
In Vitro Anticancer Potential of Avicennia marina Leave Extract and
Taurin on HeLa Cell Line: An Alternative Approach of Anticancer
Silvia Andriani
1,*
, Endang Linirin Widiastuti
2
, Evi Kurniawaty
3
, Suharyani
3
, Iffa Afiqa Khairani
4
and Dea Putri Andeska
5
1
Faculty of Health, Universitas Muhammadiyah Pringsewu, Lampung, Indonesia
2
Faculty of Math and Science, Universitas Lampung, Lampung, Indonesia
3
Faculty of Medicine, Universitas Lampung, Lampung, Indonesia
4
Faculty of Sciences, Institut Teknologi Sumatera, Lampung, Indonesia
5
Faculty of Biology, Universitas Gadjah Mada, Yogyakarta, Indonesia
Keywords: Avicennia Marina, Cytotoxic, Antiproliferative, Taurine.
Abstract: The pharmacological activities of Avicennia marina tree leaves, often known as api, have been scientifically
proven to include anti-inflammatory, analgesic, and toxicological effects. The Avicennia marina plant is
thought to have anticancer qualities since it contains high levels of flavonoids, tannins, saponins, and
alkaloids. Taurine's organic acids has antioxidant and anticancer properties, alongside mangrove plants.
Cancer is caused by excessive cell growth, which damages surrounding cells and tissues. Currently, cervical
cancer is the second most common cause of mortality among women globally. The MTT technique (3-(4, 5-
dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) on HeLa cervical cancer cell cultures showed that
leaf extract and taurine effectively inhibited cancer growth. The findings indicated that the leaf extract of api-
api and taurine had cytotoxic properties, with IC50 values of 206 ppm, 122 ppm, and 603 ppm, respectively.
In contrast, the antiproliferation test demonstrated that the api-api leaf extract and taurine exhibited a longer
period of cell division compared to the control cells, with doubling times of 72 and 19 hours, respectively.
The utilization of api-api leaf extract and taurine exhibited a deleterious impact on HeLa cells as compared to
untreated cells (control cells). Treatment also inhibited cell proliferation, as shown by the longer doubling
time of treated cells compared to control cells.
1 INTRODUCTION
Cancer results from unregulated cell proliferation
in dysfunctional tissues. Cancer ranks as the second
most prevalent cause of mortality, trailing behind
cardiovascular disease. The number of cancer-related
fatalities in 2018 was roughly 9.6 million, according
to the World Health Organization's report in 2020. It
is projected that by 2030, there will be a rise of 11.4
million deaths attributed to cancer cells (Rio, S., Suci,
2017). Indonesia has the second largest number of
cervical cancer cases globally, with a fatality rate of
50% (Kementerian Kesehatan 2020). HeLa cells are
cervical cancer cells that have been infected with the
HPV-18 virus. The current cancer treatment remains
inadequate in achieving a complete cure, primarily
due to the temporary effectiveness of chemical drug-
based treatments. These medications lack selectivity
for target cells, resulting in damage to normal cells in
the body. Exploring diverse natural resources, such as
the mangrove ecosystem, can facilitate the
development of alternative medications (Albinhassan
et al., 2021).
Mangroves are resilient plants that can adjust to
shifting environments with erratic salinity and tidal
patterns. The capacity is attributed to the production
of unique chemicals by api-api leaves (Avicennia
marina) for the purpose of adaptation. The objective
of this study is to expand the availability of
phytopharmaceuticals (medicinal plants) for the
investigation of secondary metabolites from api-api
leaves (Avicennia marina) and taurine as an
anticancer agent, focusing on the components. The
efficacy of this intervention will next be assessed on
HeLa cells, namely those derived from cervical
carcinoma (Rahman, 2021).
Andriani, S., Widiastuti, E. L., Kurniawaty, E., Suharyani, , Khairani, I. A. and Andeska, D. P.
In Vitro Anticancer Potential of Avicennia marina Leave Extract and Taurin on HeLa Cell Line: An Alternative Approach of Anticancer.
