into a test tube, let it sit Nutrient Agar at room
temperature till preparation solidifies at a 45-degree
tilt position for 10 minutes.
Bacterial rejuvenation
Bacteria Propionibacterium acnes and
Staphylococcus epidermidis swabbed into slanted
agar media, and incubated in an incubator at 37ºC for
2x24 hours.
Preparation of Mac. Farland solution
Solution H
2
SO
4
1% as much as 0.25 ml is mixed
completed using BaCl
2
solution
2
1% as much as 0.1
gram in an Erlenmeyer. Followed by shaking until a
cloudy solution is formed, then the turbidity standard
is measured with a spectrophotometer.
Preparation of bacterial suspensions
The test bacterial were taken ± 1 cycle then suspended
in a tube containing 10 ml of 0.9% NaCl solution. The
turbidity of the solution was then measured using a
spectrophotometer uv-vis with absorbance that has
been determined with standard solutions Mac.
Farland at a wavelength of 600 nm
(Mujipradana,2018).
Antibacterial Activity Test of methanol extract, n-
hexane fraction, ethyl acetate fraction and residual
fraction of propolis
The paper discs were soaked in methanol extract, n-
hexane fraction, ethyl acetate fraction and residual
fraction of propolis with concentrations 2,5%, 5%,
and 10%, clindamycin 0.1% as positive control, and
DMSO 1% as negative control for 3 minutes. Prepare
72 petri dishes, then pour in 20 mL of MHA media,
then let it solidify. Dip a sterile cotton swab into each
Propionibacterium acnes and Staphylococcus
epidermidis suspension, while it is absorbed, lift the
stick and squeeze it by pressing against the wall of the
tube. One hundred microliter bacterial suspension
was taken using a micropipette and placed in a petri
dish containing MHA media, then smeared using a
sterile cotton swab until the entire surface of the agar
media was tightly covered and left for 5-15 minutes,
therefore, bacterial suspension seeped into the agar
medium. The soaked paper disc is placed on the
surface of the MHA media which has been inoculated
with bacteria. The petri dishes were left at room
temperature for an hour before being incubated at
37°C for 24 hours and this test was repeated three
times (Pratiwi, et al., 2016). Antibacterial activity
showed by Inhibition Zone Diameter (mm).
MIC (Minimum Inhibitory Concentration) and
MBC (Minimum Bactericidal Concentration)
While observing the disc paper test, we could see an
inhibition zone that forms around the disc paper and
after obtaining the inhibition zone, the concentration
range of the inhibition zone is used to determine the
MIC and MBC using the solid dilution method.
Variations in solid dilution concentration were made
based on the smallest concentration which still
provided an inhibition zone for the antibacterial
potential test. Test bacterial suspensions and extracts
that have been dissolved according to varying
concentrations are inoculated for flat in MHA media
completed ratio of bacterial suspension, such extract
(1:1) incubated at 37°C for 24 hours. Meanwhile, the
clearest media is test media completed by the smallest
concentration accompanied by no bacterial growth
designated as MIC. The results determined as KHM
are confirmed by doing the results streak on MHA
media and incubated at 37°C for 24 hours. On the
results streak observed based on the turbidity of
bacterial growth at media and when the media still
looks clear then the results are designated as MBC
(Murtiwi, 2014).
3 RESULT AND DISCUSSION
The nest criteria of propolis use at this study which
having brittle texture and dark color, taken from Desa
Tani Harapan Loa Janan, Kutai Kartanegara, East
Kalimantan. Propolis is extracted using methanol.
The secondary metabolite groups testing were aimed
to determine the presence of secondary metabolites in
natural material samples. The results of
phytochemical screening tests on total methanol
extract of propolis can be seen in Table 1.
