
Medical Devices Used in Extreme Conditions in Pre-Hospital
Emergency Medicine: Overview of the Issue, Use Case Regarding
Mechanical Ventilation at Altitude and Advice
Carine Malle
1,* a
, Alban De Luca
2b
and Thierry Chevallier
3,4,5 c
1
Direction of Training, Research and Innovation, French Defence Health Service,
1 Place Alphonse Laveran, 75005 Paris, France
2
Archeon, 2 Chemin des Aiguillettes, 25000 Besançon, France
3
Department of Biostatistics, Epidemiology, Public Health and Innovation in Methodology (BESPIM),
CHU Nîmes, Place du Pr. Robert Debré, 30029 Nîmes, France
4
UMR 1302, Institute Desbrest of Epidemiology and Public Health, INSERM, Univ. Montpellier, Montpellier, France
5
Tech4Health-FCRIN, France
Keywords: Extreme Conditions, Medical Devices, Aeromedical Evacuation, Mechanical Ventilation.
Abstract: Pre-hospital emergency medicine sometimes involves taking care of patients in environments far different
from the hospital. Cold, heat, humidity, altitude, wind, etc. put human beings and equipment to a severe test.
What are the extreme conditions to which pre-hospital emergency medicine professionals are exposed? What
types of medical devices are particularly concerned? What are the regulations and standards in force? What
are the impacts of exposure to extreme conditions on medical devices? To answer these questions, we rely on
an analysis of the regulatory and normative context, on a scientific literature review and on a case study
involving mechanical ventilation at altitude. Finally, we share some thoughts and advice intended for health
facilities and users, in order to improve practices in terms of selection, use and monitoring of medical devices
exposed to extreme conditions. This document is illustrated with examples concerning the French defence
health service, but our approach can be applied to any entity concerned with pre-hospital emergency medicine.
1 INTRODUCTION
Pre-hospital emergency medicine focuses on caring
for seriously ill or injured patients before they reach
hospital. It calls upon various specialties:
anaesthesiology, traumatology, toxicology,
psychiatry, etc.
The increasing extension of the field of territories
open to tourism and military operations lead medical
personnel, both military and civilian, to intervene in
environments that are qualified as extreme, either
because of the climatic conditions (cold, heat,
humidity, wind, etc.) or because of the characteristics
of the point of care (aircraft, mountain, sea, etc.).
If the effects of extreme environments on human
physiology have been the subject of numerous studies
a
https://orcid.org/0000-0002-6824-1383
b
https://orcid.org/0000-0002-0911-7311
c
https://orcid.org/0000-0002-5110-6273
*
carine.malle@gmail.com
for decades, this is not the case for their effects on
drugs, and even less so on medical devices (MD).
After having made an inventory of the extreme
conditions and their impact on the MD, we will
illustrate our point with a concrete example regarding
the use of mechanical ventilation at altitude in the
context of aeromedical evacuations. Finally, we will
try to share some thoughts and advice for health care
institutions and users to improve practices in terms of
selection, use and monitoring of MD exposed to
extreme conditions.
The military medical personnel being very
frequently confronted with extreme environments, we
have chosen to illustrate our point with common
military operational situations. However, we hope
that this work will be of benefit to any health care
Malle, C., De Luca, A. and Chevallier, T.
Medical Devices Used in Extreme Conditions in Pre-Hospital Emergency Medicine: Overview of the Issue, Use Case Regarding Mechanical Ventilation at Altitude and Advice.
DOI: 10.5220/0011923100003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 1: BIODEVICES, pages 215-221
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
215

facility or caregiver practicing pre-hospital
emergency medicine.
2 EXTREME CONDITIONS IN
PRE-HOSPITAL EMERGENCY
MEDICINE
Military medical personnel routinely encounter
extreme conditions, in particular in the context of
medical care for soldiers injured in external military
operations.
2.1 Extreme Climatic Conditions
Due to these activities, military medical personnel are
occasionally faced with the practice of medicine in
extreme climatic conditions, such as cold, heat,
humidity or altitude.
In France, sub-zero temperatures are common in
high mountain areas during the winter period. At
altitude, the decrease in atmospheric pressure and the
rarefaction of the air lead to a decrease in air
temperature. The average thermal gradient is about
0.6°C every 100 m. Thus, when going from
Chamonix valley (altitude: 1100 m) to the summit of
Mont-Blanc (altitude: 4807 m), one loses about 20°C.