DOI: 10.5220/0013110000003873
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Medical Science and Health (ICOMESH 2023), pages 25-31
ISBN: 978-989-758-740-5
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
25

2 METHOD
2.1 Extract Preparation
The Api-api leaves were acquired from the Lampung
Mangrove Center (LMC) located in Labuhan
Maringgai, East Lampung. The Api-api leaves were
rinsed with flowing water. Subsequently, the drying
process is carried out by subjecting it to an oven set
at a temperature range of 30-40˚C. Following the
drying process, it is ground into a powder known as
simplicia. Simplicia is macerated in 1:10 methanol
solvent for 24 hours, 100 grams per liter. The
macerate is further strained using filter paper. A
rotary evaporator at 50°C evaporated the extract to a
thick consistency (Nurfitri, W. A., Endang, L. W., &
Endang 2019).
2.2 Hela Cell Cuture
A 10% solution of Fetal Bovine Serum (FBS) was
prepared by adding 5 ml of the solution and 0.5 ml of
Penicillin Streptomycin. This mixture was then
combined with 50 ml of Rosewell Park Memorial
Institute medium (RPMI 1640) according to CCRC
(2009). For cell counting, 10 μl of HeLa cells were
pipetted into a well plate, 10 μl of trypan blue was
added, and the cells were counted on a
hemocytometer. A living cell appears clear, while a
dead cell is red. Hemocytometer calculations are done
in 4 rooms. The subsequent calculations pertain to the
quantity of cells to be cultivated (CCRC, 2009).
Preparation of a stock solution of 10 mg
Avicennia marina extract with 1 ml 1% DMSO for
taurine in 1 ml distilled water. The original solution
was diluted to concentrations of 125 parts per million
(ppm), 100 ppm, 75 ppm, 50 ppm, and 25 ppm
(CCRC, 2009).
2.3 Cytotoxic Test Using the MTT
Method(3-(4,5-dimetiltiazol-2-il)-2,5-
difenil tetrazoliumbromida)
For the cytotoxic test, 100 μl of cells were put to each
well, with each well containing 20,000 cells (CCRC,
2009). After a 24-hour culture period, the cells are
then washed with phosphate buffer saline (PBS).
Extract and taurine were pre-concentrated and
incubated for 24 hours in each well. The solution was
discarded and subsequently washed with a PBS
solution. Combine 10 µl MTT (3-(4,5-
dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium
bromide) with 5 mg/ml phosphate buffer saline.
Subsequently, the sample was placed in a CO
2
incubator and kept at a temperature of 37°C for a
duration of 2 hours. Living cells will undergo
metabolic processes to convert MTT (3-(4,5-
Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium
bromide) into a purple compound called formazan.
The MTT reaction was halted by adding 100 µl of
100% dimethyl sulfoxide (DMSO) stopper reagent to
each well. The absorbance was measured using an
ELISA reader at a wavelength of 550 nm (CCRC,
2013).
2.4 Antiproliferative Test with the
MTT Method (3-(4,5- dimetiltiazol-
2-il)-2,5-difenil tetrazoliumbromida)
Antiproliferative assay involved 100 µl of HeLa cells
each well, totaling 20,000 cells. The incubation
process lasted for 24 hours at a temperature of 37°C
in a CO
2
incubator (CCRC, 2009). After 24 hours of
growth in well plates, cells were given 100 µl of
extract and taurine at doses of 125, 100, 75, 50, and
25 ppm. The incubation process was carried out for
24, 48, or 72 hours at a temperature of 37°C in a CO
2
incubator. Incubation was followed by PBS rinsing of
the wells. 10 µl of MTT solution (with a
concentration of 5 mg/ml in PBS) was applied to the
wells. The wells were then incubated for an additional
2 hours at 37°C in a CO
2
incubator. Active cells will
transform MTT into a purple formazan compound.
The process was halted by introducing 100 µl of
100% DMSO into each well. The measurement of
absorbance for each well was conducted using an
ELISA reader at a specific wavelength of 550 nm.
Next, statistical analysis was used to compare viable
cell counts during different incubation times (CCRC,
2013).
2.5 Data Analysis
Analysing cytotoxic test data on HeLa cells involves
calculating the proportion of viable cells. The
percentage is transformed into a probit number in
order to obtain the IC50 value. Antiproliferative test
data analysis was used to estimate the doubling time
of extract and taurine at varied doses and incubation
times. The estimates were derived using linear
regression, which involved correlating the incubation
time with the logarithm of the number of viable cells.
A statistical analysis was performed using the One-
way ANOVA test with a 95% confidence level to
assess the influence of concentration on the average
number of live cells. If there are substantial disparities
between treatments, the analysis will proceed with the
Least Significant Difference (LSD) test.
