
Shaping User-Centered Health Innovation Through Assessment
Arthur Trognon
1,2,3,*
, David Servais
3,4
, Islem Habibi
2,3
, Robert Picard
3
, Thomas Lihoreau
3,5,6
,
Lionel Pazart
3,5,6
, Sylvia Pelayo
3,7,8
, Thierry Chevallier
3,6,8
, Kathryn Ernecq
3,9
, Anaïs Garin
3,10
,
Mathias Béjean
3,10
and Denis Abraham
1,3
1
Association Innov’Autonomie, Nancy, France
2
Clinicog, Nancy, France
3
Consortium DynSanté
4
Forum des Living Labs en Santé et en Autonomie (LLSA), Paris, France
5
Inserm CIC 1431, CHU Besançon, F-25000 Besançon, France
6
Tech4Health Network - FCRIN, France
7
Université de Lille, Lille, France
8
University Hospitals of Nîmes, France
9
Frog Part of Capgemini, Paris, France
10
Paris Est Créteil University, IRG, F-94010, France
Keywords: Maturity Evaluation, Technologies for Health, Medical Devices, Computational Psychology, Innovation.
Abstract: Historically, evaluation methods for innovative projects have focused mainly on technological development
aspects. However, recent research suggests that, in the context of consumption by the general public, the
decision parameters for acceptance seem to be based more on characteristics extrinsic to technological
maturity. In the present work, we present a model for the evaluation of innovative projects, the Concept
Maturity Level Santé France model, inspired by the CML model developed by the National Aeronautics and
Space Administration and specified in the context of MedTech project development, and placing co-design
with the end-user and its ecosystem on the same level of importance as the regulatory and technological
development aspects, and giving it a theoretical and fundamental basis.
1 INTRODUCTION
Traditionally, the evaluation of innovative systems
has focused on risk prevention in three challenging
areas: performance, schedule, and budget (Mankins,
2009). Such evaluation should be incorporated in
each step of the life cycle of new systems in order to
avoid products failure and anticipate technical risks
and needs.
In order to standardize the evaluation of these
aspects of research and development, and project
programming, a number of tools have been
developed, such as the Technology Readiness Levels
(TRLs) grid, which was developed by the National
Aeronautics and Space Administration (NASA) in the
early 1970s and completed in 1995 (Mankins, 1995).
It was initially developed to standardize the
assessment of the maturity of spaceflight projects
through a technology readiness assessment (TRA)
examining key concepts, technological needs, and
demonstrability, while taking into account economic
aspects, making it possible to establish an inventory
of risks as well as to produce a standard
understanding of technological status (Dawson,
2013). However, while this model and its direct
descendants such as the Concepts Maturity Levels
model (Wessen et al., 2013) seem relevant in the
evaluation of institutional projects, it appeared
limited in the evaluation of subsequent acceptability
if the device were placed in the hands of a wider
population (Salazar &Russi-Vigoya, 2021). These
authors have highlighted that the TRL scale does not
allow the reading of the ease of use, the satisfaction
of the final user, the human performance in the use of
the device, as it does not allow the reading of the
comprehensiveness of the program or the device as
well (See et al., 2019). Thus, the grid does not seem
exhaustive in an innovation approach centered on the
user and not the technology per se. It is also of interest
to mention that TRL scale is used largely in different
Trognon, A., Servais, D., Habibi, I., Picard, R., Lihoreau, T., Pazart, L., Pelayo, S., Chevallier, T., Ernecq, K., Garin, A., Béjean, M. and Abraham, D.
Shaping User-Centered Health Innovation Through Assessment.
DOI: 10.5220/0011925800003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 1: BIODEVICES, pages 229-242
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
229

fields, for instance by funders for tender of calls in
health research, asking candidates to estimate their
pre and post project TRL level, and justifying
consequently the improvement of maturity thanks to
the activities they wish to develop thanks to the
grants.
Indeed, it would seem that the technology
development factor represents only one aspect of the
decision-making process for the acceptability of a
device by a user (Claudy et al., 2015). Consumer
Behavioral Reasoning Theory predicts that the main
barriers to acceptability of innovative devices lie in a
trade-off between the use value and the functional
barriers perceived by the user (ibid.).
However, and as highlighted by (Claudy et al.,
2015), while the number of works investigating
resistance to innovation has increased significantly in
recent years, the majority of works are conceptual and
no operating system has yet been proposed,
particularly for the French and European MedTech
ecosystem.
In response to these challenges, several key
players in the French Medtech ecosystem have
undertaken an initiative codenamed "CML Santé
France", for Concept Maturity Levels, aiming to
establish a more inclusive, legible and structured
process for collaborative innovation processes in the
French Medtech ecosystem (Béjean et al., 2021, ANR
Dynsanté). This approach differs from other existing
more 'top-down' approaches (e.g. CIMIT) in that it is
highly participatory and community-based.
Mobilizing a national network, this endeavor brings
together, since 2017, partners covering the entire
Medtech value chain, from research labs to start-ups
and SMEs, including the Clinical Investigation
Centers in Technological Innovation (Inserm CIC-IT)
of the National Institute of Health and Medical
Research (INSERM) as well as new types of actors
called "Living Labs", the initiative having been
driven by the Forum of Living Labs in Health and
Autonomy (LLSA Forum).
So far the "CML Santé France" initiative has
resulted in the formalization of a vocabulary
associated with the design process of an innovative
dispositive, from the formulation of the initial idea
(CML1) to the post-industrialization follow-up of the
solution (CML9), passing through various
intermediate evaluation stages. This process provides
a methodological framework that integrates (i) the
definition of 9 levels of maturity, (ii) concrete actions
to structure the maturation activities for each level;
(iii) a mapping of the tools and skills needed to carry
them out. All of this was integrated into a
collaborative platform developed by the start-up
Agile Solutions. Consortium DynSanté, an ANR
program, was further constituted to further develop
and test the use of CML Santé France. Dynamically
integrating the CMLs Santé, the "CML Santé Forum"
platform has been used on concrete projects since
2019.
In the present work, we will present the "CML
Santé France" model, and the extent to which it
addresses the contemporary challenges of innovation
design in a user-centered approach in the context of
Health. Through the use of CMLs, we suggest a tool
aiming to participate in facing the very actual
demanding context around particularly medical
devices, that must anticipate the complex and
multidimensional regulatory, industrial, and clinical
evidence aspects as well.