Heat exposure is a constant in some theatres of
operation, notably in the Sahel, where military
professionals are faced with temperatures
approaching 50°C. In equatorial areas, like French
Guyana, the humidity rate is comprised between 70
and 90% all year long. It should be noted that these
constraints are often combined with each other,
humidity and heat, altitude and cold, and associated
with other constraints (wind, difficult terrain, stress,
etc.).
2.2 External Military Operations
In external military operations, medical care of the
wounded soldiers is organized into four levels:
- Role 1 corresponds to the initial care of the
wounded soldiers directly on the field. Role 1 must be
mobile and responsive. Resuscitation procedures can
be performed, and the health products available are of
primary necessity. Nurses, physicians but also non-
health professionals are involved.
- Role 2 includes mobile surgical units, rapidly
deployable but with limited autonomy. They are
capable of performing resuscitation and emergency
surgical interventions, in particular haemostasis
control.
- Role 3 corresponds to a heavier and more
important surgical unit with reinforced medical,
surgical and diagnostic means. At this level, the
patient may be stabilized.
- Role 4 corresponds to hospitals located in
mainland France. The patient is evacuated when his
condition is critical or requires care that is not
available on site.
Each level is provided with medical supplies,
including specific MD. Role 1 receives mainly
“rustic” MD, i.e., light, compact, solid and easy to
use, such as portable pulse oximeters (class IIb),
tactical tourniquets (class I), bandages (class I or IIa)
or automatic bone injection guns (class IIb). In role 2,
these same MD are added to all surgical equipment
(e.g., stapler; class III). From role 3 onwards,
caregivers have all the MD commonly used in
conventional emergency medicine, such as external
defibrillators (class III) and emergency ventilators
(class IIb). Thus, each MD is associated with one (or
more) level(s) of use, which will condition the
constraints to which the MD must resist and the type
of user.
2.3 Aeromedical Evacuations
A medical evacuation (MEDEVAC) is the transfer of
a patient, carried out on a physician's prescription, in
order to provide continuity of care and treatment. It
can be performed with or without medical
accompaniment. In times of conflict, the transfer of
these patients is strongly influenced by various
factors such as the operational environment, the
climate, the length and quality of the evacuation
routes and the availability of appropriate means of
transport. In this sense, the air route is most often
chosen.
Several types of aircraft can be used depending on
the number of patients to be evacuated and the
distance to be covered, all of which are equipped with
at least one mechanical ventilator. Without adequate
dynamic correction by the ventilator or by the
physician, the decrease in barometric pressure during
the ascent to altitude is accompanied by an increase
in ventilator delivered gas volume. Depending on the
level of cabin pressurization and on the instructions
set for the ventilator (respiratory rate and fraction of
inspired oxygen (FiO
2
)), tidal volume can be
increased by up to 30%, which exposes the patient to
an increased risk of barotrauma (pneumothorax or
alveolar trauma related to excess intrathoracic
pressure) and ventilator-induced lung injury (VILI;
alveolar trauma related to too much intra-alveolar
volume sometimes responsible for secondary scar
ClinMed 2023 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
216

fibrosis). A practical example of the impact of altitude
on a mechanical ventilator is displayed in section 4.
2.4 What About CE Marking?
EU Regulation 2017/745 (Annex I. General safety
and performance requirements) is not restrictive in
terms of the environmental conditions to be met:
“7.
Devices shall be designed, manufactured and
packaged in such a way that their characteristics and
performance during their intended use are not adversely
affected during transport and storage, for example,
through fluctuations of temperature and humidity, taking
account of the instructions and information provided by
the manufacturer.”
(Chapter I, page 95)
“14.2.
Devices shall be designed and manufactured
in such a way as to remove or reduce as far as possible:
[...] (b) risks connected with reasonably foreseeable
external influences or environmental conditions, such as
magnetic fields, external electrical and electromagnetic
effects, electrostatic discharge, radiation associated with
diagnostic or therapeutic procedures, pressure,
humidity, temperature, variations in pressure and
acceleration or radio signal interferences;”
(Chapter II,
page 99).
Thus, the instructions for use remain the major
source of information regarding “i
nformation that
allows the user and/or patient to be informed of any
warnings, precautions, contra- indications, measures to
be taken and limitations of use regarding the device.”