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
26
3 RESULT AND DISCUSSION
3.1 Cytotoxic Test of Avicennia Maria
Extract
Cytotoxic studies using Avicennia marina leaf extract
and taurine against HeLa cervical cancer cells yielded
a graph connecting extract concentration to cell
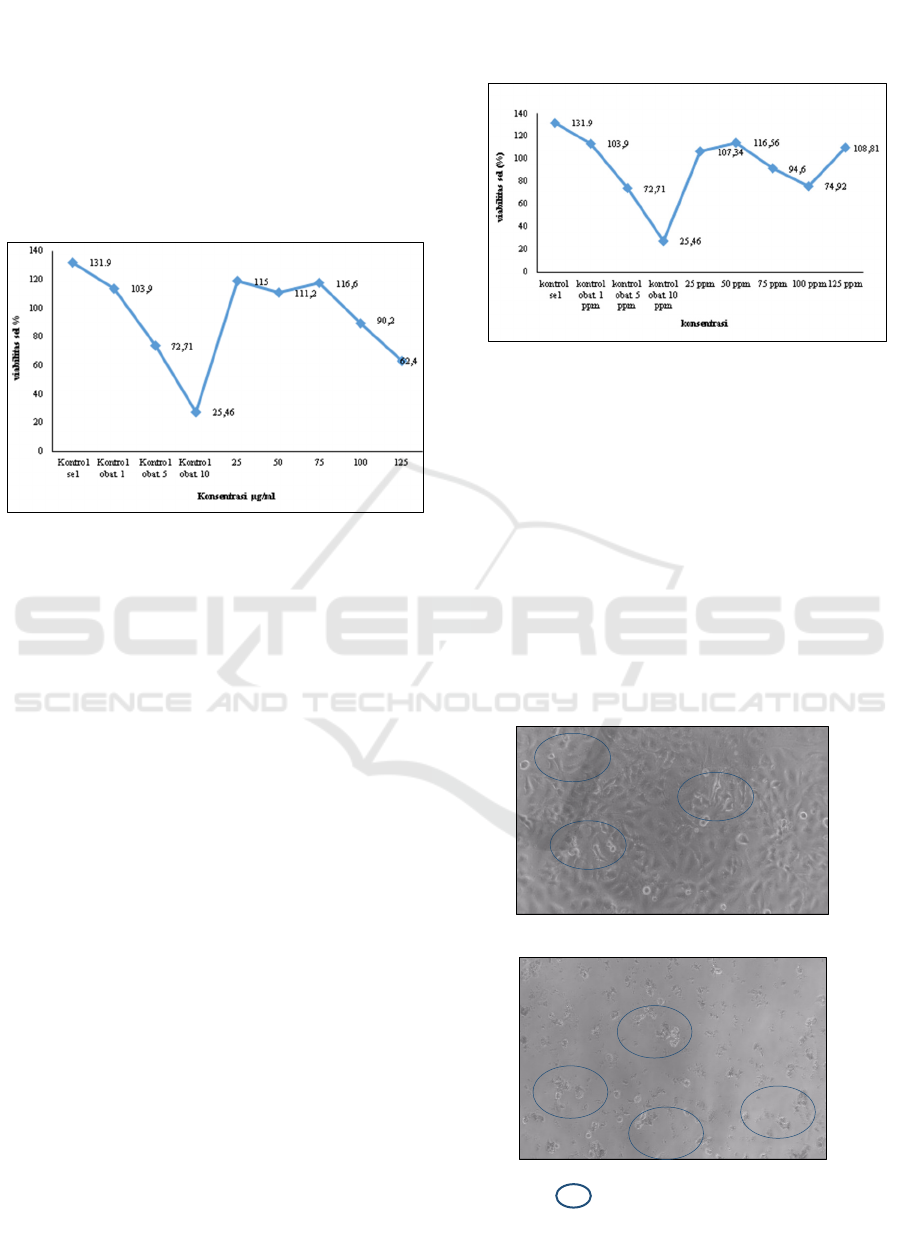
viability. Figure 1 and Figure 2 display these graphs.
Figure 1: Comparison of extract concentration with
percentage of Hela cell viability
As shown in Figure 1, Avicennia marina leaf extract
affects cell viability compared to the control group.