2 THE IMPORTANCE OF NEEDS
AND USAGE VALUE
Innovations in technology are accelerating at a rapid
pace, in a way that the user can barely get used to a
technology, the following one that has just come out.
Innovation levels of the newly developed and highly
sophisticated systems do not reflect its degree of
acceptance by consumers admitting that innovative
products mean change for consumers, and resistance
to change is a common consumer response that must
be overcome before adoption can begin and that
consumers would instead prefer efficient and easy-to-
use systems that meet their needs (Laukkanen et al.,
2007). The application of self-concept to customer
behavior suggests that customers purchase products
and/or brands that are similar to their own self-
concept (Sirgy, 1982).
Hence the importance of integrating the concept
of user acceptance and satisfaction of needs in the
marketing approach of the product (Dunphy
&Herbig, 1995). In this direction, some
manufacturers rely on Kano model (KANO et al.,
1984) which has been widely adopted by industries
and consists of classifying and prioritizing customer
needs based on how they affect customer satisfaction.
(KANO et al., 1984). This customer survey-based
model (table 1) is able to illustrate the relationship
between product performance and customer
satisfaction in four types of product attributes: (1)
must-be attributes are expected by the customers and
they lead to extreme customer dissatisfaction if they
are absent or poorly satisfied, (2) one-dimensional
attributes are those for which better fulfillment leads
to linear increment of customer satisfaction, (3)
ClinMed 2023 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
230

attractive attributes are usually unexpected by the
customers and can result in greater satisfaction if they
are available, and (4) indifferent attributes are those
that the customer is not interested in the level of their
performance (KANO et al., 1984; Xu et al., 2009).
Statistics showed that kano model-based marketing
strategies were positively influenced by the model
(Asian et al., 2019; Huang, 2017; Rotar&Kozar,
2017). On the other hand, the theory of reasoned
action suggests that consumers evaluate innovations
in regard to product attributes like relative advantage,
compatibility, and complexity, which have a strong
influence on their adoption decision (Claudy et al.,
2015b; Fishbein & Ajzen, 1977).
Before buying a product, customers have the
intention to consult different information sources
helping to decide which product to choose (Broilo et
al., 2016; Nici&Creutlein, 2017). To deal with the
increasing volume of information that may be false
and/or negatively influence customers, it may be
essential to include a usability evaluation in a
simulated environment. Such an approach reminds
the third attribute in Kano model product attributes
highlighting the importance of a pre-purchase study
englobing the product usage in a realistic
environment not only to stand out from competitors
(Joachim et al., 2018) but also to aid the consumer
decision-making (Broilo et al., 2016).
(Claudy et al., 2015b) analyzed the behavior of
users toward innovation and showed that there are 5
factors that manufacturers have not paid attention to
and which lead to user resistance to innovation. These
factors are subdivided into two categories: functional
and psychological barriers. Functional barriers
include usage, value and risk barriers that consumers
may associate with a new product or service whereas
psychological barriers are issues that consumers may
experience when innovations force them to change
existing beliefs or break with traditions and norms
(Antioco&Kleijnen, 2010; Claudy et al., 2015b).
Customers do evaluate both the reasons for and
against adoption, which can have a greater influence
on consumers' adoption decisions. This result reflects
the behavior of consumers in the market in a positive
or negative way. For example, in the first study
conducted by Claudy et al. (Claudy et al., 2015b)
reasons against adoption: high upfront costs,
perceived incompatibility with existing
infrastructure, and uncertainty regarding overall
performance; have a stronger influence on the
consumer adoption decision than reasons for
adoption: energy cost savings, environmental
benefits, and being independent from conventional
sources of energy like oil or gas; which influence
intentions only indirectly via attitudes (i.e. the
psychological tendencies that are expressed by
evaluating a particular entity with some degree of
favor or disfavor (Eagly and Chaiken, 1998)). These
results have helped managers to focus on overcoming
barriers to adoption, instead of over-emphasizing
reasons for adoption in order to improve the diffusion
of their product in the market. Unlike study 1, study
2 showed that reasons for adoption: saving money,
convenience and flexibility; have a stronger influence
on customer adoption decisions than reasons against
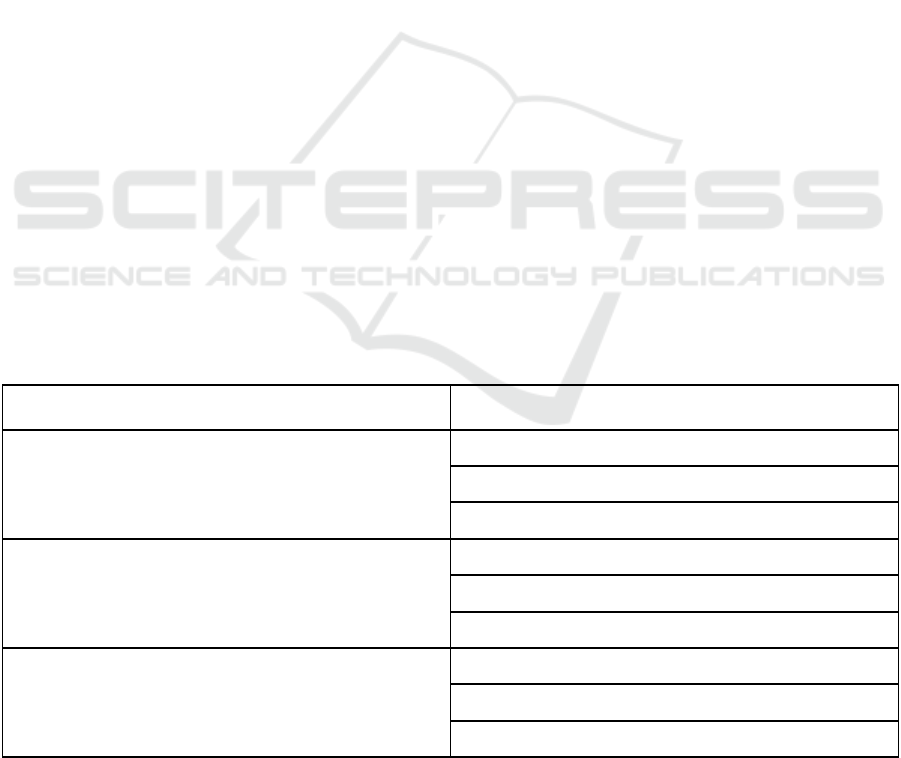
Table 1: Example of Kano items. (Xu et al., 2009).