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
146
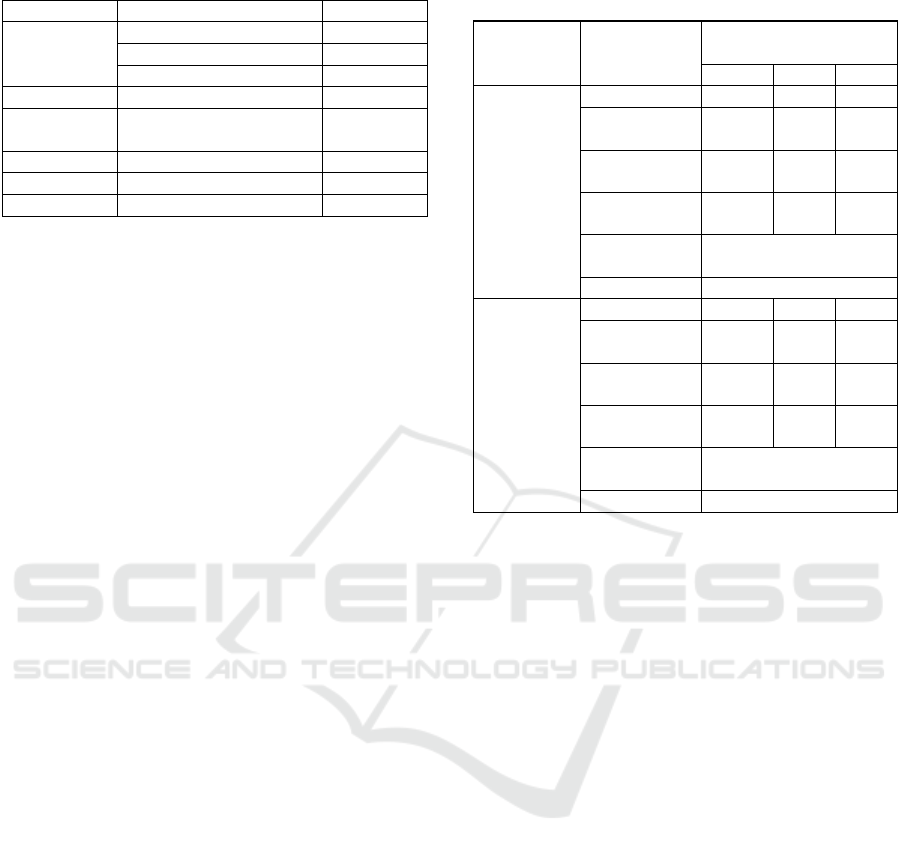
Table 1: Phytochemical Screening Test Result.
Com
p
ounds Test Result
Alkaloid
Ma
y
e
r
(
+
)
Bouchardat
(
+
)
Dragendorf (-)
Saponins HCl 2N (+)
Flavonoid
HCl + Mg + Amyl
Alcohol
(+)
Tannin FeCl
3
(+)
Fenol FeCl
3
5% (+)
Quinone NaOH 1 N (+)
Notes:
(-) = Negative result
(+) = Positive result
Groups of compounds which are suspected of
being potential antioxidants in the ethanol extract of
propolis are including flavonoids. Alkaloid, saponin.
Tannin, fenol and quinones. Flavonoid compounds in
their structure contain hydroxyl groups that can
donate hydrogen atoms to free radicals, so flavonoid
compounds have the potential as antioxidants.
Flavonoids are reducing compounds that can inhibit
many oxidation reactions. Moreover, flavonoids have
the ability as antioxidants since they can transfer an
electron to free radical compounds as well as
quinones (Ridho, 2013).
The method is based on disc diffusion for
antibacterial activity. Paper discs containing the
antimicrobial agent methanol extract of propolis were
placed on MHA media which had previously been
planted by bacteria Propionibacterium acnes and
Staphylococcus epidermidis, paper discs that have
been soaked in antimicrobial agents from methanol
extract of propolis will diffuse on MHA agar media.
The antibacterial test results on Propionibacterium
acnes and Staphylococcus epidermidis completed
various concentrations, the inhibition zone is obtained
shown at table 2.