The leaf extract, when present at a concentration of
125 ppm, exhibited the lowest viability percentage of
62.4%. This value was significantly lower compared
to the other concentrations and the control group of
cells. Administration of Avicennia marina extract at a
dosage of 125 ppm led to a greater degree of cell
inhibition than the inhibitory effect of the control drug
at a concentration of 5 ppm.
Taurine has distinct properties compared to
Avicennia marina leaf extract. Evidence indicates that
taurine elicits varying reactions based on the level of
cell viability. Taurine had the lowest percentage of
viability (74.92%) and cell inhibition (24.07%) at a
dose of 100 ppm. However, the inhibition at 100 ppm
was still below the control drug's 5 and 10 ppm effects.
When compared to the drug control at a dose of 1 ppm,
the inhibition value was still greater.
All treatments have shown considerable cytotoxic
action, which reduces test cell viability relative to the
control group. Figures 3–5 show how this cytotoxic
action alters cell shape and structure. Under normal
circumstances, HeLa cells often have a polygonal
morphology and closely interact with their
extracellular matrix. However, in the event of a
disruption or the initiation of apoptosis, the cellular
morphology and structure will undergo a
transformation. Indications of these alterations
comprise a reduction in cell dimensions and cell
contraction. Hutomo et al. (2016) have provided
additional clarification for this occurrence.
Figure 2. Relationship between taurine concentration and %
cell viability
Figure 3 (A) illustrates a significantly increased
density of viable HeLa cells in comparison to
untreated control cells. Living cells have a high
density due to cells that develop without impediments
and meet nutritional needs. The cells have a flat
epithelial morphology, with a spherical and compact
nucleus placed centrally. They possess a basal lamina
that serves to bind them to the substrate, and it is
structurally intricate. Deceased HeLa cells exhibit an
uneven shape and lack luminescence (Nurani 2011).
In the pharmacological control group, cell density
was observed to be low due to apoptosis, which
resulted in cell death.
(A)
(B)
Note: Apoptosis
Figure 3: HeLa cell morphology (A) cell control and (B)
drug control with Doxorubicin.
In Vitro Anticancer Potential of Avicennia marina Leave Extract and Taurin on HeLa Cell Line: An Alternative Approach of Anticancer
27

(A)
(B)
Note: Apoptosis
Figure 4: HeLa Cell Morphology in Api-api Leaf Extract
Treatmen (A) 25 ppm; (B) 125 ppm.
Figure 4 indicates that 25 ppm and 125 ppm
extracts significantly change cell density. The cell
density was greater at a concentration of 25 ppm
compared to 125 ppm. The cell density is relatively
low when the api-api leaf extract is present at a
concentration of 125 ppm. Certain cells undergo
apoptosis, resulting in abnormal cellular morphology.
The application of 125 ppm api-api leaf extract was
deemed efficacious in suppressing the percentage of
cell viability in comparison to other concentrations.
These findings demonstrate the toxicity of api-api leaf
extract towards HeLa carcinoma cells. At 25 ppm,
cell density was lower than control cells, but at 125
ppm, cell density was rarely visible and color and
shape changed, indicating Hela was undergoing
apoptosis. At a dosage of 125 parts per million (ppm),
it exhibits the highest level of effectiveness in
suppressing cell development and is highly toxic to
HeLa cells.
(A)
(B)
Note: Apoptosis
Figure 5: HeLa Cell Morphology in Taurin (A) 25 ppm dan
(B) 100 ppm.
Cell density exhibited a significant decrease when
treated with a concentration of 100 ppm, in contrast
to a concentration of 25 ppm. However, viable HeLa
cells were still detectable at this concentration.
Among all the concentrations tested, taurine at 100
ppm had the best response in terms of inhibiting the
development of HeLa cells. After calculating cell
viability with the extract and taurine, the IC50 value
is calculated using the reference (CCRC, 2013).
Table 2: Test Compounds' IC50 Cytotoxic Activity against
HeLa Cervical Cancer Cells
Test
compound
Concen-
tration
(ppm)
Cell
viability
(%)
IC
50
(ppm)
25 94,48 206
50 89,52
A
.marina 75 80,55
100 73,76
125 61,44
25 106,4
603
50 114,5
Taurin 75 91,4
100 75,9
125 109,9
1 113,38
12,35
Doxorubicin 5 73,71
10 27,46
IC50 is the concentration at which a drug inhibits
test cell growth by 50%. A lower IC50 value of the test
material indicates a higher level of toxicity and a better
potential for use as a medication. The American
National Cancer Institute (NCI) defines the cytotoxic
activity criterion for crude extracts as having an IC50
value of less than 30 µg/ml, as stated by (de Oliveira
et al. 2016).
