
Correlation of Lipid Profiles and Inflammatory Markers with
Visceral Fat in Young Adult Men with Normal and Higher Body
Mass Index
Mayesti Akhriani
1,*
and Wimba Widagdho Dinutanayo
2
1
Department of Nutrition, University of Aisyah Pringsewu, Lampung Province, Indonesia
2
Department of Medical Laboratory Technology, Poltekkes Kemenkes Tanjung Karang, Lampung Province, Indonesia
Keywords: Visceral Fat, Inflammatory Markers, Lipid Profiles.
Abstract: Excessive visceral fat is correlated to the increased risk of cardiovascular and metabolic diseases by increased
lipid profiles and circulation of inflammatory cytokines. Men have higher accumulated visceral fat due to
hormone and other factors. The aim of this research is to study the correlation of lipid profiles (total
cholesterol, high-density lipoprotein/HDL, low-density lipoprotein/LDL, and triglyceride/TG and
inflammatory markers (Leukocyte, Neutrophil, Lymphocyte, Erythrocyte Sediment Rate/ESR, Monocyte,
Eosinophil). The cross-sectional study recruited 37 adult men with the mean age 24 years old. BIA was used
to measure visceral fat rating (r = 0.465, p = 0.002), and samples of blood were collected following an
overnight fast. The results showed that significant correlations were found between the serum cholesterol (r
= 0.524, p = 0.001), LDL (r = 0.547, p = 0.001), triglyceride and visceral fat. From variables of inflammatory
markers, the significant positive correlations were shown between leukocyte between visceral fat (r = 0.281,
p = 0.046) and between ESR and visceral fat (r = 0.402, p = 0.007). In conclusion, statistically significant
positive correlations were found between several lipid profiles (total cholesterol, LDL and TG) and visceral
fat, and between specific inflammatory markers (leukocyte and ESR) and visceral fat.
1 INTRODUCTION
An excessive amount of visceral fat (VF) is linked to
metabolic and cardiovascular disorders and could
indicate defective subcutaneous fat that results in
ectopic fat deposition, or the build-up of unwanted
lipids in the pancreas, liver, heart, or skeletal muscle
(Lim and Meigs, 2014). In fact, visceral adipose
tissue is an important part of total body fat, and
visceral obesity is defined as an excessively increased
deposition of visceral adipose tissue. However, both
metabolic and cardiovascular diseases are linked to
this body composition (Piche et al, 2020). In order to
estimate the possible risk of developing metabolic
and cardiovascular diseases, visceral obesity must be
quantitatively assessed
Numerous techniques, including computed
tomography (CT) scanning, ultrasonography, dual-
energy X-ray absorptiometry (DXA), bioelectrical
impedance analysis (BIA), and magnetic resonance
imaging (MRI), can be used to evaluate visceral fat in
the abdominal cavity. BIA is a radiation-free,
noninvasive technique that can be used to evaluate
visceral fat in the abdominal cavity (Schwartz et al,
2017). The abdominal cavity's visceral fat is
evaluated by BIA, which also provides the visceral fat
grading level (Sukkriang et al, 2021). Therefore,
accesible and afffordable measurement of VF using
BIA could be used in many health facilities.
Compared to adult women, adult males have
lower average body fat percentages. Even with these
variations in overall body fat, adult males specifically
have higher abdominal VF depots than
premenopausal women (Nauli and Matin, 2019).
Research has attempted to ascertain the role that
testosterone plays in controlling the distribution of
body fat. Testosterone production increases
throughout puberty (about age 45) and begins to
decrease after age 20 to 30 by up to 1% annually,
reaching its lowest points in males 70 years of age
(Frank et al, 2019). Reduced testosterone levels have
been linked to an increase in the accumulation of
abdominal VF (He et al, 2018). Thus, the
accumulation of abdominal visceral fat could begin in
young age.
Akhriani, M. and Dinutanayo, W. W.
Correlation of Lipid Profiles and Inflammatory Markers with Visceral Fat in Young Adult Men with Normal and Higher Body Mass Index.