Identified at antibacterial test, the enterely
variations in concentration showed that the propolis
extract and fraction had a very active inhibitory
response to bacterial growth Propionibacterium
acnes and Staphylococcus epidermidis. Based on
measurements of the result bacterial inhibition zone,
it shows that the bacteria Staphylococcus epidermidis
which is a type of gram-positive bacteria has a larger
inhibition zone than bacteria Propionibacterium
acnes.
Based on Table 2, seems that the concentration of
propolis methanol extract has the largest inhibitory
zone diameter against the bacteria Staphylococcus
epidermidis namely a concentration of 10% which
has an average inhibitory diameter of 0 mm and the
smallest concentration at 2.5% is 9.63 mm.
Table 2: Antibacterial Activity Test Results Average Zone
of Inhibition (mm)
Bacteria Treatment
Average Inhibition
Zone Diameter (mm)
2,5% 5% 10%
P. acnes
Extract 9,34 9,67 10,17
n-Hexane
Fraction
11,83 12 12,67
Ethylacetate
Fraction
11,87 12,26 12,86
Residual
Fraction
7,03 8 9,16
Clindamycin
0.1%
22,27
DMS0 1% 0
S.
epidermidis
Extract 9,63 9,86 10
n-Hexane
Fraction
11 11,67 13,17
Ethylacetate
Fraction
11 11,85 13,27
Residual
Fraction
8,35 9,21 9,78
Clindamycin
0.1%
22,27
DMSO 1% 0
Table 2 shows the inhibitory power of propolis
extracts and fractions on bacterial growth of
Propionibacterium acnes and Staphylococcus
epidermidis has a different level of sensitivity.
Research finding by Saputera, et al (2016), the higher
the concentration of the extract used, the larger the
inhibition zone produced and it related significantly
through research results of each concentration of
2.5% < 5% < 10% completed diameter response of
the inhibition zone being successively larger.
That empirical fact shows that the methanol
extract and fractions of propolis have antibacterial
activity against acne-causing bacteria. It related
significantly to previous research by Abdullah et al,
that propolis from bees (Heterotrigona itama) from
Brunei Darussalam has antibacterial activity from 20
gram/L propolis extract against bacteria
Pseudomonas aeruginosa dan Staphylococcus
aureus. The antibacterial activity is thought to be due
to the presence of chemical compounds at the
methanol extract of propolis, according to Hotnida et
al (2011), the chemical content contained in propolis
could differ between regions, places where propolis
is honeycombed and its biological activity. It
described the flora ecosystem surrounding which
influences the chemical content of propolis.
Based on the results of phytochemical screening,
the methanol extract of propolis contains alkaloids,
saponins, tannins, flavonoids, phenols, and quinones.
Physicochemical Analyses and Antibacterial Potential of Propolis by Stingless Bee (Homotrigona apicalis) Found in East Kalimantan,
Indonesia
147

Alkaloids have an antibacterial mechanism by
inhibiting the peptidoglycan components in cells so
that the cell walls are not intact and cause cell death
(Riyanto et al, 2019). The mechanism of flavonoids
as antibacterials is by forming complex compounds
with extracellular and dissolved proteins, causing
phospholipids to be unable to maintain the shape of
the bacterial cell membrane, as a result, the bacterial
cell membrane will leak and the growth of the
bacteria will be hampered until death (Malanggi et al,
2012). Based on find research of Hotnida et al,
(2011), types of flavonoids found in propolis are
pinocembrin, respectable, quercetin, pinostrobin,
kaempferol, pinobaxin.
The mechanism of tannin as an antibacterial is to
disrupt peptidoglycan could be proved that cell wall
formation becomes imperfect and causes bacterial
cells to lyse, while the mechanism of action of
saponin is to reduce the surface tension of the
bacterial cell wall, resulting in
increased permeability or cell leakage causing
intracellular compounds to come out (Malanggi et al,
2012). The mechanism of action of high
concentrations of phenol as an antibacterial is by
penetrating and disrupting bacterial cell walls and
precipitating proteins in bacterial cells. Phenol in
lower concentrations inactivates important enzyme
systems in bacterial cells (Purwatiningsih et al 2014).
