Regulatory Positioning of an Innovative Biomaterial
for Regenerative Medicine: TissYou Project
Gwenaël Rolin
1,2 a
, Kenny Pinot
1
and Marilys Blanchy
3
1
INSERM CIC-1431, CHU Besançon, F-25000 Besançon, France
2
Univ. Franche-Comté, INSERM, EFS BFC, UMR1098, RIGHT Interactions Greffon-Hôte-Tumeur/ Ingénierie Cellulaire et
Génique, F-25000, Besançon, France
3
Rescoll
®
, Pessac, France
Keywords: Biomaterial, Electrospining, Implantable Device, European Regulation.
Abstract: New technologies make it possible to industrialize objects that can reconstruct in-vivo like extracellular
matrices. Actually, these scaffolds exhibit properties mimicking physiological tissue. The project presented
here aims at the industrial production of a new bicomposite biomaterial for skin regeneration. This “TissYou”
matrix is produced by electrospining two polymers, silk fibroin and polycaproclactone, using an innovative
process. The state of progress of the project leads us today to have in our hands a functional prototype on the
way to becoming a finished product. In order to ensure the transition of this product from R&D to a possible
medical device, the regulatory roadmap that awaits the future product should be prepared as soon as possible.
Consequently, and relying on the European regulation and its annexes, our main objective is to demonstrate
that the product meets the definition of a medical device, to precisely define the class to which it belongs, to
start a risk analysis process and definition of the standards that should be applied in the subsequent
qualification of the product. In order to stabilize the perimeter of the future indication of the product, we will
also present a questionnaire deployed among professionals in order to collect their user needs.
1 INTRODUCTION
The TissYou project aims to the industrial production