Table 2 demonstrates that api-api leaf extract,
taurine, and doxorubicin, at different doses, result in a
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
28

reduction in cell viability. This suggests that the test
chemical exhibits cytotoxic action against HeLa cells.
The regression calculations indicate that the IC50
value for api-api seed extract is 206 ppm, taurine is
603 ppm, and doxorubicin is 12.35 ppm. The IC50
value of the api-api leaf extract plus taurine is
significantly higher when compared to doxorubicin.
Table 3 indicates the doubling time values varied
across different treatment concentrations of api-api
leaf extract and taurine. Cell proliferation is measured
by linear regression equation slope in the doubling
time test. The control cell yielded a slope value of
0.0042. This value functions as a point of reference for
clusters of cells undergoing therapy. Treatment slope
lower than control cell slope increases doubling time.
However, the doubling time is reduced when the slope
is higher than that of the control cells (Meiyanto et al.,
2008). According to the information provided in Table
3, the slope values of all treatment cells are lower than
the slope values of the control cell. According to
Haryoto et al. (2013), the research shows that treated
HeLa cells have a longer period of time between each
cell division compared to untreated HeLa cells.
The doubling time of api-api leaf extract
increases with extract concentration. At 25 ppm, api-
api leaf extract doubles in time. Above 25 ppm,
doxorubicin did not produce the same doubling time
values as the control agent. This occurrence can be
ascribed to the negative coefficient in the linear
regression equation. A negative slope value indicates
the absence of growth as a result of cellular mortality
(Nurani 2011). The cells that were exposed to taurine
had diverse doubling time values, which were
significantly greater than those of the control cells.
This fact implies that api-api leaf extract and taurine
have the potential to function as anti-proliferative
agents in HeLa cervical cancer cells.
Table 3. Doubling Time Value in Antiproliferation Test
Test compound
Concentration
(ppm)
Equation of incubation time
line and log of cell number
Slope value
Doubling Time
value (hours)
A.Marina 25 0,0019x + 4,194
0,0019 170
50 0,0013x +4,232 0,0013 253
75 0,0012x + 4,168
0,0012 325
100 0,0007x + 4,145 0,0007 482
125 0,0002x + 4,136
0,0002 1884
25 0,0014x+4,2937
0,0014 218
50 0,001x + 4,3142
0,001 285
Taurin 75 0,0013x+4,2752
0,0013 249
100 0,0004x + 4,259 0,0004 852
125 0,0016x+4,2878
0,0016 195
Cell control 0 0,0021x + 4,306 0,0042 73,17
1 -0,0037x+4,3688
-0,0037 Tidak ada
Doxorubicin 5 -0,0187x + 4,462
-0,0187 Tidak ada
10 -0,0208x+4,2854
-0,0208 Tidak ada
Table 4: Average Number of Cells in Test Compound Treatment
Test Compound
Concentration
(ppm)
Number of livin
g
cells ( x 1000 Sel)
24 hours 48 hours 72 hours
Extract Avicennia
marina
25 18,9±1,48a 21,0±1,30a 26,3±0,52 a
50 18,9±0,79a 19,2 ±0,72a 23,8±0,94 a
75 16,5±0,60ab 15,6±0,92b 21,4±0,83 ab
100 15,4±0,30b 14,7±1,21b 24,1±2,88 a
125 15,0±1,34b 13,3±0,30b 18,9±0,69b
Taurin
25 21,3±0,30ab 21,8±1,42 25,7±0,60ab
50 22,9±1,48a 22,9±1,60 24,2±0,35b
75 18,3±0,66bc 22,1±0,99 23,6±1,80b
100 15,2±1,99c 21,4±3,08 19,4±1,12c
125 22,0±1,43ab 19,5±1,28 27,8±0,21a
In Vitro Anticancer Potential of Avicennia marina Leave Extract and Taurin on HeLa Cell Line: An Alternative Approach of Anticancer
29

The mean count of viable cells in all api-api leaf
extract groups varied significantly, according to One-
way ANOVA. Subsequent analyses utilizing the
Least Significant Difference (LSD) revealed that the
greatest quantity of viable cells was detected at a
concentration of 125 ppm following a 72-hour
incubation period. Conversely, the minimum value
was documented at a concentration of 100 ppm for a
duration of 24 hours. Different taurine dosages caused
changes in 24-hour and 72-hour viable cell numbers.