DOI: 10.5220/0013087500003873
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Medical Science and Health (ICOMESH 2023), pages 17-20
ISBN: 978-989-758-740-5
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
17

Studies investigating correlation lipid profiles and
inflammatory markers and VF mostly conducted in
obese adults. In fact, a study in Chinese adults showed
that an increased total cholesterol and low-density
protein was correlated with VF in participants with
normal Body Mass Index (BMI) (Lu et al, 2022).
Meanwhile, an observational study indicated that VF
in Korean adults with higher BMI was strongly
associated with white blood cells (WBC) and
neutrophil lymphocyte ratio (NLR) as inflammatory
markers (Yu et al, 2019). Thus, further research is
needed to evaluate the relationship between VF and
biomarkers of inflammatory and cardiovascular
diseases including subjects with normal BMI.
This study aimed to investigate the relationship
between VF and various serum lipid profiles (total
cholesterol, high-density lipoprotein/HDL, low-
density lipoprotein/LDL, and triglyceride/TG), and
inflammatory markers (leukocyte, neutrophil,
lymphocyte, Erythrocyte Sediment Rate/ESR,
monocyte, eosinophil) in young adult men with
normal and higher BMI.
2 MATERIALS AND METHODS
2.1 Study Design, Setting and Sample
Size
This cross-sectional study was conducted in Bandar
Lampung, Lampung Province, Indonesia, from
December 2019 to January 2020. The study was
granted by the ethics committee of Politeknik
Kesehatan Kementrian Kesehatan Tanjung Karang,
Bandar Lampung, Indonesia. A total of 37 men was
included in this study using purposive sampling. The
inclusion criteria were (i) aged 18-35 years old, (ii)
undiagnosed by type 2 diabetes, cardiovascular
diseases, metabolic syndrome and cancer, (iii) no
smoking, (iii) no fluctuated weight changes for 6
months.
2.2 Anthropometric and Biochemical
Variables
The measurement of height (cm) and weight (kg) was
collected using a stadiometer to the nearest 0.5 cm
and digital weight scale to the nearest 0.1 kg. BMI
was a result of body weight divided by the square of
body height in kg/m2. Visceral fat (VF) was
measured using BIA, a body composition analyser
(OMRON HBF 375) in standing position. VF was
rated by the BIA between 1 and 59 (low to high level
which greater visceral fat indicated a greater level.
Samples of blood were collected following an
overnight fast (>12 hours). A Hitachi 7600 Automatic
analyser (High-Technologies Corporation, Hitachi;
Tokyo, Japan) was used to test the serum levels of
total cholesterol, HDL, LDL, and TG. With the aid of
an automated blood counter system (ADVIA 120,
Bayer; Whippany, NJ, USA), total differential blood
counts were recorded as Leukocyte, Neutrophil,
Lymphocyte, ESR, Monocyte, Eosinophil
2.3 Stastical Analysis
Characteristics and variables were shown as the mean
with standard deviation (SD) for normally distributed
data. Kolmogorov-Sminrov test assessed normality of
variables. Person’s correlation analysis was used to
compute the correlation of lipid profiles and
inflammatory markers with VF rating. Data were
analyzed by SPSS version 25 (SPSS Inc., IBM,
Armonk, NY, USA) for IOS. Significance was
defined as p value below 0.05.
3 RESULTS
A total 37 males aged 24.6 years old in average were
recruited in this study. Table 1 presents the age,
anthropometric and biochemical data. The mean all
anthropometric data of male subjects were higher
than the recommendation value. The man of BMI
(24.6 ± 5.6 kg/m2) was above the cut off of normal
BMI (25-29.9 kg/m2) according to WHO. The mean
of total body fat, subcutaneous fat and muscle mass
were 26.7 ± 8.5 %, 19.3 ± 6.3 %, and 31.1 ± 3.6 %
respectively. In addition, the mean of visceral fat was
14.4 ± 8.5 %.