of an innovative bicomposite biomaterial intended for
skin regeneration. This biomaterial is produced by
electrospining, with two polymers, silk fibroin
(natural) and polycaproclactone (synthetic), thanks to
an innovative process.
The electrospinning technique is a technology
allowing the production of polymeric fibers with
controlled dimension in terms of composition, size,
diameter and orientation in the three dimensions (Liu
et al. 2021). This technology is the result of a process
developed at the end of the 19th century (Boys et al.
1887). Today, researchers have more than a hundred
years of experience on this technology, which is
experiencing enormous interest; in the wild field of
biomaterials (Wang et al. 2019). The basic
electrospining technique (Aidana et al. 2021) is based
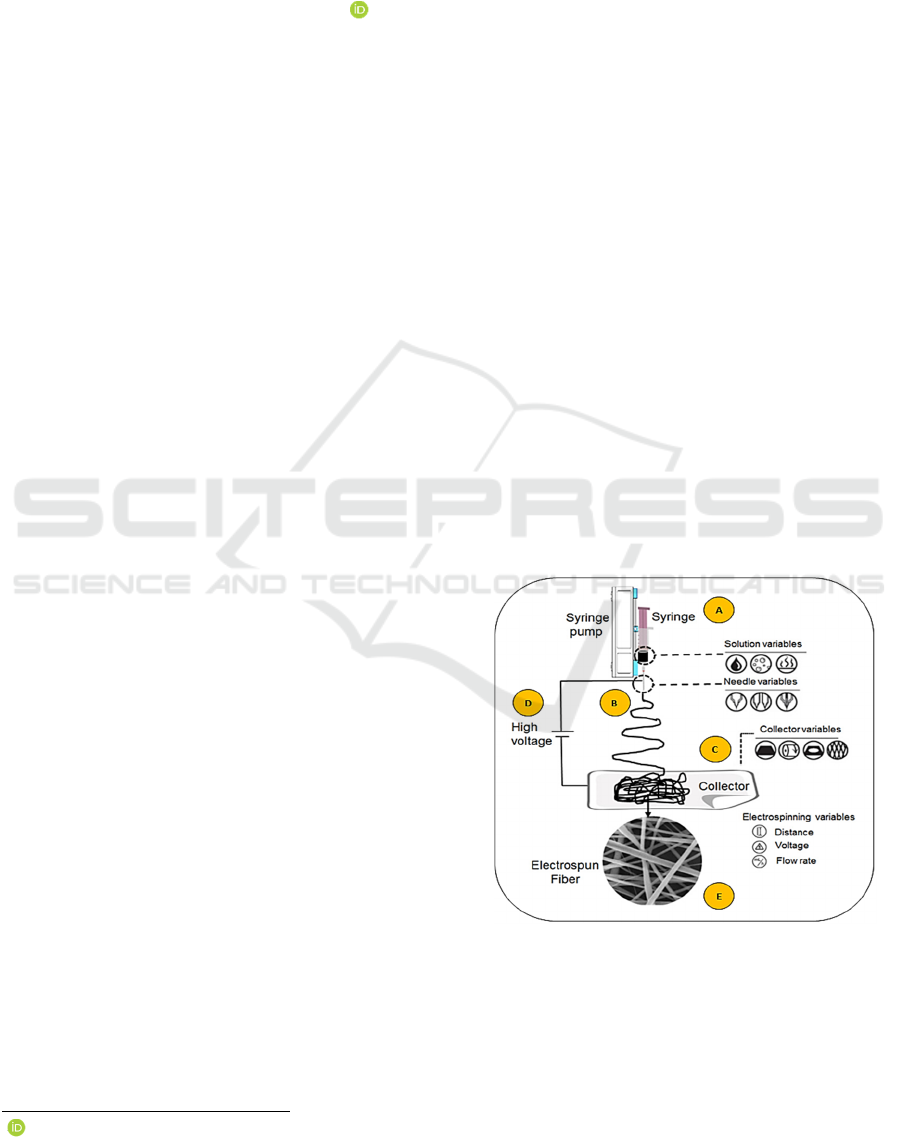
on the use of a system (Figure 1) consisting of a
syringe containing a polymer or a mixture of
polymers in solution in a solvent (A) and a needle (B)
a
https://orcid.org/0000-0002-6234-869X
Figure 1: Electrospining technique (from Liu et al., 2021).
(A) Polymers solubilised in a syringe. (B) Needle. (C)
Collector. (D) Electric field. (E) Biomaterial.
allowing the exit of the polymer towards a collecting
surface (C). An electric field is applied between the
needle and the collector (D) and induces the
Rolin, G., Pinot, K. and Blanchy, M.
Regulatory Positioning of an Innovative Biomaterial for Regenerative Medicine: TissYou Project.
DOI: 10.5220/0011926300003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 1: BIODEVICES, pages 243-249
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
243
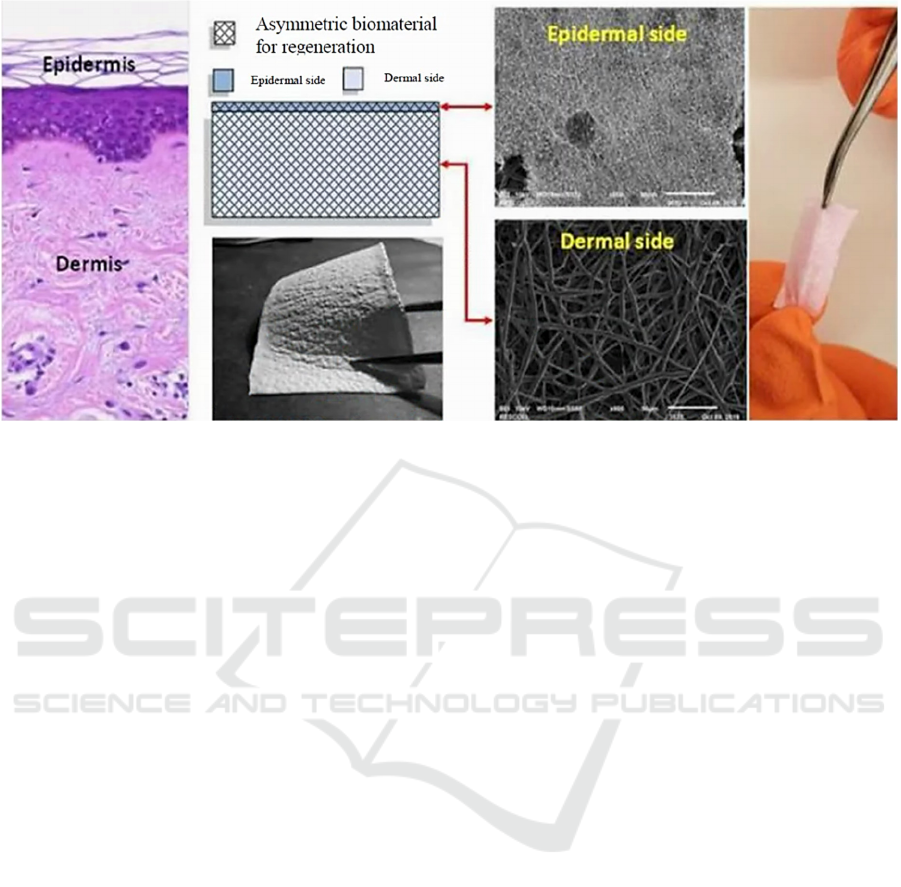
Figure 2: Concept of TissYou product composed of an epidermal and a dermal side to mimic human skin architecture.