The mechanism of action of quinones as
antibacterials is by forming complex compounds that
have properties irreversible completed by
nucleophilic amino acid residues on plasma
transmembrane proteins, cell wall polypeptides, and
enzymes found on the surface of cell membranes,
thereby disrupting the life of bacterial cells (Sapara et
al, 2016).
The SPSS statistical tests show the data tested
implemented test Kolmogorov Smirnov normally
distributed at normality test, namely completed
significance value (p>0.05). meanwhile, the
normality test on the data Pseudomonas aeruginosa
shows a significance value of 0.810 while in
Staphylococcus aureus The significance value is
0.895. The normality test with normally distributed
data is a requirement for carrying out further tests on
One Way ANOVA.
Meanwhile, homogeneity of variance test shows
the data is homogeneously related to each
significance value of Pseudomonas aeruginosa data
(p>0.005) p = 0.054, and the significance value of
Staphylococcus aureus data shows p= 0.081.
Homogeneity testing completed homogeneous data is
requirements for carrying out the One Way Anova test
and while One Way Anova at test results shows
Pseudomonas aeruginosa and Staphylococcus aerus
of each research data indicated each significance
value (p<0.05), namely p= 0.000 and it concluded
there are no similarities in each treatment group or
each concentration. This finding related significantly
to the hypothesis which described that there are
differences in the effectiveness of the antibacterial
power of the methanol extract of propolis of each
concentration on bacterial growth Pseudomonas
aeruginosa and Staphylococcus aureus.
The following test carried out after this LSD
which shows the antibacterial activity of propolis
against bacteria Pseudomonas aeruginosa, shows
significant differences at the entire concentrations
and there was a significant difference between the
antibacterial activity of propolis extract and the
positive control clindamycin. Meanwhile, bacteria
Staphylococcus aureus there was no significant
difference in the inhibition zone between
concentrations of 2.5%, 5%, and 10%. The zone of
inhibition of all propolis extract concentrations was
significantly different from the positive control
clindamycin.
MIC (Minimum Inhibitory Concentration) and
MBC (Minimum Bactericidal Concentration)
Determination of MIC (Minimum Inhibitory
Concentration) and MBC (Minimum Bactericidal
Concentration) used solid dillution method. This
main advantage method is more practical because one
concentration of the test antimicrobial agent can be
used to test more than one test microbe (Supomo et
al, 2021). The MIC and MBC testing targeted to
determine the number of doses of propolis methanol
extract that could inhibit and kill bacteria that cause
infection.
Based on finding research Nadhilla (2014),
antibacterial substances are divided into two, namely
bacteriostatic and bactericidal. Antibacterials that
have bacteriostatic activity are substances that could
inhibit the growth of bacteria, while bactericides are
substances that can kill bacteria. Variations in the test
concentrations of propolis extract and methanol
fraction were 2.5%, 5%, and 10%.
Table 3: Bacterial Growth After 1x24 hours.
Concentration
(%)
Bacteria
P. acnes S. epidermidis
2,5 --
5 - -
10 - -
Note : (-)=No bacterial growth
(+)=There is bacterial growth
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
148

Results of the first incubation show that there was
no bacterial growth at all concentrations and indicated
that MIC is the smallest concentration that could
inhibit bacterial growth, thus the smallest
concentration is 2.5% without bacterial growth
Propionibacterium acnes and Staphylococcus
epidermidis. The empirical finding shows the MIC of
methanol extract of propolis for bacteria
Propionibacterium acnes and Staphylococcus
epidermidis (w/v) is 25 mg/mL. Next, a confirmation
test was carried out by streaking again on the media
with the bacterial suspension Propionibacterium
acnes and Staphylococcus epidermidis then incubated
for 1 x 24 hours at 37°C in a row.
Table 4: Bacterial growth after re-streaking.
Concentration
(%)
Bacteria
P. acnes S. epidermidis
2,5 - -
5 - -
10 - -
Note: (-) = No bacterial growth
(+) = There is bacterial growth
Table 4 shows that after re-streaking the media
remains clear or there is no bacterial growth at all
variations in concentration, then MBC is the
minimum concentration that could kill bacteria at a
concentration of 2.5%.