The mean inflammatory markers and lipid
variables are also shown in Table 1. The mean ESR
and was 33.7 mm/h, and the mean leukocyte 9105.4
/uL. The mean total cholesterol, LDL and triglyceride
were 158.7 mg/dL, 82.4 mg/dL and 139.1 mg/dL
respectively.
Table 2 displays the correlation of inflammatory
markers and lipid profiles with visceral fat. In
variables of inflammatory markers, significant
positive correlations between ESR and visceral fat (r
= 0.402, p = 0.007). It is shown similar results
between leukocyte and visceral fat (r = 0.281, p =
0.046), although no significant were found for
lymphocyte, neutrophil, monocyte, and eosinophil
level in relation to visceral fat.
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
18
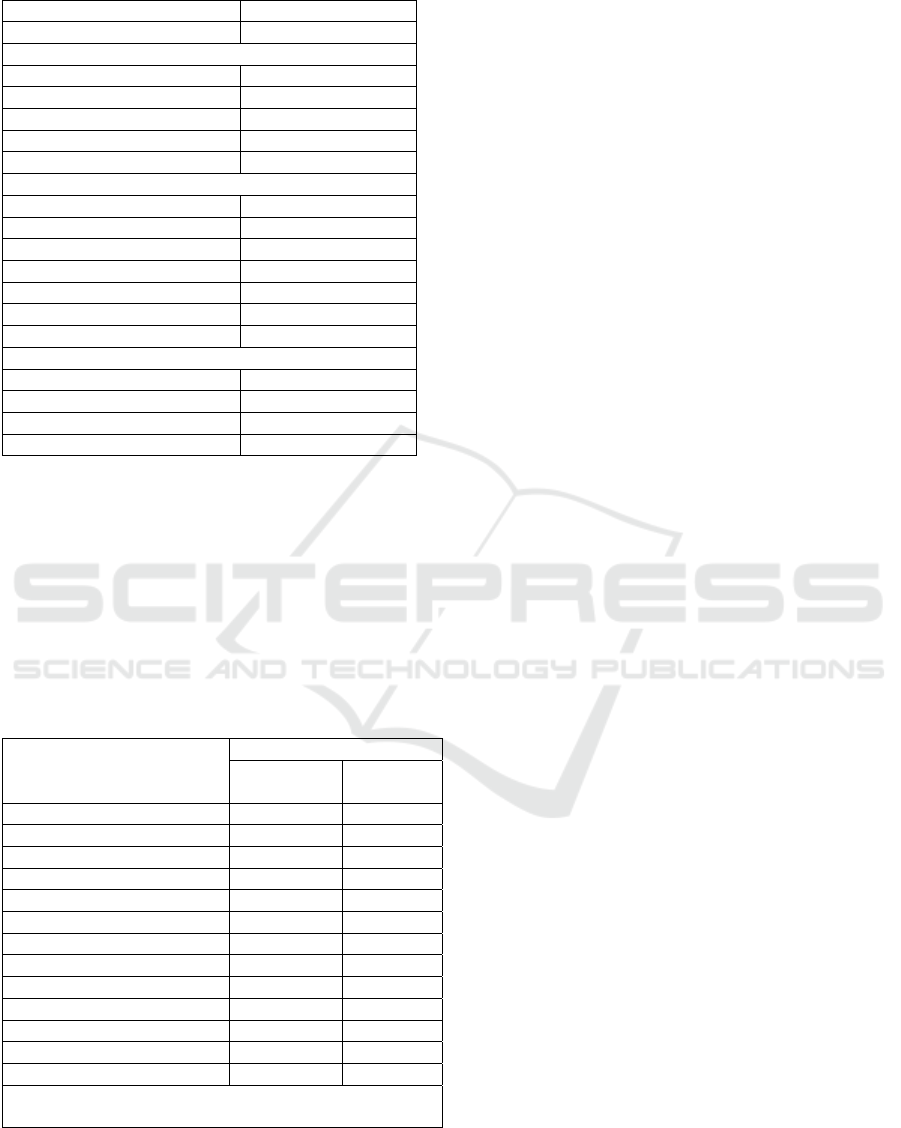
Table 1: Descriptive characteristic of subjects
Variables Mean Values
A
g
e
(y
ear
)
24.6 ± 5.6
Anthro
p
ometr
y
BMI (kg/m
2
) 30.2 ± 7.3
Body Fat Percentage (%) 26.7 ± 8.5
Visceral Fat (%) 14.4 ± 7.9
Subcutaneous Fat
(
%
)
19.3 ± 6.3
Skeletal Muscle
(
%
)
31.1 ± 3.6
Inflammation Markers
ESR (mm/h) 33.7 ± 20
Leukocyte (
/
uL) 9105.4 ± 3457.7
L
y
m
p
hoc
y
te
(
%
)
19.9 ± 7.3
Neutro
p
hil se
g
ment
(
%
)
58.2 ± 8.6
Neutro
p
hil
(
%
)
3.9 ± 2.4
Monocyte (%) 12.6 ± 4.2
Eosinophil (%) 5.4 ± 4.5
Lipid Profiles
Total Cholesterol
(
m
g
/dL
)
158.7 ± 37.9
HDL Cholesterol
(
m
g
/dL
)
48.5 ± 9.9
LDL Cholesterol
(
m
g
/dL
)
82.4 ± 32.6
Triglyceride (mg/dL) 139.1 ± 46.5
Significant positive correlations were found
between the serum total cholesterol and visceral fat (r
= 0.524, p = 0.001) and between serum LDL and
visceral fat (r = 0.547, p = 0.001). In addition, there
was a significant correlation between serum
triglyceride and visceral fat (r = 0.465, p = 0.002).
However, there was no correlation between serum
HDL and visceral fat (r = -0.232, p = 0.084)
Table 2: Correlation of Inflammatory markers and Lipid
