
Effects of Skeletonema Costatum’s Powder on the Knee Joint’s
Diameter and the Degree of Pain of Male Rat Sparague Dawley Type
Induced by Complete Freund’s Adjuvant
Astalitha Lorel Tan
ia
1,*
,
Riana Rahmawati
2
and Isnatin Miladiyah
2
1
Alumni, Faculty of Medicine, Universitas Islam Indonesia, Yogyakarta, 55584, Indonesia
2
Pharmacology Department, Faculty of Medicine, Universitas Islam Indonesia, Yogyakarta, 55584, Indonesia
Keywords: Antioxidants, Complete Freund's Adjuvant, Osteoarthritis, Skeletonema Costatum.
Abstract: Osteoarthritis is a degenerative disease caused by an abnormal inflammatory cytokine response. Complete
Freund's Adjuvant (CFA) is a chemical substance that induces osteoarthritis. Bioactive compounds in
Skeletonema costatum act as antioxidants, which could lessen inflammation. This study aims to determine
how Skeletonema costatum affects the knee joint's diameter size and the degree of pain induced by CFA in
rats. The study used thirty adult male rats (Sparague dawley), 2-3 months old, weighing 200-300 grams,
divided into five groups: negative control (K1), positive control/piroxicam (K2), and intervention groups (K1,
K2, and K3). All groups were induced with CFA, and the intervention began six weeks after induction. The
negative and positive groups received normal saline and piroxicam (10 mg/kg BW/day) for 21 days,
respectively. Skeletonema costatum was given to the intervention groups at doses of 60 mg/kg BW/day (K3),
90 mg/kg BW/day (K4), and 120 mg/kg BW/day (K5) for 21 days. The knee joint diameters and the degree
of pain were assessed using a micrometer screw and a hot water tail-flick assay on days 0, 7, 14, and 21. The
results were examined using the paired-t test, Kruskall-Wallis, and post hoc Mann-Whitney. Skeletonema
costatum lowered the diameters and pain levels of rats' knee joints before and after the trial (paired t-test,
p<0.05). All dosage groups demonstrated a beneficial effect (Kruskal Wallis, p<0.05); the higher the dose of
Skeletonema costatum, the greater the effect on reducing the knee joint's diameter and pain. A multivariate
analysis showed the reduction of knee joint diameter and pain after Skeletonema costatum's intervention was
statistically greater compared to the control/placebo group. However, this effect was lower than in the
piroxicam group (p< 0.05). Skeletonema costatum may have an anti-inflammatory effect in rats by lowering
the size of the knee joints and the degree of pain induced by CFA. Given the potential of Indonesian marine
products and the trend toward marine drug treatments, further studies are required to investigate the
inflammatory effects of Skeletonema costatum.
1 INTRODUCTION
Osteoarthritis (OA) is a chronic degenerative disease
that commonly affects older people and someone who
uses their joint too much, which causing joint
inflammation. Osteoarthritis usually affects the
interphalangeal, hip, knee, spinal, and
temporomandibular joints
(Kasper, 2015; Gs M, 2014;
Poulet, 2017; Gupta, 2017). Common symptoms of OA
include pain and limitation of daily activities due to
cartilage damage.
The incidence of OA under the age of 40 is
minimal and is commonly due to trauma. The
prevalence of OA increases between the age of 40-60
years. Globally, it is estimated that 9.6% of men and
18% of women over the age of 60 have symptomatic
osteoarthritis. According to the World Health
Organization (WHO) in 2010, OA affects more
women than men in all age groups (2.95 women per
1000 population and 1.71 men per 1000 population).
In women, the age group of 65-74 years has the
highest incidence, about 13.5 per 1000 population per
year. In men, the incidence in people over 75 years of
age is about 9 per 1000 population per year. In
Indonesia, the prevalence of OA based on
radiological findings is 15.5% in males aged 40-60
years and approximately 12.7% in females.
According to WHO, the elderly population in
Indonesia is expected to increase by 414% compared
Tania, A. L., Rahmawati, R. and Miladiyah, I.
Effects of Skeletonema Costatum’s Powder on the Knee Joint’s Diameter and the Degree of Pain of Male Rat Sparague Dawley Type Induced by Complete Freund’s Adjuvant.
DOI: 10.5220/0013084800003873
Paper published under CC license (CC BY-NC-ND 4.0)
In Proceedings of the 1st International Conference on Medical Science and Health (ICOMESH 2023), pages 5-10
ISBN: 978-989-758-740-5
Proceedings Copyright © 2025 by SCITEPRESS – Science and Technology Publications, Lda.