formation of "solid" fiber from the raw material in
solution contained in the syringe.
The electrospining technique (which can be
difficult to master technically) offers the advantage of
being extremely versatile. Indeed, each of the bricks
of the system (A, B, C, D) can be considered as a
variable on which to play to modify the final
characteristics of the biomaterial obtained. Thus, the
system input parameters can be adjusted: type,
molecular weight, viscosity, conductivity, surface
tension of the polymer(s), voltage, flow rate at the
syringe outlet, distance between the collector and the
syringe, environmental parameters of the process
(humidity, temperature). Adjusting these parameters
makes researchers control the morphology of the
obtained material, such as the orientation, the
diameter of the fibers obtained and the porosity (Zhao
et al., 2021; Wang et al., 2020). Faced with such
possibilities, electrospining is clearly considered as
one of the gold standard to produce material that can
faithfully mimic an extracellular matrix for
applications in Regenerative Medicine (Phang et al.,
2022).
Building on the interest of electrospinning for the
production of material used in the composition of
medical devices, our preclinical and clinical research
teams (Inserm UMR 1098 and Inserm CIC 1431)
have been working together for several years on R&D
phases, which have now resulted in the production of
an innovative biomaterial. We currently characterize
in vitro the scaffold that seems to reproduce the
architecture, composition and physical properties of
human skin. Grafted on the patient, should boost
tissue regeneration. Thanks to its particular
properties, this biomaterial could also be used as
artificial skin for in vitro tests and thus replace animal
models. This biomaterial is called TissYou. It is also
the name of the European project which was financed
(Eurostars, 2021-2024) and which allows the
continuation of work on a European scale. Indeed, to
allow future patients to benefit from the results of this
research, pre-clinical and clinical studies will still
have to validate the safety and efficacy of this
innovation in Regenerative Medicine. The state of
progress of the project leads us today to have in our
hands a functional prototype on the way to becoming
a finished product (Figure 2).
The objective of the work presented here was first
of all to describe the context of the progress of the
project and the short-term objectives from a
regulatory point of view. Indeed, and to ensure the
transition of this product from R&D to a possible
medical device, it is necessary to prepare as much as
possible a precise regulatory roadmap that drives the
future product development. Consequently, and
relying on the European regulation and its annexes,
the main objective of this paper was to demonstrate
that the product meets the definition of a biomaterial,
a medical device, to precisely define the class to
which it belongs, and to start a risk analysis process.
In order to stabilize the perimeter of the future
indication of the product and feed the future risk
analysis, we also worked on a questionnaire deployed
among professionals in order to collect their user
needs.
ClinMed 2023 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
244
Table 1: Justification for medical device classification.
2 METHODOLOGY
In order to position the TissYou product in the right
regulatory context, and to place our object on the
regulatory roadmap that awaits it in its regulatory
journey as a medical device, we will rely in particular
on the European regulation and its annexes to
demonstrate that the product meets the definition of a
biomaterial, and that of a medical device. Then we
will precisely define the class to which it belongs, and
will start a process of defining the standards that will
be appropriate to apply in the continuation of the
qualification of the product. In order to stabilize the
perimeter of the future indication of the device, we
will also present a questionnaire deployed among
professionals in order to collect their user needs and
the results that we have already been able to obtain.