Profiles with Visceral Fat rating
Variables
Visceral fat rating
Correlation
Coefficient
p-value
Inflammatory markers
ESR (mm/h) 0.402* 0.007
Leukoc
y
te
(/
uL
)
0.281* 0.046
L
y
m
p
hoc
y
te
(
%
)
-0.052 0.381
Neutro
p
hil se
g
ment
(
%
)
0.243 0.074
Neutrophil (%) -0.152 0.184
Monocyte (%) -0.232 0.083
Eosinophil (%) 0.005 0.488
Li
p
id Profiles
Total Cholesterol
(
m
g
/dL
)
0.524** 0.001
HDL Cholesterol (mg/dL) -0.232 0.084
LDL Cholesterol (mg/dL) 0.547** 0.001
Triglyceride (mg/dL) 0.465* 0.002
*statistically significant at P < 0.05 ** statistically
significant at P < 0.001
4 DISCUSSION
This study found that certain lipid profiles
(Cholesterol, LDL and Triglyceride) were
significantly correlated with VF in healthy and young
adult men, but not with serum HDL. This is similar to
previous studies in nondiabetic population Taiwan
(Huang et al, 2015), Chinese (Lu et al, 2022) and
Korea (Yu et al, 2019), which VF was measured using
Magnetic Resonance Imaging (MRI). Asian
population have higher risk factors for metabolic and
cardiovascular disease, since they have more total
body fat and visceral adipose tissue, despite normal
BMI (Katsuki et al, 2003).
In addition, significant correlations with VF were
only shown in ESR and leukocyte levels. Leukocyte
or White Blood Cells (WBC) and ESR has been used
to assess the risk of cardiovascular and inflammatory
diseases (Danesh et al, 1998), and has also correlated
with the increased visceral obese measured by waist-
hip ratio (Faam et al, 2014). Similar findings were
shown in the previous study that increased VF was
associated with increased inflammation (Srinivasa, et
al, 2019). The mechanism of this finding is unclear,
visceral fat could secret adipokines and pro-
inflammatory cytokines that could alter metabolism
(Deng et al, 2010). Increased leukocyte and ESR in
this study might occur due to inflammation process of
this altered metabolism.