5

to 1990 by 2025 due to the increase in life expectancy
in Indonesia.
The development of OA is influenced by many
risk factors such as: continuous mechanical stress
occurs in multiple parts of joints, inducing the release
of pro-inflammatory mediators and degradation
enzymes such as IL-1, IL-6, IL- 1 β, NO, and TNF-α,
resulting in joint inflammation (
McCance, 2014).
Inflammation results from an imbalance between
pro-inflammatory and anti-inflammatory cytokines.
Pro-inflammatory mediators induce the formation of
reactive oxidative stress (ROS), leading to
exacerbated inflammation. Another consequence of
the release of pro-inflammatory mediators is that the
chondrocyte components in the turnover process
become imbalance and the joint undergoes excessive
destructive processes leading to chondrocyte
apoptosis (
McCance, 2014). Increased chondrocyte
apoptosis reduces the number of proteoglycans in
cartilage. Bonds between collagens weaken due to
decreased synthesis of type II collagen and increased
collagen degradation.
Molecules resulting from the breakdown of
collagen and proteoglycans are destroyed by synovial
macrophages causing an increase in the number of
pro-inflammatory cytokines. These cytokines bind to
chondrocyte receptors and trigger the release of
metalloproteinase (MMP), inhibiting type II collagen
production and continuing cartilage degradation. In
response to mechanical and biochemical stimuli,
chondrocytes overproduce its MMP, collagenase,
stromelysin, and gelatinase, causing greater joint
damage. Metalloproteinase also trigger the release of
ROS, namely hydrogen peroxide, hypochlorite ion,
hydroxyl radical, or superoxide anion
5
. Chondrocytes
normally produce small amounts of nitric oxide (NO)
and superoxide anion. These two ions will form
peroxynitrite and hydrogen peroxide. Hydrogen
peroxide can convert its form to hydroxyl radical,
forming lipid peroxide in chondrocytes and causing
further degradation (Gupta, 2017).
Our cells can naturally reduce excess ROS by
using antioxidants. Antioxidants are divided into two
groups: enzymatic and non-enzymatic. Antioxidants
use superoxide dismutase (SOD), catalase (CAT),
glutathione peroxidase (GPX) and some fat- and
water soluble small molecules to prevent the
formation of ROS. It acts as a ROS scavenger by
repairing damage that has occurred (Gupta, 2017; Sun
AR, 2017). However, when the antioxidant capacity
is reduced, ROS damage the extracellular matrix,
nucleus, and cell membrane, leading to cell death,
causing dead cells to release oxidative molecules,
triggering the release of synovial macrophages, pro-
inflammatory cytokines, ROS and MMP, which lead
to chronic inflammation (Sun AR, 2017).
Initial treatment of OA uses an oral non-steroidal
anti-inflammatory drug (NSAID) such as piroxicam.
It aims to reduce the inflammatory response that
occurs in the joints. Additionally, corticosteroid
injections, oral administration of glucosamine and
chondroitin sulfate can reduce joint inflammation. It
acts as an anti-inflammatory agent, causes a
scavenging effect, and plays a role in lipid peroxidase
mechanism by exploiting the mechanism of reducing
ROS production (Gunawan SG, 2016). Because
NSAID therapy adversely affects the gastrointestinal
system when taken long term (Gunawan SG, 2016).
Another alternative from a marine alga, Skeletonema
costatum, may help reduce the inflammatory effects
of OA.
Antioxidants are protective agents which inhibit
disease progression and the formation of ROS from
chondrocytes, thus reducing the progression of OA.
Skeletonema costatum is a microalga belonging to the
class Coscinodiscophyceae and the Skeletonemacea
family (Miyashita K, 2009). This microalgae contains
antioxidants such as carotenes and unsaturated fatty
acids. The types of carotene they contain include
fucoxanthin and astaxanthin (Miyashita K, 2009; Foo
SC, 2017). The total fucoxanthin contained in this
microalgae is 0.36 ± 0.00 with a total carotene of 0.97
± 0.24 (Foo SC, 2017). While levels of omega 3:
0.911 – 3.738%; omega 6: 15.591 – 38.002% and
omega 9: 0.292 – 15.112% (Erlina A, 2004). The
antioxidants in Skeletonema costatum act as anti-
inflammatory agents by reducing intracellular ROS,
SOD, and NO levels, increasing type II collagen
synthesis and enabling joint repair.