3 ANALYSIS
3.1 Regulatory Position
3.1.1 Is our Product a Biomaterial?
According to the definition of the European Society
for Biomaterials (European Society for Biomaterials),
a biomaterial is defined as a : “Material intended to
be in interaction with a biological system with the aim
of evaluating, treating, increasing or replace a tissue,
organ or function of the human body”. TissYou is a
material intended to interact with the human body, the
skin in particular, with the aim of supplementing skin
healing. The notions of interaction, replacement and
supplementation of a function of the organism are
very present. The TissYou product can therefore be
qualified as a biomaterial.
3.1.2 Is our Biomaterial a Medical Device?
According to European regulation MDR
2017/745/EU which entered into force on May 26,
2021, a medical device meets the following
definition. Is considered as a “medical device”:
Any instrument, device, equipment, software,
implant, reagent, material or other article, intended by
the manufacturer to be used, alone or in combination,
in humans for one or more of the following specific
medical purposes:
• Diagnosis, prevention, control, prediction,
prognosis, treatment or alleviation of a disease,
• Diagnosis, control, treatment, mitigation of
injury or disability or compensation thereof,
• Investigation, replacement or modification of
an anatomical structure or function or of a
physiological or pathological process or state,
• Communication of information by means of in
vitro examination of samples from the human
Article - UE 2017/745 Justification
Article 2 Section 1
Medical device
The biomaterial is a material, intended for use in humans, with the aim of treating
traumatic wounds with extensive loss of substance, by replacing by replacing or
modifying the physiological process of healing. The biomaterial meets the
definition of a “medical device”.
Article 2 Section 2
Accessory
The biomaterial is a “medical device” and is therefore not a “accessory”.
Article 2 Section 3
Custom-made device
The device is not expressly manufactured according to a prescription and intended
for a single patient. It is not a “custom-made device”
Article 2 Section 4
Active device
The operation of the device does not depend on a source of energy other than that
generated by the human body for this purpose or by gravity. It is not an “active
device”.
Article 2 Section 5
Implantable device
The device aims to replace the skin on an injured surface. It is an “implantable
device”.
Article 2 Section 6
Invasive device
The device is applied to the surface of a wound or damaged skin and penetrates the
body through its surface. It is an “invasive device”.
Annexe VIII 2,2 –
Invasive device, surgical type
The device is an invasive device but is not an “invasive surgical-type device”.
Regulatory Positioning of an Innovative Biomaterial for Regenerative Medicine: TissYou Project
245
body, including organ, blood and tissue
donations,
and whose principal intended action in or on the
human body is not obtained by pharmacological or
immunological means or by metabolism, but whose
function can be assisted by such means.»
With the help of the European regulation and the
articles that make it up, we have chosen as a strategy
for determining membership in the category of
medical devices the creation of a checklist in order to
answer point by point the questions that must be
asked (Table I). In the light of the answers provided
according to the characteristics noted, the TissYou
biomaterial can be considered as a medical device. It
is not an accessory of DM nor of a custom-made or
active DM. On the other hand, TissYou is an
implantable MD, invasive but not of the surgical type.
3.1.3 What Class of Risk for TissYou ?
MD classification, as defined by European
legislation, is a risk-based system that takes into
account the vulnerability of the human body and the
potential risks associated with the devices.
This approach uses a set of criteria that can be
combined in various ways to determine classification,
such as duration of body contact, degree of
invasiveness, local vs systemic effect, potential
toxicity, body part affected by use of the device and
whether the device depends on an energy source.
The "classification rules" set out in Annex VIII of
Regulation (EU) 2017/745 relating to medical
devices (MDR) were used and transcribed in the form
of a checklist (Table II) in order to determine the class
of membership of our medical device “TissYou”.
Thus, for each of the 22 stated rules, the notion of
applicability has been defined and justified with
regard to our product. In accordance with the
answers provided, the TissYou medical device can
be classified as a class III medical device, the class
with the highest level of risk. Indeed, the device is an
implantable device, absorbed in whole or in large
part, and is intended to undergo a physico-chemical
transformation in and by the body. Furthermore, the
device is composed of combinations of substances
intended to be introduced into the human body by
application to damaged skin and absorbed by the
human body or dispersed locally therein. The
substances in question, or the products of their
metabolism, are systemically absorbed by the human
body in accordance with the intended purpose of the
Device.