Healthy individuals from Asia with increased
visceral fat should concern on this condition,
especially men (He et al, 2018). This is caused that a
large number of studies, including this study,
indicates higher visceral fat could be an independent
predictor of pro-inflammatory cytokines and
component of metabolic syndrome, leading to
cardiovascular diseases. According to these findings,
biochemical assessments of lipid profiles and
inflammatory markers could be a part of
cardiometabolic screening (Arakaki et al, 2018).
Indeed, preventive efforts of CVD risk should include
lifestyle modification such as limited alcohol and
saturated fat intake, along with weight management
and limited smoking habit (Traversy and Chaput,
2015).
We acknowledge that there were several
limitations in this study, First, it was unable to
determine causality or causation due to its
observational cross-sectional design, and any
potential confounding variables were unable to be
controlled. Secondly, the number of subjects were
limited, which might not be represent the population.
Third, different adipokines or other inflammatory
mediators were not collected in this study. Despite
Correlation of Lipid Profiles and Inflammatory Markers with Visceral Fat in Young Adult Men with Normal and Higher Body Mass Index
19

these limitations, studies investigating correlation of
visceral fat in young and healthy adult men with lipid
profiles and inflammatory markers are still limited,
thus this study could be the baseline to conduct the
similar studies with the retrospective design.
5 CONCLUSIONS
This study indicated that statistically significant
positive correlations were found between several
lipid profiles (total cholesterol, LDL and TG) and
visceral fat, and between specific inflammatory
markers (leukocyte and ESR) and visceral fat.
REFERENCES
Arakaki, S., Maeshiro, T., Hokama, a., Hoshino, K.,
Maruwaka, S., Higashiarakawa, M., Parrott, G., Hirata,
T., Kinjo, K., & Fujita, J. (2016). Factors Associated
with Visceral Fat Accumulation in the General
Population in Okinawa, Japan. World Journal of
Gastrointestinal Pharmacology and Therapeutics, 7(2),
261–267. Https://Doi.Org/10.4292/Wjgpt.V7.I2.261
Danesh, J., Collins, R., Appleby, P., & Peto, R. (1998).
Association of Fibrinogen, C-Reactive Protein,
Albumin, or Leukocyte Count with Coronary Heart
Disease: Meta-Analyses of Prospective Studies. JAMA,
279(18), 1477–1482.
Https://Doi.Org/10.1001/Jama.279.18.1477
Deng, Y., & Scherer, P. E. (2010). Adipokines as Novel
Biomarkers and Regulators of the Metabolic Syndrome.
Annals of the New York Academy of Sciences, 1212,
E1–E19. Https://Doi.Org/10.1111/J.1749-
6632.2010.05875.X
Faam B, Zarkesh M, Daneshpour MS, Et Al.the Association
between Inflammatory Markers and Obesity-Related
Factors in Tehranian Adults: Tehran Lipid and Glucose
Study. Iran J Basic Med Sci 2014;17:577–82. [PMC
Free Article] [Pubmed] [Google Scholar] [Ref List]
Faam, B., Zarkesh, M., Daneshpour, M. S., Azizi, F., &
Hedayati, M. (2014). the Association between
Inflammatory Markers and Obesity-Related Factors in
Tehranian Adults: Tehran Lipid and Glucose Study.
Iranian Journal of Basic Medical Sciences, 17(8), 577–
582.
Frank, a. P., De Souza Santos, R., Palmer, B. F., & Clegg,
D. J. (2019). Determinants of Body Fat Distribution in
Humans May Provide Insight about Obesity-Related
Health Risks. Journal of Lipid Research, 60(10), 1710–
1719. Https://Doi.Org/10.1194/Jlr.R086975
He, Z., Rankinen, T., Leon, a. S., Skinner, J. S., Tchernof,
a., & Bouchard, C. (2018). Plasma Steroids, Body
Composition, and Fat Distribution: Effects of Age, Sex,
and Exercise Training. International Journal of Obesity
(2005), 42(7), 1366–1377.