In the present study, CFA induction can induce
inflammation due to OA after 6 weeks after intra-
articular injection. No research explains the anti-
inflammatory activity by Skeletonema costatum,
based on data sources, so it is necessary to research to
examine the effect of Skeletonema costatum on the
diameter of the knee joint and the value of pain in rats
induced by Complete Freund's Adjuvant
(CFA).
2 METHOD
This type of research was conducted using a
laboratory experimental method with a pre-post-
study design and a post-test-only control group
design. The research was conducted in January-
February 2020 at the Nutrition Laboratory of the
Center for Food and Nutrition Studies, Gadjah Mada
University, and obtained research ethics permit from
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
6
the Ethics Committee of the Faculty of Medicine,
Public Health and Nursing, Gadjah Mada University
with protocol number KE/0851/07/2019.
The inclusion criteria used in this study were adult
male rats of the Sprague Dawley strain, aged 3-4
months and weighing 200-300 grams, the rats were
obtained from the same breeding grounds with the
same food and drink, the rats were healthy and had no
marked defects with the animal trying to move
actively, the fur looks clean and doesn't fall out.
Meanwhile, the exclusion criteria were sick and dead
rats during the study.
The grouping of the sample was carried out using
the simple random sampling method. The size of the
research sample is determined by the Federer
formula.
Thirty adult male rats (Sparague dawley), 2-3
months old, weighing 200-300 grams, were utilized
in the study and were divided into five groups:
negative control (K1), positive control (K2), and
intervention groups (K1, K2, and K3). CFA was used
to induce all the groups, and the intervention started
six weeks after induction. Normal saline and
piroxicam 10 mg/kg BW/day for 21 days were
administered to the negative and positive groups.
Skeletonema costatum was administered to the
intervention groups for 21 days at various dosages of
60 mg/kg BW/day (K3), 90 mg/kg BW/day (K4), and
120 mg/kg BW/day (K5). On days 0, 7, 14, and 21,
the groups joint diameter and pain levels were
measured using a micrometer screw and a hot water
tail-flick assay. A paired-t test, Kruskall-Wallis, and
post hoc Mann-Whitney were used to examine the
results. On the 21st day, the groups were terminated.
SPSS software version 20.0 for Windows (SPSS
Inc. Chicago, USA). Paired-t test (data are normally
distributed) and Wilcoxon test (data are not normally
distributed) were performed to confirm the difference
in mean size of right knee joint diameters in rats
before and after treatment (Sastroasmoro, 2011)
To confirm the decrease in joint diameter and
comparison of pain response between groups, the
non-parametric Kruskal-Wallis tes was performed
because the requirements of the Anova test were not
met. To identify significant differences in each group,
we performed Mann-Whitney as a follow-up test to
Kruskall Wallis.
3 RESULTS
3.1 Measurement of Rat Right Knee
Joint Diameter
Right knee joint diameters in rats were measured
using a screw micrometer calibrated based on the
Clinical Assessment of Experimentally Induced
Osteoarthritis Rat Model In Relation To Time to see
OA before and after treatment. Table 1 observed
diameter of the right knee joints of rats in the five
groups.
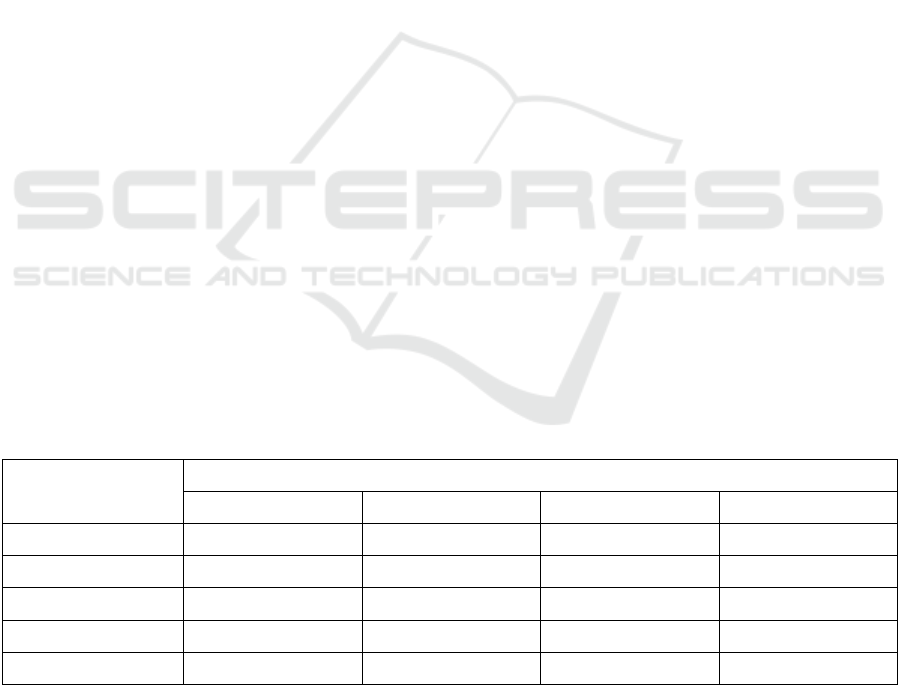
Based on the test results, the right knee joint
diameter of rat in the negative control group (K1)
tended to increase. On the other hand, the positive
control group (K2) (piroxicam 10 mg/kg BW) and all
dose treatment groups (K3, K4, K5) decreased the
diameter of the right knee joint of rats. A decrease in
the diameter of the rat's right knee joint began to be
seen on day 7.