4 CONCLUSIONS
Research indicated that bee propolis extracts and
fractions of Homotrigona apicalis can inhibit
bacterial growth. Methanol extract of propolis has
inhibitory power against bacteria Propionibacterium
acnes and Staphylococcus epidermidis at
concentrations of 2.5%, 5%, and 10%. MIC and MBC
of propolis methanol extract on bacteria
Propionibacterium acnes and Staphylococcus
epidermidis of 25 mg/mL. Propolis extract has the
potential to be an active ingredient in cosmetics as an
antibacterial Propionibacterium acnes and
Staphylococcus epidermidis cause of acne.
ACKNOWLEDGEMENT
Special thanks to Mr. Prof. Enos Tangke Arung, MP,
Ph.D., as the Promoter who has provided a lot of
direction, guidance, motivational contribution, and
very valuable suggestions. Mr. Prof. Irawan Wijaya
Kesuma, MP, Ph.D. and Mrs. Dr. Dewi Sondari, M.Si
as the promoter who guided and directed the author.
Lecturers and staff of the Forestry Science Doctoral
Study Program, Faculty of Forestry, Mulawarman
University.
REFERENCES
Achyani., Wicandra, D. 2019. Practical Tips for Cultivating
Trigona Bees (Heterotrigona itama). Laduny Alifatama:
Lampung.
Alexandra, G. M., Alina, M. H. 2018. Therapeutic Probiotic
Unconventional Food. Elsevier. 2(1). 141-150.
Anisa, N., Najib, S. Z. 2022. Phytochemical Screening and
Determination of Total Concentrations of Flavonoid
Phenols and Tannins in Kersen Leaves (Muntingia
calabura L.). Indonesian Journal Pharmaceutical and
Herbal Medicine. 2(1). 96-103.
Blaskovich, M. A. T. et al., 2019. In vitro Antimicrobial
Activity of Acne Drugs Against Skin-Associated
Bacteria. Scientific Reports, 9(1). doi: 10.1038/s41598-
019-50746-4.
Campos, J. F., dos Santos, U. P., Da, R. P. S., and others.
2015. Antimicrobial, Antioxidant, Antiinflammatory,
and Cytotoxic Activities of Propolis from The Stingless
Bee Tetragonisca fiebrigi (jataí). Evidence-Based
Complementary and Alternative Medicine (15). 1-11.
Chessa, D., Ganau, G., & Mazzarello, V., 2015. An
overview of Staphylococcus epidermidis and
Staphylococcus aureus with a focus on developing
countries. Journal of Infection in Developing Countries,
9(6), pp. 547–550, doi: 10.3855/jidc.6923.
Christensen, G. J. M. et al., 2016. Antagonism between
Staphylococcus epidermidis and Propionibacterium
acnes and its genomic basis. BMC Genomics. BioMed
Central Ltd., 17(1). doi: 10.1186/s12864-016-2489-5.
De Farias, J. H. C., Reis, A. S., Arajo, M. A. R. 2014. Effects
of Stingless Bee Propolis on Experimental Asthma.
Evidence Based Complementary and Alternative Medi-
cine. (14). 1-8.
E. A. Ridho. 2013. “Uji Aktivitas Antioksidan Ekstrak
Metanol Buah Lakum (Cayratiatrifolia) dengan Metode
DPPH (2,2-Difenil-1-Pikrilhidrazil)”. Thesis.
Pontianak: Universitas Tanjungpura.
Francoy, T. M., Silva, R. A. O., Nunes, S. P. 2019. Gen-der
Identification of Five Genera of Stingless Bees (api-dae,
meliponini) Based on Wing morphology Genet.
Molecular Research. 8(1). 207-214.
Galderma. 2014. Media Center Antibiotic Resistance and
Acne Treatment Tackling the Challenge. Galderma
Media Journal Center. 1-5.