3.1.4 What Intended Use for TissYou?
The skin is an essential organ for maintaining the
homeostasis of the human body as a whole. However,
being the first barrier against the external
environment, skin tissue can be easily damaged,
leading to the formation of an injury. The severity of
these injuries is variable and depends on the layers of
skin affected.
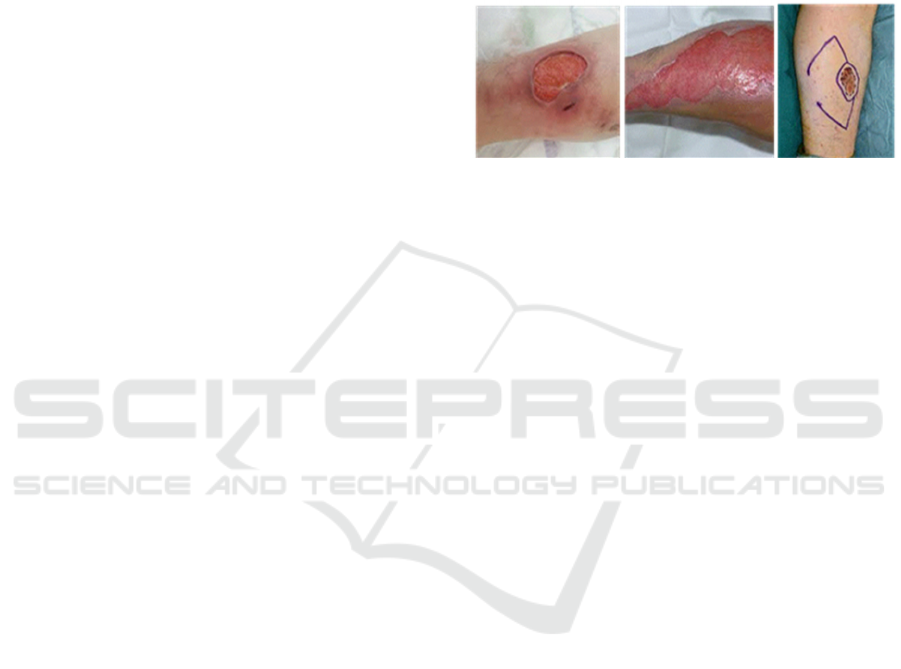
Figure 3: Example of clinical indication addressed by
TissYou. From left to right: leg ulcer, burn, and skin cancer.
In response to this injury, a physiological repair
process takes place: healing. Healing is an extremely
complex and regulated phenomenon that can be
summarized in 4 major phases: hemostasis,
inflammatory phase, proliferative phase, and
remodeling phase. During serious injuries, the dermis
is no longer able to regenerate properly. One of the
therapeutic management strategies may then consist
of the use of equivalent dermis / regeneration matrix.
Three main clinical indications (Figure 3) have been
considered in terms of application of our future
medical device TissYou: chronic wounds, burns,
reconstructive surgery after surgical resection.
Depending on the intended indication, the
properties of the biomaterial may not be the same.
From a regulatory point of view, the risk analysis,
which will have to be carried out to feed the
documentation relating to our medical device in order
to converge towards its CE marking, will also greatly
depend on the clinical indication. In the current state
of development of the TissYou biomaterial, a firm
indication has not yet been chosen. Nevertheless, for
the reasons mentioned above, and in order not to slow
down the progress of the translational project, this
choice must be made quickly.
Today the project team working around TissYou
does not include a committee of clinical experts. As
part of the work presented here, we then wrote and
distributed a questionnaire intended for future users /
endusers of our biomaterial. This questionnaire
(Figure 4) aimed to achieve several objectives:
• Define the best indication for TissYou
according to user needs,
ClinMed 2023 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
246

Table 2: Justification of the risk class assigned to the device (for full definition: 2017/745:
http://data.europa.eu/eli/reg/2017/745/oj).
Classification rules
(Appendix VIII)
Applicability Justification
1 to 4: Non-invasive device Non applicable The device is an invasive device.
5: Invasive devices with
respect to bod
y
orifices
Non applicable The device is unrelated to body orifices and is not a surgical
t
y
pe invasive device.