Table 1: Rat’s right knee joint diameter on days 0 to 21
Treatment Group
Mean diameter of right knee joint ± SD (mm)
Day-0 Day-7 Day-14 Day-21
K1 9.07±0.45 9.13±0.35 9.39±0.26 9.49±0.23
K2 8.62±0.33 6.48±0.35 3.97±0.44 2.50±0.23
K3 8.64±0.28 8.23±0.07 7.37±0.29 5.40±0.20
K4 8.77±0.50 8.00±0.07 6.51±0.29 4.58±0.21
K5 8.70±0.25 7.53±0.41 5.11±0.20 3.11±0.22
The paired-t test in table 2 was performed to
confirm size group mean difference in the diameter of
the right knee joint (data for all groups were normally
distributed). A Kruskall-Walis test was then
performed to confirm significant comparisons
between groups (data not normally distributed until
day 7). Then proceed to the Mann-Whitney test to
check for significant differences between groups
(table 4).
Effects of Skeletonema Costatum’s Powder on the Knee Joint’s Diameter and the Degree of Pain of Male Rat Sparague Dawley Type
Induced by Complete Freund’s Adjuvant
7
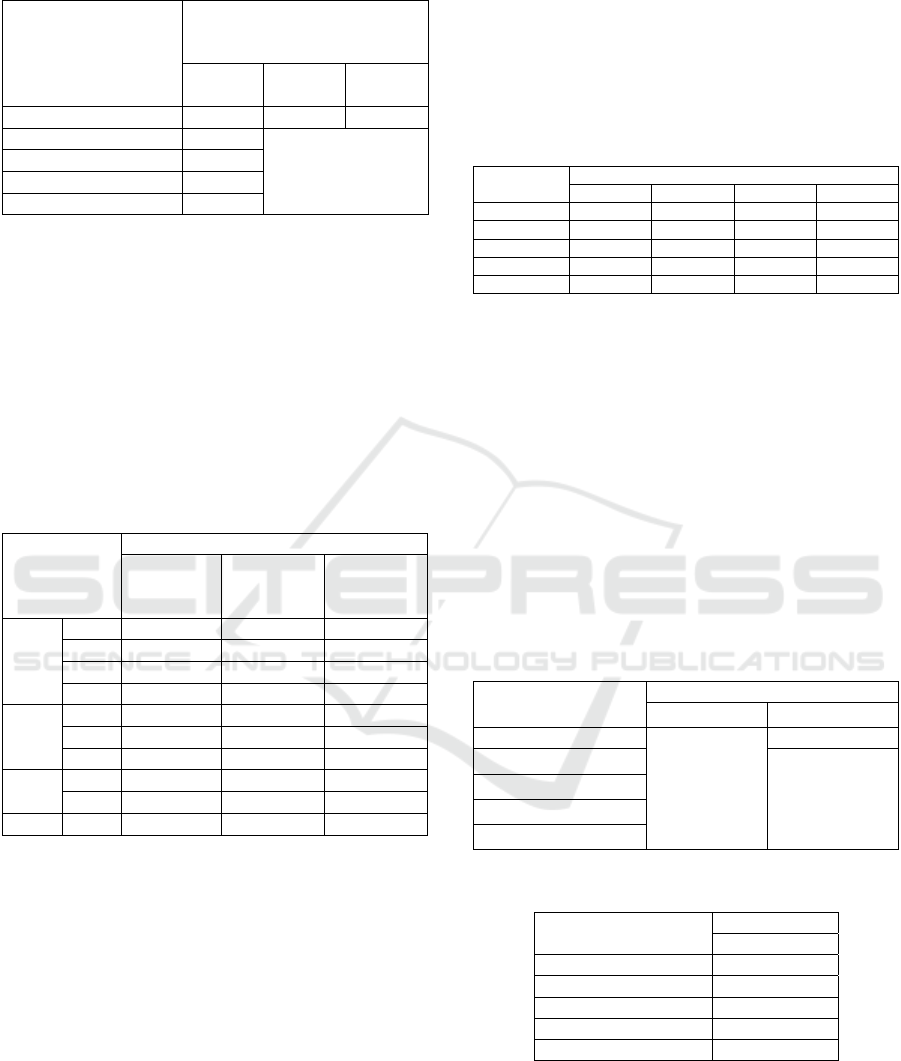
Table 2: Test results paired -t-test on groups rat’s right knee
joint diameter.