(Chapter III, page 106), although design may provide
useful feedback to users
3 IMPACTS OF EXTREME
CONDITIONS ON MD USED IN
EMERGENCY MEDICINE
3.1 Literature Review
Kämäräinen et al. (2012) assessed the resistance of
various single-use MD mainly composed of plastic
materials, such as endotracheal tubes, suction
catheters, and infusers, to a 15-minute exposure to a
temperature of -21.5°C. Resistance was assessed via
a manual stress test designed to mimic normal pre-
hospital use. The authors observed a loss of flexibility
that led in some cases to the rupture of tubes and
catheters. A comparative study of several oxygen
concentrators showed that storage for 24 hours at -
35°C significantly impaired the ability of portable
oxygen concentrators to maintain FiO
2
at set point
(Blakeman et al. 2016).
In the early 1990s, as part of the development of
heliborne medical evacuations in the United States,
Bruckart and colleagues (1993) evaluated 34 MD,
including defibrillators, ventilators, infusion pumps
and vital signs monitoring devices under various
environmental conditions (in accordance with the
environmental tests described in the American
military standard MIL-STD 810D): altitude (15,000
ft, or 4,572 m), heat, cold, humidity and vibrations.
One third of the MD failed at least one environmental
test, with the failure consisting of a “visible” device
failure. A “visible” failure was defined as a MD that
completely stops working, a display screen that goes
out, a battery that discharges, an alarm that sounds
without reason, etc. In the absence of a performance
evaluation of MD, a dysfunction affecting the
measurement by the sensors would probably not be
identified by these tests. The compliance of two thirds
of the devices evaluated with environmental
standards does not guarantee the safety of patients
treated with these devices in extreme conditions.
Since then, more recent studies have compared
various models of the same type of MD at altitude,
either with the aim of determining the most “suitable”
of them, or with the aim of understanding the cause
of malfunction identified in current practice. For
example, in a comparative study of 4 capnographs
exposed to increasing altitude, one device failed as
early as 3650 m and only one device was still
functional at 5470 m (Pattinson et al. 2004). Few
published studies have not stopped at listing failures
but have actually assessed the performance of MD.
For example, in 2019, a comparative study of 5
syringe pumps showed that miniature models, which
are more easily transportable, were less accurate than
standard-sized models in terms of infusion rate
accuracy as early as 1700 m (Blancher et al. 2019).
Regarding transport ventilators, several studies (e.g.,
Rodriguez et al., 2009; Blakeman et al, 2014;
Boussen et al., 2014) have shown a decrease in the
accuracy of volume delivered by some MD at
altitude, even on MD with altitude-compensating
features. Thus, Boussen's team compared 6
ventilators at moderate altitudes (1500 and 2500 m).
If 4 of them proved to be efficient (average relative
error of the delivered tidal volume <10%), they
showed however that the exposure to a moderate
altitude led to an increase of 30% of the tidal volume
(for a FiO
2
of 100%) on one of the recent models and
whose use at altitude (up to 3500 m approximately)
was not contraindicated by the manufacturer. It
should be noted that some articles do not mention the
use of measurement sensors independent of those of
the MD, which suggests that the results are based on
Medical Devices Used in Extreme Conditions in Pre-Hospital Emergency Medicine: Overview of the Issue, Use Case Regarding
Mechanical Ventilation at Altitude and Advice
217
the data displayed by the MD itself without
verification of their accuracy.
Although these studies present relatively
concerning results, we do not know whether they
have had any real impact on the MD tested (e.g.,
changes to the design or the manual).
3.2 A Vital Risk for Patient
Due to the fragility of electronic components, active
MD seem to be particularly at risk of malfunctioning
under extreme conditions. Whether the failure results
in an obvious malfunction (e.g., complete shutdown,
display failure) or one that is more difficult to detect
(e.g., measurement error), there is a vital risk for the
patient.
The other types of MD are not spared: hardening,
deformation (shrinking or swelling), rupture,
oxidation, corrosion of materials, delamination of
composite materials, condensation, air bubble
formation, loss of seal, etc. (Janno & Degiovanni,
2018; Parent, 2017) are some of the potential
consequences of exposure of any MD to extreme
conditions. Continuous or repeated exposure to
extreme conditions also participates in the accelerated
aging of MD, which requires specific maintenance
procedures. It therefore seems essential that MD
intended to be used in extreme conditions be
evaluated under these conditions during preclinical
testing. Standards have been established to harmonize
practices.