6 et 7: Transcient/ shor-
term use
Non applicable The device is intended for long-term use (more than 30
days) because, the scaffold will be graft on the skin and will
be biodegraded by the organism over time (> degradation
rate > 30 da
y
s).
8: Implantable device
classification?
Applicable
Classe III
The device is an implantable device, absorbed in whole or
in large part and is intended to undergo chemical
transformation in the bod
y
.
9 to 13: Active device Non applicable The device is not an active device.
14: Drug incorporation Non applicable The device does not incorporate any substance that could be
considered a dru
g
.
15: Contraception or STD
p
revention
Non applicable The device is not used for contraception or to prevent the
transmission of STDs.
16: Desinfection or
sterilization
Non applicable The device is not intended to disinfect, clean, rinse or
moisturize contact lenses or Class IIa devices.
17: X-ray radiation Non applicable The device is not intended to record diagnostic images
g
enerated b
y
X-ra
y
irradiation.
18: Tissue or cell origin Non applicable The device is not manufactured from tissues or cells of
human or animal ori
g
in or their derivatives.
19: Nanomaterial
incorporation
Non applicable The device does not incorporate nanomaterials and does not
consist of them.
20: Medicinal product
inhalation
Non applicable The device is not related to the body orifices and are not
intended to administer medicinal product.
21: Human body absorption Applicable
Classe III
The device is composed of combinations of substances
intended to be introduced into the human body by
application to damaged skin and absorbed by the human
body or dispersed locally in it. The substances in question,
or the products of their metabolism, are systemically
absorbed by the human body in accordance with the
intended purpose of the device.
22: Active device Non applicable The device is not an active device.
• Collect information on user expectations
vs competitiors,
• Obtain data allowing us to develop our
biomaterial in order to meet closely their needs,
• Propose to clinicians to join us as an
expert,
• Propose to the clinicians contacted to be
those who will participate in the first clinical and
usability trials,
• Obtaining other user contacts with a
“close by close” strategy, with clinicians sending
us the contact details of potentially interested
colleagues.
Regulatory Positioning of an Innovative Biomaterial for Regenerative Medicine: TissYou Project
247

To date, our questionnaire was distributed to 12
clinicians (Dermatologists and Plastic surgeons)
working at the Besançon University Hospital or at the
Nord Franche-Comté Hospital in Trevennans
(France). It was sent in Word format as well as in
Googleform to facilitate data extraction.
Among the responding clinicians, 1/3 have
already used dermal substitutes and 2/3 have never
experienced such device. The dermal substitutes
usually used are Integra® and Matriderm®. The
indications leading to the use of a dermal substitute
are: preparation for thin skin grafts and resurfacing.
The complications encountered were superinfection,
lack of integration of the biomaterial and detachment
of the basement. Regarding the manipulation of the
dermal substitute, the surgeon is not the only user.
Nurses and interns may also be required to handle it.
The reasons that hinder practitioners from using
dermal substitutes are the lack of easy availability of
such medical devices, the lack of habit of use on this
subject, their cost and the difficult match with the
topography of deep wounds. The reasons that could
lead clinicians to use more dermal substitutes are: a
shortening of the healing time, better functional and
aesthetic results, the management of wounds, an
effective and permanent discharge of chronic
wounds, the treatment of a superficial wound, the fact
that the device is available, ergonomic and at a low
cost. Clinicians would also prefer the device to be
ready-to-use and not sutured.
4 CONCLUSION
TissYou is a bicomposite biomaterial produced by an
optimized electrospinning technique. The material is
said to be bicomposite because it is composed of two
polymers: a natural and a synthetic one. In order to
ensure the transition of this product from R&D to a
possible medical device, the regulatory roadmap that
awaits the future product should be prepared as much
as possible.
To achieve this objective, we have shown here
that our product meets the definition of a biomaterial
and more specifically that of a medical device within
the meaning of European Regulation 2017/745.
Appendix VIII and the positioning of our product
have enabled us to show that our product consists of
a class III, invasive and implantable medical device.
Nevertheless, there remains uncertainty about the
positioning of medical devices as an invasive product
and about the proposed definition of invasiveness.