In addition, the taurine concentration reached its peak
at 125 ppm after 72 hours, whereas the lowest level
was measured at 100 ppm after 24 hours.
The anticancer action of the active chemicals in
the methanol extract of api-api seeds (Avicennia
marina) may be attributed to various probable
pathways. A. marina, a natural source abundant in
medicinal properties, has been recognized for its
potential to function as an anti-cancer agent. Api-api
seeds consist of a diverse range of chemical
constituents, including cyclic triterpenoids,
flavonoids, iridoids, naphthaquinones, polyphenols,
polysaccharides, and steroids. The majority of these
substances have demonstrated substantial anticancer
efficacy, so validating the promise of A. marina as a
natural agent in cancer treatment. The reference is
from Tian et al. (2020). Studies conducted on various
solid tumor models, both in vivo and in vitro, have
demonstrated that the activity of the treatment is
dependent on the dosage. Furthermore, the treatment
exhibits selectivity towards cancer cells, hence
minimizing the occurrence of adverse effects caused
by non-specific distribution.
Antioxidant activity depends on extract phenolic
and flavonoid concentration. Higher phenolic content
increases antioxidant activity (Gaffar et al. 2022). The
active chemical component acts as an inhibitor of
signal transduction. Growth factor-induced signal
transduction begins with external stimulation and is
subsequently detected by receptors. Signal
transduction cascades can be hindered by a variety of
test compounds, including phosphatase inhibitors and
kinase inhibitors. Flavonoids, like ATP, can interfere
with the phosphorylation process, leading to its
inhibition (Meiyanto et al., 2008). Saponin
compounds possess the capability to inhibit the
synthesis of Bcl-2. Bcl-2 is a protein with anti-
apoptotic properties, which means it prevents cell
death and promotes cell proliferation (Nitami, 2019).
Research has shown that the use of Avicennia marina
extract can trigger apoptosis in cancer cells and
enhance the expression of p53 in these cells
(Momtazi-Borojeni, 2013).
4 CONCLUSION
1. Methanol extract from api-api leaves and taurine
have a cytotoxic effect on HeLa cervical cancer
cells.
2. Methanol extract of api-api leaves and taurine
inhibit HeLa cervical cancer cell growth, as
evidenced by slower cell doubling rates compared
to controls.
3. Test compounds showed variations in cytotoxic
and antiproliferative activity. Api-api leaf extract
stands out with higher activity against HeLa
cervical cancer cells than taurine.
REFERENCES
Albinhassan, TahaniH et al. 2021. Anticancer, Anti-
Proliferative Activity of Avicennia Marina Plant
Extracts.” Journal of Cancer Research and
Therapeutics 17(4): 879.
https://journals.lww.com/10.4103/jcrt.JCRT_659_19.
CCRC (Cancer Chemoprevention Research Center). 2009.
Prosedur Kultur Sel. Fakultas Farmasi Universitas
Gadjah Mada. Yogyakarta
CCRC (Cancer Chemoprevention Research Center). 2009.
Prosedur Perhitungan Sel. Fakultas Farmasi
Universitas Gadjah Mada. Yogyakarta.
CCRC (Cancer Chemoprevention Research Center). 2009.
Prosedur Preparasi Sampel. Fakultas Farmasi
Universitas Gadjah Mada. Yogyakarta.
CCRC (Cancer Chemoprevention Research Center). 2009.
Prosedur Uji Proliferasi Sel (Doubling Time). Fakultas
Farmasi Universitas Gadjah Mada. Yogyakarta.
CCRC (Cancer Chemoprevention Research Center). 2013.
Prosedur Uji Sitotoksik. Fakultas Farmasi Universitas
Gadjah Mada. Yogyakarta.
Gaffar, Shabarni et al. 2022. “Aktivitas Antioksidan Dan
Sitotoksik Terhadap Sel Kanker HeLa Dari Ekstrak
Daun Vernonia Amygdalina (Asteraceae).” Chimica et
Natura Acta 10(1): 6–14.
https://jurnal.unpad.ac.id/jcena/article/view/36779.