3.3 Main Applicable Standards
Regarding Extreme Environments
and Their Limits
Even if they are not mandatory, standards allow to
meet certain requirements of the applicable
regulations.
These standards are of 2 types: horizontal
standards that concern development and
manufacturing processes, risk analysis, clinical
investigations and quality assurance systems, and
vertical standards that concern specific MD. They are
continually evolving because of the constant
evolution of MD.
If the conformity of MD to these standards is
important to take into account, one must however be
aware of their limits. A first limitation is the existence
of several standards depending on the country and the
context (notably civil/military, air/land). A second
limitation is the relative freedom left to manufacturers
in the choice of tests performed to claim compliance
with these standards. With regard to altitude, for
example, the military standards AECTP-230 and
MIL-STD-810 indicate different exposure levels that
can be investigated, but it is up to the manufacturer to
choose which level to apply to test his MD. Thus, the
manufacturer may claim compliance with a military
aeronautical standard even though the MD has only
been evaluated at moderate altitudes (e.g., 2500 m).
A third limitation lies in the interpretation of test
results. Most of these standards remain superficial as
to the evidence of performance and safety that must
be provided. For example, one can see that the
standard for transport ventilators (ISO/IEC 10651-
3:1997 “Lung ventilators for medical use - Part 3:
Particular requirements for emergency and transport
ventilators”) requires at a minimum that the ventilator
“continue to function” under extreme conditions:
“Extreme conditions [...] Note - The ventilator might
continue to function but outside the specified
tolerances.” (6.8.3.e, page 7).
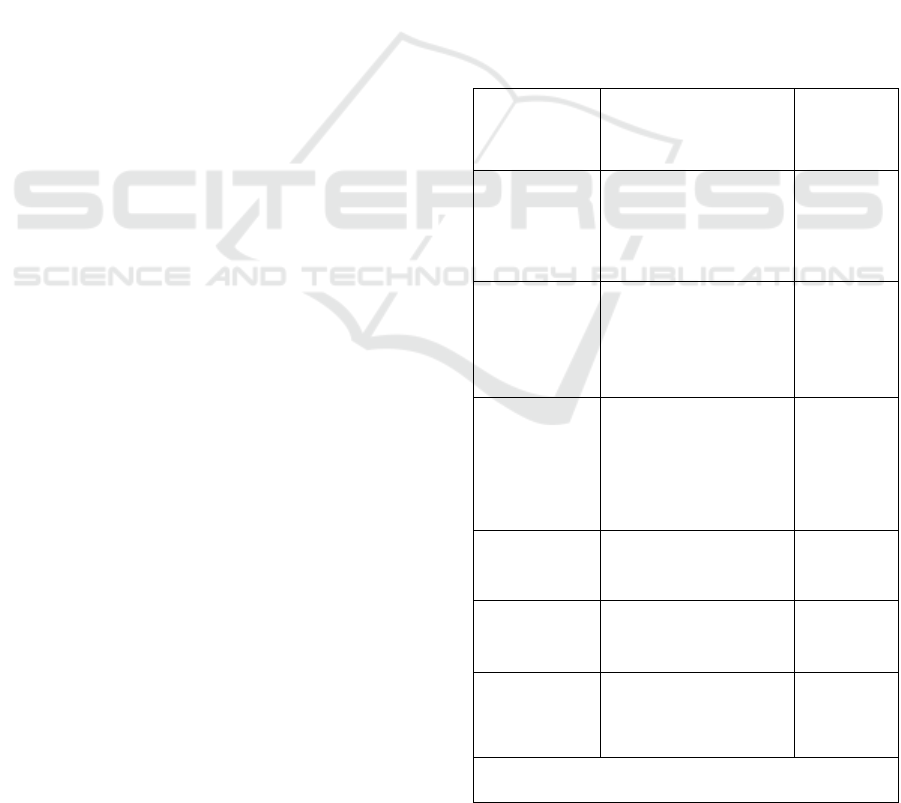
Table 1: Main applicable standards relative to MD used in
extreme conditions.