Kano question Answer
Functional form of the question
(e.g., if the car has airbags, how do you feel?)
● I like it that way
● It must be that way
● I am neutral
● I can live with it that way
● I dislike it that way
Dysfunctional form of the question
(e.g., if the car does not have airbags, how do you feel?)
● I like it that way
● It must be that way
● I am neutral
● I can live with it that way
● I dislike it that way
Shaping User-Centered Health Innovation Through Assessment
231

ClinMed 2023 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
232
adoption: availability and security; and that reasons
against adoption influence customer decisions only
indirectly via attitudes. Reasons for and against
adoption were elicited by a group of nearly equal
numbers of males and females, and it included
different age groups and educational levels (Claudy
et al., 2015b). These findings insist on integrating the
customer self-concept in the marketing strategies at
the pre-purchase phase to help businesses in
identifying the required needs they must fulfill
(basic), characteristics they should be competitive
with (i.e. performance) and the advantages making a
differential in the eyes of the customer (i.e.
excitement) (Tontini, 2007).
Specifically for medical devices we can also cites
the “IEC 62366 standard for usability engineering to
medical devices”, as well as the medical device
regulation (MDR) (EU) 2017/745 that cites explicitly
the importance of usability and users, to take into
account also for pre clinical and clinical evaluation.
3 THE FRENCH CML HEALTH
MODEL
The French CML Health (CMLH) model is an
iterative reading grid that decomposes the innovation
process into three interdependent axes: needs,
technology, and programmatic. It is therefore a direct
descendant of the original CML model, which
integrates these last two domains, by completing
it with an equivalent user-centered axis. It also
specifies the two original domains of technology
and programmatic in order to adapt them to the
French and European regulatory specificities in terms
of research methodology and data management
(Table 1).
3.1 Technological Maturity
An example of the milestones used to assign maturity
levels on the technology axis is shown in Table 2.
The first axis of the CML Santé France model comes
from the direct heritage of the TRL model mentioned
above. It assesses the development of technological
concepts, the management of the products that it
allows to obtain, as well as their ownership, by being
formalized on three axes: technological development,
data management, and intellectual property. The first
axis of technological development allows us to
gradually assess, on a scale of 1 to 9, the development
processes from the evaluation of the bibliographic
state of the art, through its critical functionality
simulations, to the management of the product life
cycle. The second axis permits us to appreciate the
way in which the project leaders will manage the data
resulting from their own devices, from the R&D data
(including bibliographic) to the protocols allowing
their protection as well as the automation of the
product life cycle data. Finally, its last axis of
intellectual property gives an insight about the
competitive study, from the monitoring of existing
patents to the management of infringements that
could emerge.
Table 2: Factorial structure of the French CMLH model
Domain Sub-domain
Needs
Usa
g
e
Marke
t
Clinicalproofs
Technology
Technicaldevelopmen
t
Data mana
g
emen
t
Intellectualpropert
y
Schedule
Pro
j
ect mana
g
emen
t
Re
g
ulation
Fundin
g
Shaping User-Centered Health Innovation Through Assessment
233
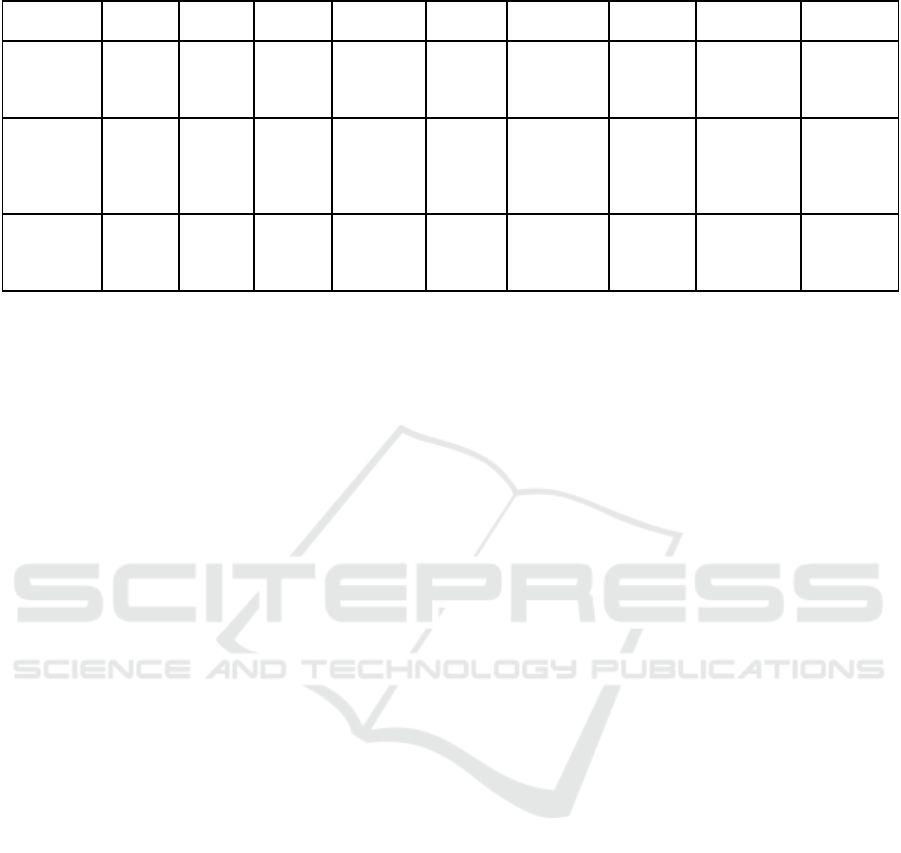
Table 3: Example of the different maturity levels for each sub-area of the "Technological Maturity" domain.