Gusmawarni, V., Wardaniati, I. 2021. Antibacterial Activity
Test of Propolis Ethanol Extract AgainstStreptococcus
Mutans, Journal Pharmacy Higea. 3(2). 115-123.
Handayani, F., Apriliana, A., Natalia, H. 2019.
Characterization and Phytochemical Screening of
Selutui Puka Leaf Simplicia (Tabernaemontana
Physicochemical Analyses and Antibacterial Potential of Propolis by Stingless Bee (Homotrigona apicalis) Found in East Kalimantan,
Indonesia
149

Macracarpa Jack). Ibn Sina Scientific Journal. 4(1). 49-
58.
Hermalinda, R., Irham, T., Zairin, N. H. 2019. Total
Flavonoid Contenet Analysis of Ramania Leaves
Extract Using Ethanol, Methanol and N Hexane as
Solvent. Journal Tooth. 4(1). 60-63.
Hotnida, C. H., Siregar., Asnath, M., Fuah and Yoke, O.
2011. Propolis, Honey and Multi-Efficacy. Self-help:
Jakarta.
Lestari, F., Andriani, S. 2020. Phytochemical Plants
Efficacious in Traditional Medicine in South
Kalimantan and Central Kalimantan.Galam Journal.
2(1). 79-92.
Lopes, A. J. O., Vasconcelos, C. C., Pereira, F. A. N., Silva,
R. H. M. 2019. Anti Inflammatory and Activity and
Antinociceptive Activity of Pollen Extract Collected by
Stingless Bee Melipona fasciculata. International
Journal. Moleculer Science. 20(4). 512.
Lutpiatina, L. 2015. Effectiveness of Kelutut Bee Propolis
Extract (Trigona spp) in Inhibiting GrowthSalmonella
typhi, Staphylococcus aureus andCandida albicans.
Journal of Health Scales. 6(1). 6-13.
Malanggi, L. P., Meiske, S. S., Jessy, J. E. P. 2012.
Determination of tannin content and antioxidant activity
test of avocado seed extract (Persea americanaMill).
UNSRAT MIPA Journal. 1, 5-10.
Mardiah. 2017. Resistance TestStaphylococcus aureus
Against Antibiotics, Amoxillin, Tetracyclin and
Propolis.Journal of Natural and Environmental
Sciences. (8) 16. 1-6.
Mayangsari, A. 2013. Minimum Inhibitory Concentration
(MIC) and Minimum Kill Concentration (MBC) of
Lawang Propolis Extract Against Fusobacterium
nucleatum. Thesis. Faculty of Dentistry, Airlangga
University. Surabaya.
Murtiwi, M. T. 2014. Antibacterial Activity of Leaf Ethanol
Extract (Macaranga tanarius(L.) Mull. Arg.) Against
(Streptococcus pyogenes) ATCC 19615. Thesis. Sanata
Dharma University, Yogyakarta.
Mujipradana, 2018. Antibacterial Activity of Ascidian
Herdmania momus Extract on Human Pathogenic
Microbes, Pharmaceutical Scientific Journal, 7 (3),
2302-2493.
Nadhilla, N. F. 2014. The Activity of Antibacterial Agent of
Honey Against Staphylococcus aureus. Journal
Majority. 3(7). 94-101.
Nakase, K., Nakaminami, H., Takenaka, Y., Hayashi, N.,
Kawashima,M., & Noguchi, N., 2014. Relationship
between the severity of acne vulgaris and antimicrobial
resistance of bacteria isolated from acne lesions in a
hospital in Japan. Journal of Medical Microbiology,
63(Part 5), pp. 721–728, doi: 10.1099/jmm.0.067611-0.
Nafisah, M., Tukiran., Suyatno., Nurul, H. 2014
Phytochemical Screening Test on Hexane, Chloroform,
and Methanol Extracts from Patikan Kebo Plants
(Euphorbia hirta). Proceedings of the National Seminar
on Chemistry, State University of Surabaya. (2). 279-
286.