Group Pair P Value,
Confidence Interval Value
(CI) 95%
Days 0
and 7
Days 0
and 14
Days 0
and 21
K1 before
–
K1 afte
r
0.527
_
0 .029 * 0 .029 *
K2 before
–
K2 afte
r
0,000 * 0.000 *
K3 before
–
K3 afte
r
0 .021 *
K4 before
–
K4 afte
r
0.013 *
K5 before
–
K5 afte
r
0.004 *
Table 2 shows that joint diameter remained at day
7 and increased significantly at day 14 and 21 in
group K1 (p<0.05). For K2 and all test groups (K3,
K4, and K5) the mean joint diameter was significantly
decreased on day 7, 14, and 21 (p<0.05). From the
data in tables 1 and 2, we concluded, Skeletonema
costatum (60 mg / kgBB; 90 mg / kgBB; 120 mg /
kgBB) significantly reduced the size of diameter of
right knee joint as seen in the piroxicam-treated
group.
Table 3: Mann -Whitney post hoc test in groups of rat’s right
knee joint diameter.
Intergroup
Relations
P value
Decline
(0-7 days)
Decline
(0-14
da
y
s
)
Decline
(0-21
da
y
s
)
K1 K2 0.004* 0.004* 0.004*
K3 0.004* 0.004* 0.004*
K4 0.004* 0.004* 0.004*
K5 0.004* 0.004* 0.004*
K2 K3 0.004* 0.004* 0.004*
K4 0.004* 0.004* 0.004*
K5 0.005* 0.004* 0.008*
K3 K4 0.004* 0.004* 0.004*
K5 0.004* 0.004* 0.004*
K4 K5 0.004* 0.004* 0.004*
The results of the Kruskall-Walis test revealed
that there was a significant difference (p<0.05) in the
mean reduction in diameter of the right knee joints of
rats between groups in all groups. Table 3 shows a
significant (p<0.05) mean reduction in diameter of
the right knee joint of rats in each group. The negative
control (K1) is significantly different from the
positive control (K2) or test group (K3, K4, K5).
Although the right knee joint diameters of rats are
decreased, Table 3 shows that the mean decrease in
diameter in the various test groups is significant in the
positive control group (K2). In the study groups,
significant differences in joint diameter reduction
were observed between the 60 mg/kg BW (K3), 90
mg/kg BW (K4), and 120 mg/kg BW (K5).
3.2 Measuring Pain Response of Rats
Assessment of the degree of pain in rats using the hot
water tail-flick assay. The longer the rat lifts its tail
from the water bath, the better the pain response.
Below is a table of the observed pain scores of the rats
in the five groups.
Table 4: Pain response rats on days 0 to 21.
Treatment
Group
Rat
p
ain score ± SD (seconds)
Day-0 Day-7 Day-14 Day-21
K1 0.42±0.07 0.32±0.04 0.25±0.40 0.23±0.03
K2 0.45±0.92 0.92±0.06 1.08±0.06 1.98±0.05
K3 0.45±0.05 0.51±0.02 0.66±0.03 0.78±0.05
K4 0.48±0.07 0.62±0.03 0.86±0.04 0.96±0.02
K5 0.45±0.03 0.80±0.04 0.95±0.04 1.47±0.22
Based on table 4, the pain scores of rats in the
negative control group (K1) tended to decrease or
showed no improvement in pain response with tail
raising. In the positive control group (piroxicam 10
mg/kg BW) and all dose treatment groups (60 mg/kg
BW; 90 mg/kg BW and 120 mg/kg BW), there was
an increase in the tail-lifthing time which began to
appear on the day 7.