CML1 CML2 CML3 CML4 CML5 CML6 CML7 CML8 CML9
Technological
development
State of
the art
Theorizing Functional
simulation
Software
demonstrator
Prototype
alpha
Technological
analysis for
improvement
Automation
of function
testing
Software bug
reports and
corrections
Product
lifecycle
management
Data
management
/ R&D data
collection
Software
data
structure
Cybersecurity Data
availability
Exploitation
of clinical
data
Data server
access
Implementation
of data
collection
devices
Production of
material-
epidemiology
data
Intellectual
property
Patent
monitoring
Patent-in-
principle
Specific
patents
Freedom of
operation
Process
patents
Intellectual
property of
clinical data
/ / Competitive
intelligence
3.2 Programmatic Maturity
An example of the milestones used to assign maturity
levels to the Programmatic axis is presented in Table
3.
The second axis of the CML Santé France model
is the first real adaptation of the CML model as
developed by NASA. It conceptually takes up the
project management axis as well as part of its
regulatory axis, specifying it for the European and
French particularities which are organized for the
devices themselves (everything for access to market,
under the CE mark certification), but also for the
researches that focus on. The idea is also to follow the
“Good Clinical Practices” (GCP) and all ethical
principles protecting the individual that participates
in a research, from all kinds of risks when he or she is
involved in research aimed at acquiring new
biological or medical knowledge. It should be
remembered that researches are organized and carried
out on healthy or sick volunteers with a view to
developing new knowledge in the biological or
medical fields, and that the regulatory framework in
France is based on the european regulations, and with
recent updates for innovation that claims a medical
devices status (“clinical investigations” categories
proposed by ANSM french authority). It is also a
matter of ensuring that the methods for collecting and
processing health data comply with the General Data
Protection Regulation (GDPR) as well as the French
reference research methodologies (MR-00X), which
range from level 1 to 3.
Thus, the Programmatic axis of the CML Santé
France model enables the programmatic maturity of
the project to be assessed in three areas: project
management, regulatory aspects, and financial
aspects. The first axis, project management, is used to
assess the consortium formed for the project, from the
identification of the pilot to the renewal of the
development partnership, including the creation of
Test Beds, by assessing the nature of the partnerships
created. The second axis, called regulation, evaluates
the programmatic maturity from the first legal
investigation surrounding the project to the renewal
of the CE mark, including product risk analysis and
compliance with European (e.g. MDR, RGPD…) and
French (i.e. ethics; clinical investigations for medical
devices…) regulatory constraints. Finally, the last
axis, called financing, allows for a gradual evaluation
of the financial aspects, ranging from the
identification of potential sources of financing to the
updating of business economic assumptions in line
with the real-life use data of the device.
3.3 Needs Maturity
An example of the milestones selected for the
assignment of maturity levels on the Need axis are
presented in Table 4.
Finally, the last axis of the CML Santé France model
is the real innovation of the consortium in the
specification of the CML model as described by
NASA. It integrates the elements of the theory of
consumer behavior and in particular the jargon of its
barriers within the CML grid and thus makes it
possible to evaluate maturity in terms of needs on
three axes: uses, market, and clinical evidence.
Therefore, the first axis, Uses, provides an insight into
the value of use of the device as well as a metric of
the development process to ensure that the user has
been put at the center of the product development in
terms of uses. This axis allows us to gradually assess
its development from the identification of the social
context and in terms of public health, to the methods
of evaluation of the quality of care perceived by the
patient (PREM), through the performance of
ClinMed 2023 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
234
acceptability studies. This axis thus makes it possible
to verify the removal of certain functional barriers, in
particular the conflict with usage patterns as
described by (Ram &Sheth, 1989). The second axis,
called the market axis, allows us to obtain information
on the competitive study that was carried out in terms
of uses, in particular from the establishment of a
review of the market literature to the evaluation of the
multicentricity of the market segments, including the
respective market access strategies. This axis thus
allows us to verify the removal of functional value
barriers as described by (Molesworth &Suortti,
2002), in particular by verifying the uniqueness of the
value proposition conveyed by the device. Finally, the
last axis, called Clinical Proof, makes it possible to
assess the quality of the clinical investigation that the
device has undergone, up to the establishment of
fundamental proofs through an exhaustive analysis of
the literature, up to the formalization of the processes
for evaluating the quality of the results of the device
as perceived by the patient (PROM). It is this last axis
that will manage the functional barrier of uncertainty
as described by (Stone &Grønhaug, 1993) and which
occurs when end-users have only limited access to
devices under development.
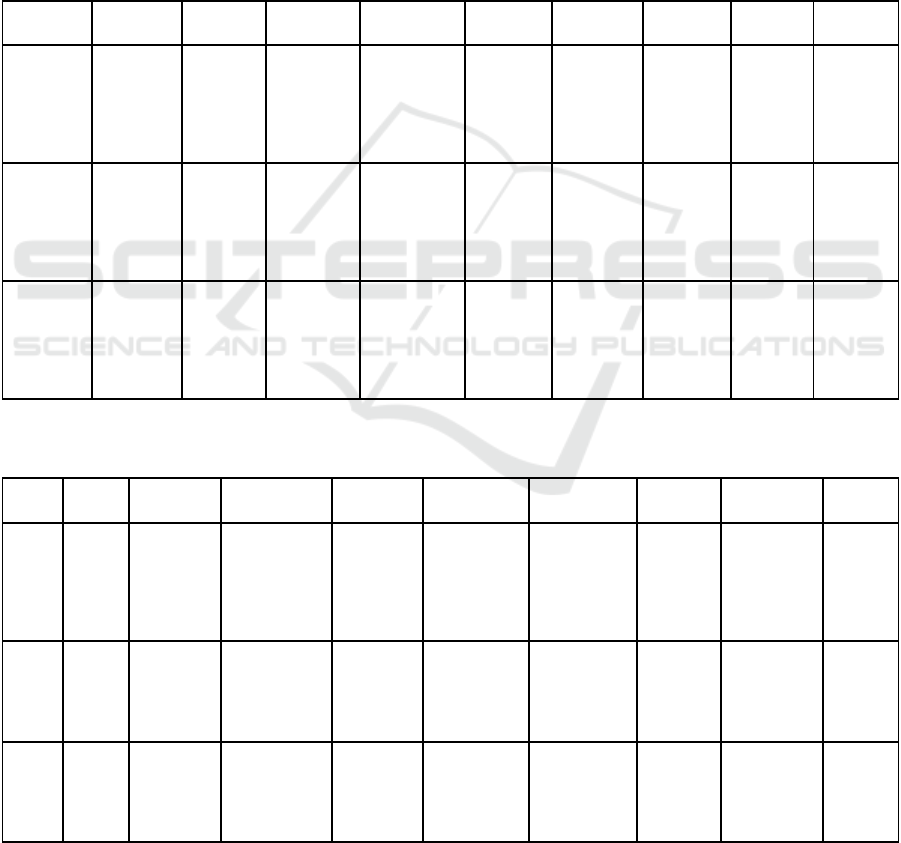
Table 4 : Example of the different maturity levels for each sub-area of the "Technological Maturity" domain.