Haryoto et al. 2013. “Aktivitas Sitotoksik Ekstrak Etanol
Tumbuhan Sala (Cynometra Ramiflora Linn) Terhadap
Sel HeLa, T47D Dan WiDR.” Jurnal Penelitian Saintek
18(AKTIVITAS SITOTOKSIK EKSTRAK ETANOL
TUMBUHAN SALA AKTIVITAS SITOTOKSIK
EKSTRAK ETANOL TUMBUHAN SALA
(Cynometra ramiflora Linn) TERHADAP SEL HeLa,
T47D dan WiDR): 21–28.
Hastuti, Endah Dwi, Fina Irodatul Afiyah, and Munifatul
Izzati. 2023. “Potensi Mangrove Avicennia Marina
(Forsk.) Sebagai Agen Fitoremidiasi Kadmium (Cd) Di
Tambak Dan Laut Mangunharjo, Kecamatan Tugu,
Kota Semarang.” Buletin Anatomi dan Fisiologi 8(1):
71–78.
https://ejournal2.undip.ac.id/index.php/baf/article/view
/13743.
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
30

Kementerian Kesehatan. 2020. “Panduan Penatalaksanaan
Kanker Serviks.” Kementerian Kesehatan Republik
Indonesia. Kanker.kemkes.go.id.
Meiyanto, E., R. A. Susidarti, S. Handayani, and F Rahmi.
2008. “Ekstrak Etanolik Biji Buah Pinang (Areca
Cathecu L.) Mampu Menghambat Proliferasi Dan
Memacu Apoptosis Sel MCF-7.” Majalah Farmasi
Indonesia 19(1): 12–19.
Momtazi-Borojeni, Amir Abbas, Mandana Behbahani, and
Hojjat Sadeghi-Aliabadi. 2013. “Antiproliferative
Activity and Apoptosis Induction of Crude Extract and
Fractions of Avicennia Marina.” Iranian Journal of
Basic Medical Sciences 16(11): 1203–8.
Nurani, Laela Hayu. 2011. “Cytotoxicity, Antiproliferatif
Assays, and Expresion of P53 and BCl2 of Ethanolic
Fraction from Tea (Camellia Sinensis (L.) O.K.) Leaves
Infuse to HeLa Cells.” Majalah Obat Tradisional 16(1):
2011.
Nurfitri, W. A., Endang, L. W., dan Endang, N. C. 2019.
“Efek Ekstrak Metanol Daun (Acanthus Ilicifolius L.)
Serta Buah Jeruju Dan Taurin Dalam Menurunkan
Kadar Glukos Darah Dan Kolesterol Serta Fertilitas
Mencit Jantan (Mus Musculus L.) Yang Diinduksi
Aloksan.” In Prosiding Seminar Nasional Tumbuhan
Obat Indonesia Ke-55. Magelang,.
de Oliveira, Pollyanna Francielli et al. 2016. “Study of the
Cytotoxic Activity of Styrax Camporum Extract and Its
Chemical Markers, Egonol and Homoegonol.”
Cytotechnology 68(4): 1597–1602.
Rahman, Mahbubur. 2021. “The Effect Of Dosage Of
Mangrove Leaf Extract Avicennia Marina On The
Viability Of Hela Cells.” Journal of Stem Cell Research
and Tissue Engineering 5(1): 41.
Rio, S., Suci, E. S.T. 2017. “Persepsi Tentang Kanker
Serviks Dan Upaya Prevensinya Pada Perempuan Yang
Memiliki Keluarga Dengan Riwayat Kanker.” Jurnal
Kesehatan Reproduksi 4(3): 159–69.
Siska Febdian Nitami, Rifki Febriansah. 2019.
“Penambatan Molekular Senyawa Tangeretin Dan
Kampferol Pada Protein Antiapoptosis Bcl-XL: Studi In
Silico.” Acta Pharmaciae Indonesia 7(2): 42–50.
Tian, Shan et al. 2020. “Anti-Cancer Activity of
Biosynthesized Silver Nanoparticles Using Avicennia
Marina against A549 Lung Cancer Cells through
ROS/Mitochondrial Damages.” Saudi Journal of
Biological Sciences 27(11): 3018–24.
https://doi.org/10.1016/j.sjbs.2020.08.029.
WHO. 2020. “Cervical Cancer.” World Health
Organization. www.who.int.
In Vitro Anticancer Potential of Avicennia marina Leave Extract and Taurin on HeLa Cell Line: An Alternative Approach of Anticancer
31