Paired-t test (normally distributed data on days 7
and 21) and Wilcoxon (non-normally distributed data
on day 14) were used to count difference in mean pain
response before and after treatmnet. Tables 5 and 6
show the averages of various test results.
Table 5: Test results paired -t-test on groups pain response
rat for days 0 and 7 and days 0 and 21
Group Pair
P value
Days 0 and 7 Days 0 and 21
K1 before
–
K1 afte
r
0,000 *
0.003 *
_
K2 before
–
K2 afte
r
0.000 *
K3 before
–
K3 afte
r
K4 before
–
K4 afte
r
K5 before
–
K5 afte
r
Table 6: Wilcoxon test results on groups pain response rat
for days 0 and 14.
Group Pair
P value
Da
y
s 0 and 14
K1 before
–
K1 afte
r
0.027 * _
K2 before
–
K2 afte
r
0 .024 *
K3 before
–
K3 afte
r
0.027 *
_
K4 before
–
K4 afte
r
0.027 *
K5 before
–
K5 afte
r
0.028 *
Tables 5 and 6 for the negative control group (K1)
show a reduction in time pain response on days 7, 14,
or 21. This is linear with the data of measurement of
the right knee joint diameter of rats (table 1) which
illustrates enhanced inflammation in the K1 group.
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
8
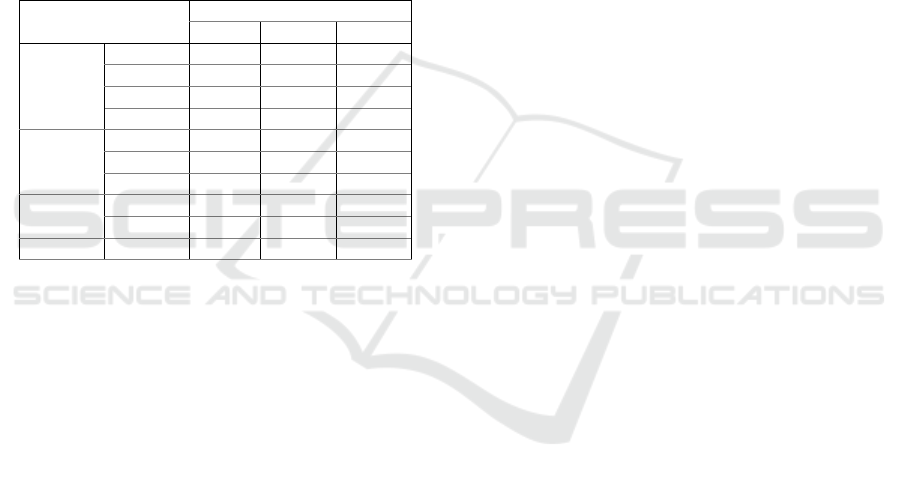
Otherwise, the means pain scores before and after
treatment, the positive control group (K2) or all test
groups (K3, K4, and K5) were significantly different
(p<0.05) in improving tail lift time in rats that
indicates the pain response is improved.
The other test is to compare the average of
decreased score pain on days 7, 14, and 21 using the
Kruskall-Wallis test. The data for day 14 are not
normally distributed and therefore did not meet the
requirements for the Anova test. According to the
results of the Kruskall-Walis test, there was a
significant difference between groups in all post
treatment observation periods (p<0.05). To see which
groups differed, Mann-Whitney performed a post hoc
test.
Table 7: Further test results post hoc Mann-Whitney on the
pain response group.
Intergroup Relations
P value
Day-7 Day-14 Day-21
K1
K2 0.004* 0.004* 0.004*
K3 0.003* 0.004* 0.004*
K4 0.004* 0.004* 0.004*
K5 0.004* 0.004* 0.004*
K2
K3 0.003* 0.004* 0.004*
K4 0.004* 0.004* 0.004*
K5 0.012* 0.005* 0.004*
K3
K4 0.003* 0.004* 0.003*
K5 0.003* 0.004* 0.004*
K4 K5 0.004* 0.004* 0.004*
Table 7 shows this significant difference in pain
score in all groups. Regarding the effective reduction
of right knee joint diameter in rats, this study shows
that the groups (K3, K4, and K5) are significantly
different from the negative control group (K1) or the
positive control group (K2). There are also significant
differences between the Skeletonema costatum
groups (60 mg/kg BW (K3); 90 mg/kg BW (K4), and
120 mg/kg BW (K5)).