CML1 CML2 CML3 CML4 CML5 CML6 CML7 CML8 CML9
Project
mangement
Driver
identification
Initial
analysis of
the project
risk
Tests beds Identification
of
complementa
ry skills
required
Detailed
development
plan of the
solution
Update of
project
elements and
risks
Identification
of marketing
and sales
skills
Closing of
the project
Review of
industrial
development
partnerships
Regulation Regulatory
framework
RGPD
Compliance
/ Ethical
analysis of the
product
Collection
of regulatory
data for
clinical
investigation
Consolidation
of the
technical file
for deposit
CE mark file Regulatory
framework
for data use
Renewal of
the CE
marking
Funding Identification
of funding
sources
Preparation
of the
business
plan
Demonstrator
financing plan
Formalization
of the business
plan
Financial
modeling
Minimum
Viable
Business
Model
Series A
Capital
Raising
Updating
economic
assumptions
with real-life
data
Table 5: Example of the different maturity levels for each sub-area of the "Need Maturity" domain (PREM : Patient Reported
Experience Measure ; PROM : Patient Reported Outcomes Measure).
CML1 CML2 CML3 CML4 CML5 CML6 CML7 CML8 CML9
Usage Social
and
public
health
context
Qualification
of a practice
situation
justifying the
need
Co-construction
of adapted
usage scenario
UX/UI lab
evaluations
Definition of the
usage
industrialization
scheme
Usability and
acceptability
assessment
Ecological
evaluation
of a pre-
series
Real-life
organizational
impact study
Quality
control
PREM
Market Review
of the
market
literature
Identification
of the value
proposition
Product
positioning and
expected impact
Quantificatio
n
of the
expected
impact
Market access
strategy
Characterization
of the device on
the basis of
usage surveys
Marketing
elements
(deployment,
export)
Refinement of
go-to-market
strategies by
customer type
Marketing
on
different
markets
Clinical
proofs
Review
of the
clinical
literature
Identification
of the
medical need
Clinical strategy Preliminary
clinical trials
Analysis of
clinical trials
Drafting of the
study report
(publication)
Multi-center
clinical trials
Medico-
economic
studies
Quality
control
PROM
Shaping User-Centered Health Innovation Through Assessment
235

3.4 An Example of the Implementation
of the Grid in a Health Project
In order to illustrate the use of the grid, we will now
present, as an example, some of the results obtained
during a project appraisal that was recently conducted
during the validation studies of the CML Health
questionnaire. They concern a particularly mature
project, but with some rooms for improvement
localized majoritarily at median and very advanced
CML levels.
3.4.1 General Maturity
Results for the self-reported General Maturity
measures for the sample project (anonymized) are
available in Figure 1. Data suggest that the project is
at the CML9 level, with an important homogeneity on
the needs and programmatic domains.
Regarding the general maturity and taking into
account all the sub-domains (Figure 1; Top-Left), the
self-reported data from the project leader suggest that
the entire sample project is at CML9 (M
G
=9), as the
project has exceeded the critical milestones on all the
maturity domains and sub-factors.
Furthermore, regarding maturity by CML domain
and considering the nine CML levels (Figure 1, Top-
Right), the self-reported data show that the most
mature and homogeneous domain is the Needs
domain [µ=5.67±.63], followed by the Programmatic
domain which seems slightly less mature and
homogeneous in its overall development
[µ=5.49±.81]. Finally, the data suggest that the most
heterogeneous domain in its development is the one
covering Technology, despite a higher average
maturity [µ=5.57±.89].
3.4.2 Focus on the Heterogeneity: Looking
at the Technological Domain
The results for the self-reported maturity measures of
the Technology domain are available in Figure 2. The
data suggest that the project is at the CML8 level in
the technology domain, with notable heterogeneity in
its constituent factors.
Regarding the maturity of the Technology domain
and considering all the factors that constitute it
(Figure 2; Top-Left), the self-reported data by the
project owner suggest that the sample project is at
CML8 level (M
T
=8), given that the project could not
exceed the critical milestones of CML9 at the level of
data management and intellectual property.
Regarding the maturity of the Technology domain
by CML factor and considering the nine CML levels
(Figure 2; Top-Right), the self-reported data show
that the most mature and homogeneous factor is
Technology Development [µ=5.81±.39], and whose
factorial CML level is 9. This factor thus contrasts
with the factors measuring data management
[µ=5.2±1.39] and intellectual property [µ=5.25±1.16]
whose data suggest a slightly lower level of maturity
(factorial CMLs at 8) with a more heterogeneous
development.
Regarding the maturity of the Technology
domain, for each CML level and each constituent
factor (Figure 2; Bottom), the self-reported data
suggest several possible areas of improvement.
Regarding intellectual property, these are mainly at
the CML1 level [µ
pi
=4.5±.5]. This contrasts with the
data management factor, where the areas of
improvement are more likely to be found in CML5
[µ
gd
=4±0] and CML6 [µ
gd
=4±0] as well as in CML9
[µ=5.09±1.04] regarding data management
[µ
gd
=3.75±2] and intellectual property specifically
[µ
IP
=3±0].
Once the data was reported on the grid,
recommendations could be made to the project leader.
It was suggested for this specific domain to focus on
the production of solution exploitation data and to
update its competitive intelligence on services and
patents.
4 SHAPING USER-CENTERED
INNOVATION THROUGH
ASSESSMENT: THE LIVING
LAB MODEL
Now that we have described a model capable of a
priori catching the different parameters for estimating
the maturity of a health project, the framework in
which it can be used will be detailed and to formalize
the approach for its effective implementation.