Publisher Standard title
Scope of
application
NATO
STANAG 4370
“Environmental
testing”
MD used in
the military
field (in
NATO
countries)
Department of
Defense, USA
MIL-STD-810E
“Environment
engineering
considerations and
laboratory tests”
MD used in
the US
army
Special
Committee
135 (SC-135)
DO-160G
“Environmental
conditions and test
procedures for airborne
equipment”
On-board
MD in
aircraft
International
standard
ISO/IEC 60601-1-
2:2014
Active MD
International
standard
ISO/IEC 60068-2-6
MD
exposed to
vibrations
International
standard
ISO/IEC 60068-2-27
MD
exposed to
shocks
NATO: North Atlantic Treaty Organization
STANAG: Standard Agreement
ClinMed 2023 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
218
4 CASE STUDY: MECHANICAL
VENTILATION IN
AEROMEDICAL EVACUATION
4.1 LTV® 1200
The LTV® 1200 (Care Fusion, San Diego, USA) is
currently present in French MEDEVAC aircrafts. It is
a turbine ventilator that can operate in both controlled
and spontaneous mode with inspiratory support.
The LTV® 1200 ventilator is intended to provide
continuous or intermittent ventilatory support for the
care of persons requiring mechanical ventilation. The
ventilator is a restricted MD intended for use by
qualified and trained personnel under the direction of
a physician. Specifically, the ventilator is applicable
to adult and paediatric patients weighing at least 11
pounds (5 kg). The ventilator is suitable for use in
institutions, at home or in transport.
The temperature must be between +5 and +40°C
and the relative humidity between 15% and 95%. The
device complies with the international standard IEC
68-2-27 for shock resistance, the international
standards IEC 68-2-6 and IEC 68-2-34 for vibration
resistance and the US military standard MIL-STD-
810E for shock resistance in ground and helicopter
transport. The device has also been approved by the
FDA as a “transport ventilator” and the leaflet states
that the LTV® 1200 is “suitable for use in institutional,
home, or transport settings”. However, the
manufacturer does not claim the standard for transport
ventilators (ISO/IEC 10651-3:1997 “Lung ventilators
for medical use - Part 3: Particular requirements for
emergency and transport ventilators”). While the
leaflet refers to the device's ability to automatically
adapt tidal volume in response to increasing altitude,
no indication is given regarding the altitude range at
which the device should be used.
4.2 Performance Evaluation of the
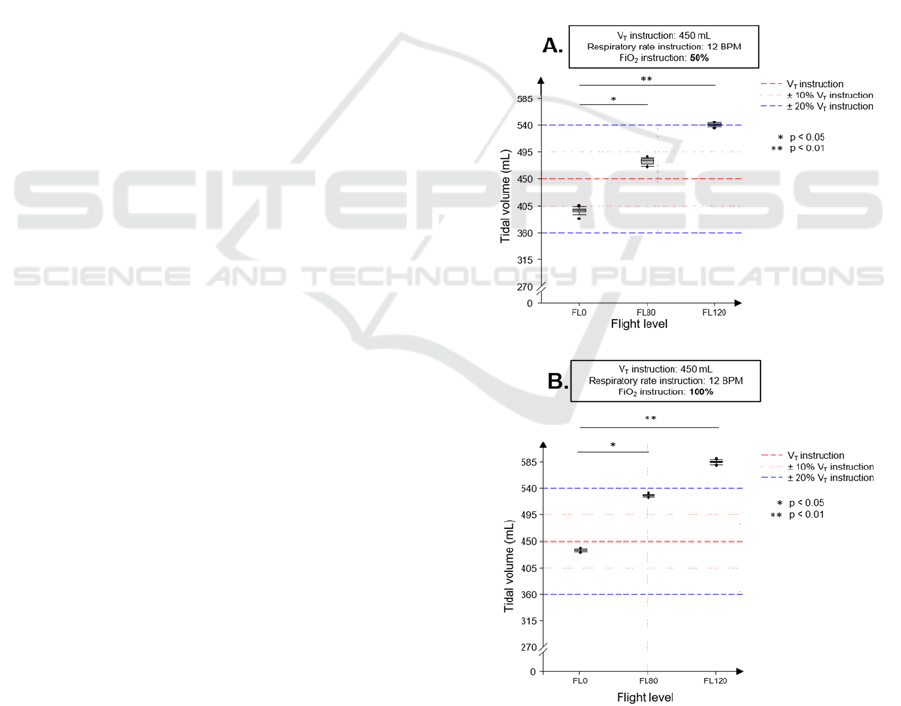
LTV® 1200 at Altitude
In 2012, the French defence health service conducted
a study comparing the performance of 3 ventilators,
including the LTV® 1200, at simulated altitude in a
hypobaric chamber. The performance of the
ventilators on an artificial lung was measured at
ground level (FL0), at 2400 m (FL80) and at 3600 m
(FL120).