Interpretation of the definition may not be as clear as
expected with respect to the use of the device on
injured skin and the interface with the inside and
outside of the body. To contribute to progress on this
subject, a study of the competition has been initiated
in order to compile the information available, in
particular via the ANSM's documentary base to
parallelize us with the devices of the same type
already on the market.
Still based on the regulations, and thanks to
appendix II (section 6.1 and 6.2), we will also list the
verification and validation elements to be provided in
the technical documentation of our future MD. Thus,
the output data from appendix II will allow us to
orient ourselves towards the standards that will have
to be followed and respected within the framework of
our work, in particular standard 10993 devoted to the
biological evaluation of medical devices. Other
standards have also been identified which would also
be applicable to the future DM TissYou (data not
shown). This non-exhaustive list will be completed,
in particular in parallel with the performance of a risk
analysis. This analysis may begin as soon as the
indication for use of the TissYou device is fixed.
Indeed, for the time being, the indication for use is not
defined. Several avenues have been suggested
(chronic wounds, burns, reconstructive surgery) as
shown previously.
In order to converge towards a choice based on the
desire to respond strongly to an unmet clinical need,
we decided during this work to build and distribute a
questionnaire to health professionals with several
objectives: to collect information to develop our
biomaterial, better meet their needs, choose the right
indication, create a network of experts. The data
collection is not finished but already many relevant
answers and comments allow us today to validate
some of our technical choices (thickness and
mechanical properties), to rule out indications not
favorable to the use of our product (chronic wound),
to identify possible indications. The choice of an
indication, and therefore of specific claims, will help
define what preclinical steps will be taken and which
remain ahead of us, namely the trials in small and
large animals that we will have to conduct soon.
In conclusion, the work presented has triggered a
regulatory switch in the way the TissYou project is
viewed until today. Indeed, the available prototype,
and soon to become a finished product, only has a
chance of becoming a medical device with a place on
the market if it meets a strong clinical need. This is
the challenge of positioning our product quickly in
front of a precise indication. We will thus be able to
take a further step on the regulatory roadmap which
will lead us to carry out work contributing to meeting
the requirements of the regulations, to demonstrating
ClinMed 2023 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
248

the safety and then the effectiveness of our class III
medical device and to being able to file medium term
a CE marking dossier.
ACKNOWLEDGEMENTS
TissYou Project “Industrial production of a
biomimetic matrix for cutaneous regenerative
medicine and in vitro research (n°E!115719)” is
funded by Eurostars-2 from European union (Horizon
2020) and BPI France.
REFERENCES
Aidana Y., Wang Y., Li J., Chang S., Wang K., Yu D.-G.
Fast dissolution electrospun medicated nanofibers for
effective delivery of poorly water-soluble drugs. Curr.
Drug deliv. 2021;18
Boys CV On the Production, Properties, and Some
Suggested Uses of the Finest Threads. Proc. Phys. Soc.
London 1887, 9, 8201319.
Liu X, Xu H, Zhang M, Yu DG. Electrospun Medicated
Nanofibers for Wound Healing: Review. Membranes
(Basel). 2021;11(10):770.
Phang SJ, Basak S, Teh HX, Packirisamy G, Fauzi MB,
Kuppusamy UR, Neo YP, Looi ML. Advancements in
Extracellular Matrix-Based Biomaterials and
Biofabrication of 3D Organotypic Skin Models. ACS
Biomater Sci Eng. 2022 Aug 8;8(8):3220-3241.
(UE) 2017/45 : https://eur-lex.europa.eu/legal-content/FR/
TXT/PDF/?uri=CELEX:32017R0745
Wang C., Wang J., Zeng L., Qiao Z., Liu X., Liu H., Zhang
J., Ding J. Fabrication of electrospun polymer
nanofibers with diverse morphologies. Molecules.
2019; 24:834.
Wang M., Yu D.-G., Li X., Williams G.R. The development
and bio-applications of multifluid electrospinning.
Mater. Highlights. 2020;1:1–13.
Zhao K., Kang S.X., Yang Y.Y., Yu D.G. Electrospun
functional nanofiber membrane for antibiotic removal
in water: Review. Polymers. 2021;13:1–33.
Regulatory Positioning of an Innovative Biomaterial for Regenerative Medicine: TissYou Project
249