4 DISCUSSION
This study showed that the negative control group has
a decrease in pain response and an increase in the
diameter of right knee joint rat, that is, there was
inflammation caused by OA by Complete Freund's
Adjuvants (CFA). While in the positive control group
(piroxicam 10 mg/ kg BW) and in all treatment
groups (Skeletonema costatum 60 mg/ kg BW; 90
mg/kg BW; 120 mg/ kg BW) there was an increase in
pain response along with a decrease in the size of the
right knee joint diameter of the rats(p<0.05).
This study, also demonstrated that Skeletonema
costatum has the effect of reducting joint diameter
and improving pain response before and after
treatment (paired t-test, p<0.05). Although efficacy
was seen in all groups of Skeletonema costatum, it has
been reported that the higher the Skeletonema
costatum dose, the greater the reduction in joint
diameter and the greater the improvement in pain
response. Reduction in right knee joint diameter of
rats and improvement of pain response after
administration of Skeletonema costatum was better
than negative control, but anot as good as using
piroxicam compared with positive control.
It reduces inflammatory markers since
Skeletonema costatum acts as an anti-inflammatory
agent. This is achived by inhibiting the release of
cytokine by macrophages and neutrophils because
Skeletonema costatum contains fatty acids, trace
elements, and antioxidants. Antioxidants act as
inhibitors of inflammation by reducing the formation
of pro-inflammatory cytokine (Health L, 2008). The
inhibition of inflammation was supported by
researchers who staid that the methanol extract of
Skeletonema costatum produced the highest phenolic
content compared with the hexane extract which was
0.644 mg / gallic acid equivalent (GAE)/g, where the
phenolic content of the hexane extract wass 0.392 mg
GAE/g (Health L, 2008). In addition, it said that the
free radical scavenging activity of the Skeletonema
costatum methanol extract is 59% at a concentration
of 3.2 mg/ml (Lenin T, 2015).
The anti-inflammatory and antioxidant effects
may be related to vitamins contained in Skeletonema
costatum. Vitamins are trace minerals that play a role
in metabolism, cell repair, immunity, and more.
Vitamin content of Skeletonema costatum, among
other things; vitamin A 141 µg, vitamin D 11 µg,
vitamin E 108 µg, vitamin K 5.5 µg, vitamin B1 710
µg, B2 37 µg, B6 134 µg, B12 117 µg, vitamin C 59
µg and vitamin PP 511 µg (Roeck-holtzhauer, 1991).
Vitamins A, C, and E have antioxidant roles in
reducing reactive oxidative stress (ROS). According
to the 2012 Framingham Cohort Study, vitamin E can
reduce the progression of OA in men, but has no
significant effect on the incidence of OA (Roeck-
holtzhauer, 1991). At the same time, high doses of
vitamin C can slow the progression of OA induced by
surgery in guinea pigs because vitamin C has a
protective effect on cartilage by stimulating collagen
and proteoglycans (Roeck-holtzhauer, 1991).
In this study, the best anti-inflammatory effect of
Skeletonema costatum was achieved after day 21 and
using the highest dose of 120 mg/kg BW. Consistent
with study Andari (2016) it was also found that
lemuru fish oil which is rich in omega-3 and omega-
6 had the best time to reduce TNF-α in joint cartilage
Effects of Skeletonema Costatum’s Powder on the Knee Joint’s Diameter and the Degree of Pain of Male Rat Sparague Dawley Type
Induced by Complete Freund’s Adjuvant
9

induced by CFA on day 21. In agreement with the
study of Bahtiar, it was shown that the longer the
administration of Skeletonema costatum, the higher
the antioxidant level in the tissue and the better the
ability of Skeletonema costatum to inhibit the
inflammatory process (Bahtiar A, 2011).
To the researchers’ knowledge, there are currently
no publications on the benefits of Skeletonema
costatum as an anti-inflammatory agent in OA. The
results of this study could lead to a pilot study on the
efficacy of Skeletonema costatum as an anti-
inflammatory agent. In this study, the anti-
inflammatory effects of Skeletonema costatum were
not yet comparable to the administration of piroxicam
as the standard, but using a higher dose of
Skeletonema costatum, the effect was improved with
a decrease in the diameter of the right knee joint
diameter in rats. It can be observed in pain response.
This could inform the next study to determine the
dose of Skeletonema costatum to be tested.