Https://Doi.Org/10.1038/S41366-018-0033-1
Katsuki, a., Sumida, Y., Urakawa, H., Gabazza, E. C.,
Murashima, S., Maruyama, N., Morioka, K., Nakatani,
K., Yano, Y., & Adachi, Y. (2003). Increased Visceral
Fat and Serum Levels of Triglyceride Are Associated
with Insulin Resistance in Japanese Metabolically
Obese, Normal Weight Subjects with Normal Glucose
Tolerance. Diabetes Care, 26(8), 2341–2344.
Https://Doi.Org/10.2337/Diacare.26.8.2341
Lim, S., & Meigs, J. B. (2014). Links between Ectopic Fat
and Vascular Disease in Humans. Arteriosclerosis,
Thrombosis, and Vascular Biology, 34(9), 1820–1826.
Https://Doi.Org/10.1161/ATVBAHA.114.303035
Lu, Y., Li, N., Kamishima, T., Jia, P., Zhou, D., Hind, K.,
Sutherland, K., & Cheng, X. (2022). Visceral Obesity
and Lipid Profiles in Chinese Adults with Normal and
High Body Mass Index. Diagnostics (Basel,
Switzerland), 12(10), 2522.
Https://Doi.Org/10.3390/Diagnostics12102522
Nauli, a. M., & Matin, S. (2019). Why Do Men Accumulate
Abdominal Visceral Fat?. Frontiers in Physiology, 10,
1486. Https://Doi.Org/10.3389/Fphys.2019.01486
Piché, M. E., Tchernof, a., & Després, J. P. (2020). Obesity
Phenotypes, Diabetes, and Cardiovascular Diseases.
Circulation Research, 126(11), 1477–1500.
Https://Doi.Org/10.1161/CIRCRESAHA.120.316101
Schwartz, M. W., Seeley, R. J., Zeltser, L. M., Drewnowski,
a., Ravussin, E., Redman, L. M., & Leibel, R. L. (2017).
Obesity Pathogenesis: an Endocrine Society Scientific
Statement. Endocrine Reviews, 38(4), 267–296.
Https://Doi.Org/10.1210/Er.2017-00111
Srinivasa, S., Fitch, K. V., Torriani, M., Zanni, M. V.,
Defilippi, C., Christenson, R., Maehler, P., Looby, S.
E., Lo, J., & Grinspoon, S. K. (2019). Relationship of
Visceral and Subcutaneous Adipose Depots to Markers
of Arterial Injury and Inflammation among Individuals
with HIV. AIDS (London, England), 33(2), 229–236.
Sukkriang, N., Chanprasertpinyo, W., Wattanapisit, a.,
Punsawad, C., Thamrongrat, N., & Sangpoom, S.
(2021). Correlation of Body Visceral Fat Rating with
Serum Lipid Profile and Fasting Blood Sugar in Obese
Adults using a Noninvasive Machine. Heliyon, 7(2),
E06264.
Https://Doi.Org/10.1016/J.Heliyon.2021.E06264
Traversy, G., & Chaput, J. P. (2015). Alcohol Consumption
and Obesity: an Update. Current Obesity Reports, 4(1),
122–130. Https://Doi.Org/10.1007/S13679-014-0129-
4
Yoon K.H., Lee J.H., Kim J.W., Cho J.H., Choi Y.H., Ko
S.H., Zimmet P., Son H.Y. Epidemic Obesity and Type
2 Diabetes in Asia. Lancet. 2006;368:1681–1688.
Doi: 10.1016/S0140-6736(06)69703-1.[Pubmed]
[Crossref] [Google Scholar]
Yu, J. Y., Choi, W. J., Lee, H. S., & Lee, J. W. (2019).
Relationship between Inflammatory Markers and
Visceral Obesity in Obese and Overweight Korean
Adults: an Observational Study. Medicine, 98(9),
E14740.
Https://Doi.Org/10.1097/MD.0000000000014740
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
20