In the current paper, we propose that the Living
Lab model, which emphasizes the collaboration
between the user and the designer throughout the
design process, is an effective method for developing
new devices. This model is based on iterative
evaluations, which are repeated over time, and
incorporates methodological techniques from both
the exact and social sciences. This approach allows us
to identify and understand the facilitators and
constraints associated with the use of the device, as
well as to ensure that these factors are taken into
account in future versions of the device under
examination. Additionally, this model allows for a
ClinMed 2023 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
236
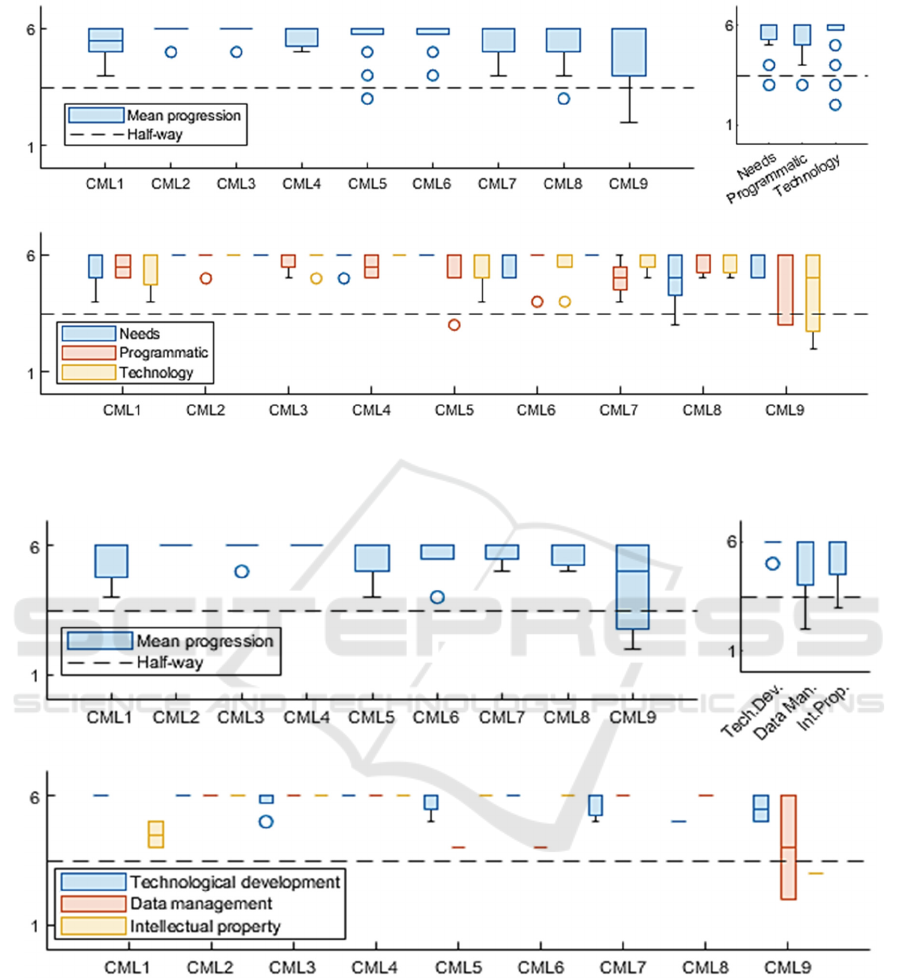
Figure 1: Dashboard of global maturity by level in self-assessment by the project leader (Association Innov’Autonomie -
DynSanté Concept Maturity Levels Questionnaire 180-items ; data taken from psychometric validation project runned by
Association Innov’Autonomie for illustration purpose). [Circle : outliers data].
Figure 2: Dashboard of Technological maturity by level in self-assessment by the project leader (Association
Innov’Autonomie - DynSanté Concept Maturity Levels Questionnaire 180-items ; data taken from psychometric validation
project runned by Association Innov’Autonomie for illustration purpose). [Circle : outliers data].
more efficient and effective development process, as
it allows for constant feedback and improvements
based on user needs and preferences (Zipfel et al.,
2022).
Generally speaking, a Living Lab is defined as a
place that practices user-centered research metho-
dologies to develop by co-design, use test, and
implement MedTech innovations in real-life contexts;
Shaping User-Centered Health Innovation Through Assessment
237

ClinMed 2023 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
238
with a focus on placing the user
1
at the center of the
creation process and all of its stakeholders, such as
caregivers, academics, and entrepreneurs (Ballon et
al., 2005; Leminen et al., 2012; Pallot et al., 2010;
Veeckman et al., 2013).
A recent meta-analysis (Zipfel et al., 2022)
concluded that Living Labs methodologies had a
positive impact on the acceptability of the system and
the subsequent feasibility of the procedure and made
it possible to predict the perenniality of the
implementation which will be carried out later, as
observed previously by (Mulder et al., 2008). In
general, this work and its precursors have shown that
the implementation of a Living Lab evaluation
methodology allows us to hope for a better
subsequent adoption of the device (Kim & Chung,
2017).
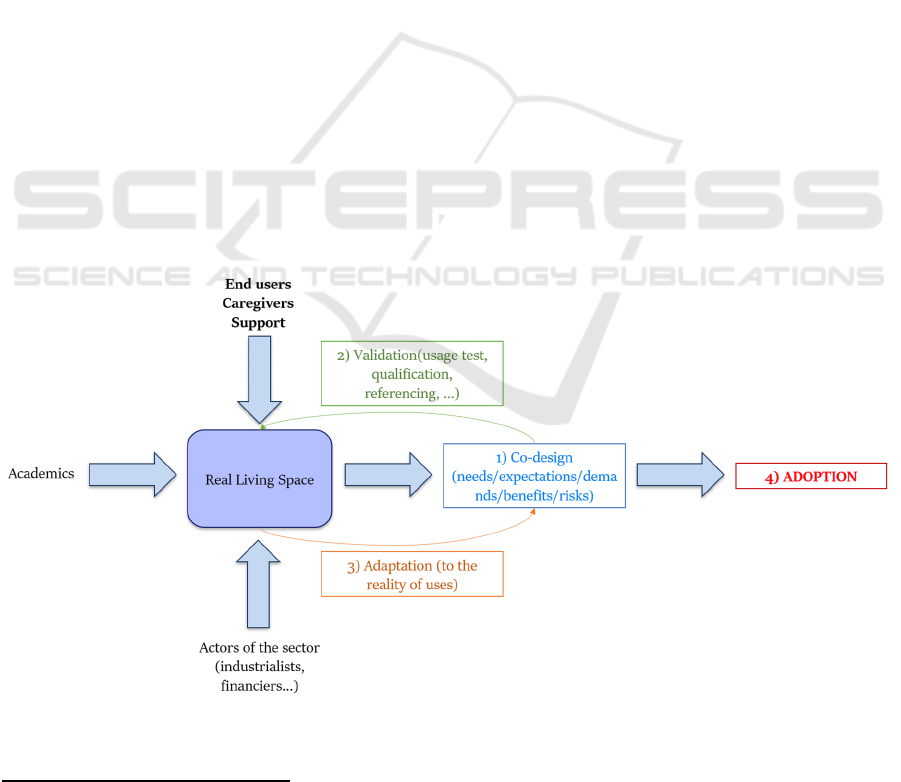
Figure 3 describes the process of developing an
innovative solution in the Living Lab approach. The
first preparatory condition is to bring together all the
actors of the project ecosystem, from the end-users
with their caregivers, the academics, and the main
actors of the sector (e.g. industrials, funders...)