Nazri., Mohd, N. A. A., Ahmat, N., Adnan, A., Mohamad
Syed, S.A., & Ruzaina Syaripah, S. A. 2011. In Vitro
Antibacterial and Radical Scavenging Activities of
Malaysian Table Salad.African Journal of
Biotechnology. 10(30).
Neves, J.R., Francesconi, F., Costa, A. Ribeiro, B.M.,
Follador, I., & Almeida, L.M.C., 2015.
Propionibacterium acnes and bacterial resistance. Surg
Cosmet Dermatol, 7(3 Suppl 1): S27-38
Pratiwi, L., Kusharyanti, I. 2016. Water Henna Gel
Formulation (Irresistible balsamLim.)
AgainstPropionibacterium acnes andStaphylococcus
epidermidis. Pharmaceutical Sciences and Research.
1(1). 30-45.
Pujirahayu., Niken., Ritonga. 2014. Properties And
Flavonoids Content in Propolis of Some Extraction
Method of Raw Propolis. International Journal of
Pharmacy and Pharmaceutical Sciences. 6(6). 338-340.
Purwatiningsih, T. I., Yustina, Y. S., and Widodo. 2014.
Activity of Phenolic Compounds in Noni Fruit (Morinda
citrifolia) As a natural antibacterial for bacteria that
cause mastitis.Livestock Bulletin. 38(1). 59-64.
Rismawati, S. N., Ismiyati. 2017. The Effect of PH
Variations on Flavonoid Levels in Propolis Extraction
and Their Characteristics as Antimicrobials.Journal
Conversion. 6(2). 90.
Riyanto, E. F., Nurjanah, A. N., Ismi, S. N., Suhartati R.
2019. Inhibitory Power of Ethanol Extract of Butterfly
Flower (Clitoria Ternatea L) Against Food Spoiling
Bacteria.Health Journal. 19. 218–225.
Rosyidi, D., Radiati, E. L., Minarti, S., Mustakim, M.,
Susilo, A., Jaya, F., Azis, A. 2018. Comparison of the
Antioxidant Properties of Propolis in Two Types of Bees
(Apis mellifera andTrigona sp.) in Mojokerto and Batu,
East Java, Indonesia.Journal of Animal Products
Science and Technology. 13(2). 108–117.
Sapara, T. U., Waworuntu, O., Juliatri. 2016. Antibacterial
Effectiveness of Henna Leaf Extract (Irresistible
balsamL.) Against GrowthPorphyromonas gingivalis.
Pharmaceutical Scientific Journal. 5(4). 10-17.
Saputera, M. M. A., Marpaung, T. W. A., Ayuchecaria, N.
2019. Minimum Inhibitory Concentration (MIC)
Ethanol Extract Content of Batang Bajakah Tampala
(Spatholobus littoralis Hassk) Against
BacteriaEscherichia coli Via the Well
Method.Manuntung Scientific Journal. 5(2), 167-173.
Syafrizal., Hariani, N., Budiman. 2016. Phytochemical,
Toxicity and Antioxidant Analysis of Pollen Extracts
(bee pollen) beesa trigon spp. Proceeding of
Mulawarman Pharmaceutical. (16). 408-416.
Supomo, E.S.Syamsul, A.Apriliana, C.Saleh, Erwin and D.
Lestari. 2019. Antioxidant Assay of Dayak Onion
(Eleutherine palmifolia) VIA DPPH (1,1-Difenil-2-
Pikrilhidrazil) And Bslt Test for Its Active Fraction.
Rasayan Journal of Chemistry, 12(3),1340-1346.
Utami, E. R., Rosa, Y. 2021. Antibacterial Activity of
Ethanol Extract of Wangi Pandan Leaves (Pandanus
amaryfolius) to Staphylococcus aureus. Scientific health
journal. 1(11). 61-71.
Walsh, T. R., Efthimiou, J., Dréno, B. 2016. Systematic
Review of Antibiotic Resistance in Acne an Increasing
Topical and Oral Threat. Lancet Inf Di. 16(3). 23-33.
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
150