5 CONCLUSIONS
Based on the study and results, it can be concluded
that the administration of Skeletonema costatum
reduced the diameter of the right knee joint in rats and
improved the pain response before and after treatment
(paired t-test, p<0.05). Although effects were
observed in all groups of Skeletonema costatum
groups, higher dose of Skeletonema costatum
reported improved reduction in joint diameter and
improvement in pain response (closer to the positive
control). The mean reduction in right knee joint
diameter and improvement in pain response in rats
following administration of Skeletonema costatum is
superior to the negative control (no treatment), but not
as good as piroxicam (10 mg/kg BW) as a positive
control (p<0.05).
Further investigation into the inflammatory
effects of Skeletonema costatum needs to be carried
out especially to determine the therapeutic dose of
Skeletonema costatum, the time needed to reach
maximum therapeutic levels, and the toxicity level of
Skeletonema costatum. Apart from that, given the
potential of Indonesia, which is rich in marine
products, and the trend toward marine drug
treatments, further studies are essential to study the
effect of Skeletonema costatum on other types of
diseases and other organs, for example, metabolic
syndrome and cancer.
REFERENCES
Andari K., 2016. The Effect of Lemuru Fish Oil (Sardinella
longiceps) on Cartilage Tumor Necrosis Factor (TNF-
a) Expression Induced by Complete Freund's Adjuvant.
University of Jember.
Bahtiar A, Nakamura T, Kishida K, Katsura J, Nitta M,
Ishida-Kitagawa N, Ogawa T, Takeya T., 2011. The l-
Ser analog # 290 promotes bone recovery in OP and RA
mice. Pharmacological Research. 1;64(3):203-9.
Erlina A, Amini S, Endrawati H, Zainuri M., 2004.
Nutritive Study of Natural Feed Phytoplankton in Mass
Cultivation Systems. 9(4):206–10.
Foo SC, Yusoff FM, Ismail M, Basri M, Yau SK, Khong
NMH, et al., 2017. Antioxidant capacities of
fucoxanthin-producing algae as influenced by their
carotenoid and phenolic contents. J Biotechnol.
241:175–83.
Gs M, Mologhianu G., 2014. Osteoarthritis pathogenesis –
a complex process that involves the entire joint.
7(1):37–41.
Gunawan SG, Setiabudi R, Nafrialdi, 2016. Instiaty.
Pharmacology and Therapy. 6th ed. Gunawan SG,
Setiabudi R, Nafrialdi, Instiaty, editors. Jakarta: FKUI
Issuing Agency;.
Gupta E Das, Ng WR, Wong SF, Bhurhanudeen AK, Yeap
S., 2017. Correlation of serum cartilage oligometric
matrix protein ( COMP ) and interleukin-16 ( IL- 16 )
levels with disease severity in primary knee
osteoarthritis: A pilot study in a Malaysian population.
16:1–13.
Health L., 2008. Lipids in Health and Disease. 49th ed.
Peter J. Quinn, Wang X, editors. Springer US. 603 p.
Kasper D, Fauci A, Hauser S, Longo D, Larry JJ, Joseph L.,
2015. Harrison's Principles of Internal Medicine. 19th
ed. 3000 p.
Lenin T, Sangeetha SPJ, Veerapandiyan N, Sampathkumar
P., 2015. Antioxidant Potentials of Marine Diatom
Skeletonema costatum. Int J Adv Multidiscip Res.3(2):
35–9.
McCance KL, Huether SE., 2014. Pathophysiology The
Biologic Basis for Disease in Adults and Children. 7th
ed. Elsevier Ltd.
Miyashita K., 2009. Function of marine carotenoids. Nutr
Forums.61:136–46.
Poulet B., 2017. Models to define the stages of articular
cartilage degradation in osteoarthritis development. Int
J Exp Pathol. 98(3):120–6.
Roeck-holtzhauer Y De, Quere I, Claire C., 1991. Vitamin
analysis of five planktonic microalgae and one
macroalgae. 259–64.
Sastroasmoro S, Ismael S., 2011. Fundamentals of Clinical
Research Methodology 4th Edition. 4th ed. Jakarta:
Sagung Seto.
Sun AR, Panchal SK, Friis T, Sekar S, Crawford R, Brown
L, et al., 2017. Obesity-associated metabolic syndrome
spontaneously induces infiltration of pro-inflammatory
macrophage in synovium and promotes
osteoarthritis.1–22.
ICOMESH 2023 - INTERNATIONAL CONFERENCE ON MEDICAL SCIENCE AND HEALTH
10