This study showed that the tidal volume (V
T
)
delivered by the LTV® 1200 at FL80 and FL120 was
significantly increased compared to the ground
measurement. Whatever the altitude, the Vt delivered
never respected the V
T
set point (450 or 700 ml). If
the measurements were within the ± 20% margin
provided by the ISO/IEC 10651-3 standard at FL0
and FL80, this was no longer the case at FL120. For
a set point of 450 ml (breathing rate = 12 breaths per
minute; FiO
2
= 50%), the ventilator delivered an
average of 540 ml at FL120 (figure 1A). Furthermore,
when the FiO
2
set point was increased from 50 to
100%, the V
T
was even higher, increasing to an
average of 585 ml (figure 1B). Similar results were
observed with a tidal volume set point of 700 ml. This
study concluded that the LTV® 1200 did not meet the
stability criteria necessary for a transport ventilator
(Forsans, 2012). However, the LTV® 1200 does still
equip airborne MEDEVAC today. This study
illustrates the unsuitability of certain medical MD for
the environment in which they are used. As patient
safety is at stake, we would like to share some
thoughts and advice for health facilities and users.
Figure 1: LTV® 1200 performance at altitude.
A and B parts display two different sets of instructions
(framed text). BPM: breaths per minute; FiO
2
: fraction of
inspired oxygen; V
T
: tidal volume.
Medical Devices Used in Extreme Conditions in Pre-Hospital Emergency Medicine: Overview of the Issue, Use Case Regarding
Mechanical Ventilation at Altitude and Advice
219

5 CONSIDERATIONS AND
ADVICE FOR HEALTH
FACILITIES AND USERS
Before purchase by the health facility, the analysis of
requirements (by biomedical engineers) should be
based on general recommendations (in particular the
WHO technical series on MD: “Assessment of
medical device requirements”) and specific
recommendations for each type of MD, and should
include all the constraints to which the device is
intended to be exposed. The choice of a MD over
another should be based on reliable, verifiable
information that is the responsibility of the
manufacturer (instructions for use) and not on a sales
pitch.
After purchase by the health facility, in the
absence of specific recommendations from the
manufacturer for the planned use, health facilities
should ensure that the performance and safety of the
device under extreme conditions are evaluated before
use. This may involve different types of tests: pre-
clinical tests, usability evaluations or even clinical
investigations as defined in the EU Regulations
2017/745 and 2017/746. Monitoring should include
traceability of conditions of use and the collection of
safety information related to these (extreme)
conditions of exposure/use. Finally, user training
should include awareness of the impact of extreme
conditions on the device (figure 2).
6 CONCLUSIONS
To conclude, we show that pre-hospital emergency
medicine is inseparable from the notion of “extreme
conditions”, particularly in the French defence health
service. The types of MD concerned are very diverse
and of all classes. Only the instructions for use
provide reliable information about the conditions
supported by a given device. The claim of conformity
to environmental standards must be analysed with
care and is in no way a guarantee of the performance
and/or safety of the MD. The scientific literature on
the impact of extreme conditions on MD is relatively
poor and official recommendations in terms of
exposure to extreme conditions are almost non-
existent. One interpretation of this finding could be
the rarity of malfunctions, but we also suspect an
under-reporting of incidents associated with a strong
publication bias. Finally, the use case we described
illustrates in a masterly way the gap that can exist
between the needs of caregivers and the equipment
they actually have. It also highlights the lack of
communication within a healthcare institution
between the medical personnel who use MD and the
department in charge of selecting and purchasing
them.
Figure 2: Tips for health facilities and users.
This problematic raises ethical questions. How
should the user behave when confronted with the
emergency care of a patient with a medical device in
unexpected extreme conditions? Use the medical
device anyway and risk sanctions? Not to use it at the
risk of letting the patient's condition deteriorate? How
should he report the incident to his hierarchy? In our
ClinMed 2023 - Special Session on European Regulations for Medical Devices: What Are the Lessons Learned after 1 Year of
Implementation?