directly in the end-user's living place. The co-creation
process (1) then begins. It can take the form of
interviews or focus groups to highlight the needs of
the population in all their ecosystemic complexity as
well as their constraints of use, the main grievances
as well as to have a first estimation of the resultant
benefit/risk balance.
Once the device and its usage network have been
modeled for the first time, a validation phase (2) takes
place. It can take the form of technical tests (i.e. tests
on the basis of procedures to be followed) ensuring
the usability of the essential functions and especially
of use tests. A major importance must be given to the
evaluation methods in technical tests and usability
tests in order to ensure their interpretability and their
reproducibility on several subjects or several samples
and any hypothesis must be tested on a statistical
level.
Once the validation phase is completed, the
adaptation phase (3) begins. Its objective is to modify
the device in order to adapt its use to the constraints
of use that were identified in (2). Indeed, the first
circle of co-design, which was reduced, probably
contains biases that constrained the generalisability of
the device to a larger population (i.e. such as a
population of a market segment). These biases must
be corrected by the parameters measured during the
tests.
Thus, we understand that phases (2) and (3) are
cyclic, and that they allow to correct the device
iteratively until arriving at a satisfactory version on
the technical level (i.e. as evidenced by the technical
test) and on the usage level (i.e. as evidenced by the
usage test).
Figure 3: Co-creation process implemented in the Living Lab approach.
1
As a subject of experimentation rather than as a rights
holder in terms of intellectual property.
Shaping User-Centered Health Innovation Through Assessment
239

ClinMed 2023 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
240

5 DISCUSSION AND
PERSPECTIVES
The objective of this work was to establish a
fundamental bibliographic link between successful
implementation and acceptability of a MedTech
innovation, as well as to provide a methodological
framework for applying this fundamental knowledge
in practice.
We first described the canonical models used by
our contemporaries, and in particular the TRL and
CML models developed by NASA (Mankins, 1995;
Wessen et al., 2013), which assess the maturity of a
project on technology development and proof-of-
concept metrics in the aerospace field. However,, we
have also highlighted through elements of the
literature that the acceptability and use of an
innovative MedTech device was not primarily based
on its intrinsic characteristics (Claudy et al., 2015),
contrary to spatial projects, but is indeed based on
criteria related to the consumer himself as well as his
usage traditions (Antioco&Kleijnen, 2010), where
the innovative parameter may even become a strong
barrier to the use of the device due to its perceived
uncertainty of use (Agarwal & Teas, 2001; Stone
&Grønhaug, 1993).
Thus, the TRL and CML models as canonically
described do not seem relevant for the evaluation of
consumer-related parameters rather than intrinsic
device parameters. The TRL and CML models are
particularly interesting for institutional evaluation
and the development of solutions for industries. The
TRL and CML models are particularly interesting for
institutional evaluation and lead to the development
of solutions for industries. These grids allow us to
arrive at solutions but they do not take enough into
account the acceptability and the use of these
solutions once developed..
We have therefore proposed a variant of the CML
model, the CML Santé France model, which
specializes the classical CML scales centered on
Technology by also opening its reading grid to the
elements of French and European regulations
(standards for scientific research methodology,
ethics, RGPD), and above all by granting a capital
place to the evaluation of the place of the end-user
and his ecosystem. Indeed, the CML Santé France
levels constitute a reference framework that does not
replace the interaction between the coach and the
entrepreneur, but provides to the coach a way to save
time, a knowledge base, a shared language, and to the
entrepreneur a rich, structured result that can be
shared and understood by other coaches or experts
who have adopted the method. The French field of
study is particularly interesting because regulation in
terms of health innovation is particularly heavy and
complex. This legislation is constantly evolving and
this is why there is a significant need for support in
this field. Even if there is a European will to define
common schemes for all countries, the regulation is
different from one country to another and must
therefore be adapted by country.
The use of this approach allows us to measure
maturity, to help companies optimize their evolution.
The goal is to carry out this type of assessment within
a controlled time frame, and to renew it at regular
intervals (for example, every year) to continue to
support the choices and decisions of start-ups.
In this sense, the CML Santé France
evaluation methodology seems particularly adapted
to the co-design methodological approach resulting
from Living Labs, where the user is placed at the
center of a creation process in partnership with all his
potential ecosystem (caregivers, funders...) and
making it possible to adapt the device to the reality of
the uses in an iterative fashion. Now that the
framework for the development of the system and a
relevant evaluation model have been described in the
literature, further research will focus on the
construction of CML Santé France tools for project
leaders and the experts who evaluate them. For the
time being, two standardized repeatable evaluation
questionnaires are being constructed, one for project
leaders, allowing them to quickly assess their level of
CML Santé France on all dimensions; the other for
expert evaluators of projects, enabling them to assess
the level of CML Santé France maturity on the basis
of a short oral presentation (i.e. pitch) by a project
actor. The data from these events are now being
analyzed as part of the psychometric validation of the
scale, in order to ensure the content and divergent
validity of the CML Santé France model as evaluated
in the construction of psychometric questionnaires for
clinical purposes (Gonzalez et al., 2021; Messick,
1989; Schmeiser et al., 2006). Moreover, its ease of
use and its effectiveness in comparison to existing
models is yet to be demonstrated in a large sample of
non-expert end-users.