220

view, in the same way that exceptions to the
collection of patient’s consent in emergency
situations have been established, the unplanned use of
a medical device in the emergency context should be
the subject of reflection so as to result in rules of good
practice ensuring protection of both patients and
users. A major limitation of this article is that we have
not found any tangible evidence (incident reports,
product recalls, clinical investigation results, etc.) to
prove that this problem is a clinical reality. However,
we have collected several testimonies from French
defence health service caregivers who have
encountered difficulties in the use of MD in an
operational context. This paradox raises questions.
Our hypothesis is that incidents related to extreme
conditions are under-reported by users, in particular
because it is considered that the issue is not related to
the device but is the responsibility of the user who has
not followed the instructions for use. It seems crucial
to encourage the reporting of these incidents, without
implicating the manufacturer's responsibility, in order
to measure their frequency and severity in real life.
ACKNOWLEDGEMENTS
We would like to thank the entire teaching staff of the
“Développement d’une nouvelle technologie de
santé: pourquoi, quand et comment réaliser les
études cliniques?” degree for giving me the occasion
to work on this fascinating topic and the Direction of
training, research and innovation of the French
defence health service for their strong support. We
also thank the medical and paramedical staff of the
French defence health service for their relevant
feedback.
REFERENCES
Blakeman, T., Britton, T., Rodriquez, D. Jr., & Branson, R.
(2014). Performance of portable ventilators at altitude.
Journal of trauma and acute care surgery,
77(3 Suppl 2):S151-5. https://doi.org/10.1097/TA.
0000000000000379
Blakeman, T. C., Rodriquez, D. Jr., Britton, T. J.,
Johannigman J. A., Petro, M. C., & Branson, R. D.
(2016). Evaluation of Oxygen Concentrators and
Chemical Oxygen Generators at Altitude and
Temperature Extremes. Military Medicine, 181(5
Suppl):160-8. https://doi.org/10.7205/MILMED-D-15-
00130
Blancher, M., Repellin M., Maignan, M., Clapé, C., Perrin,
A., Labarère, J., Debaty, G., & Viglino, D. (2019).
Accuracy of low-weight versus standard syringe
infusion pump devices depending on altitude.
Scandinavian Journal of Trauma Resuscitation and
Emergency Medicine, 11;27(1):65. https://doi.org/10.
1186/s13049-019-0643-1
Boussen, S., Coulange M., Fournier M., Gainnier M.,
Michelet P., Micoli C., & Negrel L. (2014). Evaluation
of transport ventilators at mild simulated altitude: a
bench study in a hypobaric chamber. Respiratory Care,
59(8):1233-41. https://doi.org/10.4187/respcare.02985
Forsans, E. (2012). Ventilation en altitude : Performances
de trois ventilateurs [Thèse de médecine, Ecole du Val-
de-Grâce].
Jannot, Y., & Degiovanni, A. (2018). Mesure des propriétés
thermiques des matériaux. Iste editions. ISBN 978-1-
78405-423-6
Kämäräinen, A., Virta, J., Yliherne, S., & Virkkunen, I.
Evaluation of the effects of low temperature on
performance of standard non-electric medical
equipment at -21.5°C (-6.7°F) - a descriptive study on
a real life challenge in the prehospital setting. (2012).
Resuscitation, 83(12):1517-20. https://doi.org/10.1016/
j.resuscitation.2012.05.007
Parent, G. (2017). Evaluation de la durée de vie de
composants électroniques de puissance commerciaux
soumis à plusieurs tests de vieillissement et
détermination des mécanismes de défaillance [Thèse de
sciences, Université de Toulouse].
Pattinson, K., Myers, S., & Gardner-Thorpe, C. (2004).
Problems with capnography at high altitude.
Anaesthesia, 59(1):69-72. https://doi.org/10.1111/j.
1365-2044.2004.03447.x
Rodriguez, D. Jr., Branson, R. D., Dorlac, W., Dorlac, G.,
Barnes, S. A., & Johannigman, J.A. (2099). Effects of
simulated altitude on ventilator performance. Journal of
trauma and acute care surgery, 66(4 Suppl):S172-7.
https://doi.org/10.1097/TA.0b013e31819cdbd1
Medical Devices Used in Extreme Conditions in Pre-Hospital Emergency Medicine: Overview of the Issue, Use Case Regarding
Mechanical Ventilation at Altitude and Advice
221