ACKNOWLEDGEMENT
We would like to thank all those who contributed to
the preparation of the DYNSANTÉ project: Pierre-
Yves Traynard, Pôle ETP, Willy Allegre,
Cowork’Hit, Anne-Claude Lefevre, Cowork’HIt. The
project received a grant from the National Research
Agency to support the networking of partners
Shaping User-Centered Health Innovation Through Assessment
241

REFERENCES
Agarwal, S., & Teas, R. K. (2001). Perceived value :
Mediating role of perceived risk. Journal of Marketing
theory and Practice, 9(4), 1‑14.
Antioco, M., & Kleijnen, M. (2010). Consumer adoption of
technological innovations : Effects of psychological
and functional barriers in a lack of content versus a
presence of content situation. European Journal of
Marketing, 44(11/12), 1700‑1724. https://doi.org/
10.1108/03090561011079846
Asian, S., Pool, J. K., Nazarpour, A., &Tabaeeian, R. A.
(2019). On the importance of service performance and
customer satisfaction in third-party logistics selection:
An application of Kano model. Benchmarking: An
International Journal.
Ballon, P., Pierson, J., &Delaere, S. (2005). Test and
experimentation platforms for broadband innovation :
Examining European practice. Available at SSRN
1331557.
Broilo, P. L., Espartel, L. B., & Basso, K. (2016). Pre-
purchase information search: too many sources to
choose. Journal of Research in Interactive marketing.
Claudy, M. C., Garcia, R., & O’Driscoll, A. (2015).
Consumer resistance to innovation—A behavioral
reasoning perspective. Journal of the Academy of
Marketing Science, 43(4), 528‑544.
https://doi.org/10.1007/s11747-014-0399-0
Dawson, G. (2013). Soldier heroes: British adventure,
empire and the imagining of masculinities. Routledge.
Dunphy, S., &Herbig, P. A. (1995). Acceptance of
innovations: the customer is the key!. The Journal of
High Technology Management Research, 6(2), 193-
209.
Eagly, A. H., &Chaiken, S. (1998). Attitude structure and
function. In D. T. Gilbert, S. T. Fiske, & G. Lindzey
(Eds.), Handbook of social psychology (pp. 269–322).
New York: McGraw-Hill.
Gonzalez, O., MacKinnon, D. P., & Muniz, F. B. (2021).
Extrinsic convergent validity evidence to prevent jingle
and jangle fallacies. Multivariate Behavioral Research,
56(1), 3‑19.
Joachim, V., Spieth, P., & Heidenreich, S. (2018). Active
innovation resistance: An empirical study on functional
and psychological barriers to innovation adoption in
different contexts. Industrial Marketing Management,
71, 95-107.
Kano, N., &Seraku, K. (1984). F. and Tsuji, S..(1984).
Attractive Quality and Must-be Quality. The Journal of
the Japanese Society for Quality Control, 14(2).
Kim, J. S., & Chung, G. H. (2017). Implementing
innovations within organizations : A systematic review
and research agenda. Innovation, 19(3), 372‑399.
Laukkanen, T. (2007). Internet vs mobile banking:
comparing customer value perceptions. Business
process management journal, 13(6), 788-797.
Leminen, S., Westerlund, M., &Nyström, A.-G. (2012).
Living labs as open-innovation networks.
Mankins, J. C. (1995). Technology readiness levels. White
Paper, April, 6(1995), 1995.
Messick, S. (1989). Validity. Em r. Linn (org.), educational
measurement.(13-103). New York, NY: American
Council on Education and Macmillan Publishing
Company
.
Molesworth, M., &Suortti, J.-P. (2002). Buying cars
online : The adoption of the web for high‐involvement,
high‐cost purchases. Journal of Consumer Behaviour:
An International Research Review, 2(2), 155‑168.
Mulder, I., Velthausz, D., & Kriens, M. (2008). The living
labs harmonization cube : Communicating living lab’s
essentials. The Electronic Journal for Virtual
Organizations and Networks, 10, 1‑14.
Pallot, M., Trousse, B., Senach, B., &Scapin, D. (2010).
Living lab research landscape : From user centred
design and user experience towards user cocreation.
First European Summer School" Living Labs".
Ram, S., &Sheth, J. N. (1989). Consumer resistance to
innovations : The marketing problem and its solutions.
Journal of consumer marketing, 6(2), 5‑14.
Salazar, G., &Russi-Vigoya, M. N. (2021). Technology
readiness level as the foundation of human readiness
level. Ergonomics in Design, 29(4), 25-29.
Schmeiser, C. B., Welch, C. J., & Brennan, R. L. (2006).
Educational measurement. American Council on
Education and Praeger Publishers, Westport, CT.
See, A., Roller, S., Kiela, D., & Weston, J. (2019). What
makes a good conversation? how controllable attributes
affect human judgments. arXiv preprint
arXiv:1902.08654.
Sirgy, M. J. (1982). Self-concept in consumer behavior: A
critical review. Journal of consumer research, 9(3), 287-
300.
Stone, R. N., &Grønhaug, K. (1993). Perceived risk :
Further considerations for the marketing discipline.
European Journal of marketing, 27(3), 39‑50.
Tontini, G. (2007). Integrating the Kano model and QFD
for designing new products. Total Quality
Management, 18(6), 599-612.
Veeckman, C., Schuurman, D., Leminen, S., &Westerlund,
M. (2013). Linking living lab characteristics and their
outcomes : Towards a conceptual framework.
Technology Innovation Management Review, 3(12
december), 6‑15.
Wessen, R., Borden, C. S., Ziemer, J. K., Moeller, R. C.,
Ervin, J., & Lang, J. (2013). Space mission concept
development using concept maturity levels. AIAA
Space 2013 Conference and Exposition, 5454.
Xu, H., & Gupta, S. (2009). The effects of privacy concerns
and personal innovativeness on potential and
experienced customers’ adoption of location-based
services. Electronic Markets, 19, 137-149.
Zipfel, N., Horreh, B., Hulshof, C. T. J., de Boer, A. G. E.
M., & van der Burg-Vermeulen, S. J. (2022). The
relationship between the living lab approach and
successful implementation of healthcare innovations:
An integrative review. BMJ Open, 12(6), e058630.
https://doi.org/10.1136/bmjopen-2021-058630
ClinMed 2023 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
242
