
Shape-based Features Investigation for Preneoplastic Lesions on Cervical
Cancer Diagnosis
Daniela C. Terra
1,4 a
, Adriano C. Lisboa
2 b
, Mariana T. Rezende
3 c
, Claudia M. Carneiro
3 d
and Andrea G. C. Bianchi
4 e
1
Department of Computing, Federal Institute of Minas Gerais, Ouro Branco, MG, Brazil
2
Research Department, GAIA, Belo Horizonte, MG, Brazil
3
Clinical Analysis Department, Federal University of Ouro Preto, Ouro Preto, MG, Brazil
4
Department of Computing, Federal University of Ouro Preto, Ouro Preto, MG, Brazil
Keywords:
Cervical Cancer, Image Classification, Morphological Features, Features Selection, XGBoost Classifier.
Abstract:
The diagnosis of cervical lesions is an interpretative process carried out by specialists based on cellular in-
formation from the nucleus and cytoplasm. Some authors have used cell nucleus detection and segmentation
algorithms to support the computer-assisted diagnosis process. These approaches are based on the assump-
tion that the nucleus contains the most important information for lesion detection. This work investigates the
influence of morphological information from the nucleus, cytoplasm, and both on cervical cell diagnosis. Ex-
periments were performed to analyze 3,233 real cells extracting from each one 200 attributes related to size,
shape, and edge contours. Results showed that morphological attributes could accurately represent lesions in
binary and ternary classifications. However, identifying specific cell anomalies like Bethesda System classes
requires adding new attributes such as texture.
1 INTRODUCTION
Cervical cancer is the fourth most common cancer
in women after breast, colorectal, and lung cancer.
In 2018 about 570,000 women were diagnosed with
the cervical disease, and 311,000 of them died due to
malignancy tumors worldwide (Das, 2021). This oc-
curs even with slow progress from precursor lesions
to the cancer stage. Thus, the cure of malignancy
cases depends on a timely diagnosis or screening for
pre-neoplastic lesions. If detected early, the prognosis
can be substantially improved with effective treatment
(Williams, 2021).
A Pap smear is a cost-effective technique widely
used to prevent cervical cancer. Under the micro-
scope, professionals identifying suspicious cell struc-
tures following diagnosis protocols internationally
adopted such as the Bethesda System (Nayar and
Wilbur, 2015). The main disadvantage of such man-
a
https://orcid.org/0000-0002-2828-8275
b
https://orcid.org/0000-0001-5773-2200
c
https://orcid.org/0000-0002-9514-9312
d
https://orcid.org/0000-0002-6002-857X
e
https://orcid.org/0000-0001-7949-1188
ual analysis is the high rate of false negatives. Screen-
ing and diagnosis are subject to misinterpretation by
visual habituation and a need for expertise.
Computer-aided diagnostics can reduce errors and
increase productivity in cancer screening. Propos-
als for automated cytology include solutions to detect
(Diniz et al., 2021c; Li et al., 2021), segment (Umadi
et al., 2020; Teixeira et al., 2022; Zhao et al., 2022),
and automate the screening of cell lesions.
Automatic cervical lesion classification follows
cell detection or segmentation. The solutions of-
ten employ features extraction related to cell size
and shape such as area, perimeter, elongation (ma-
jor/major axes), circularity, and nucleus-cytoplasm
ratios (Jantzen et al., 2005; Marinakis et al., 2009;
Chankong et al., 2014; Dong et al., 2020; Yakkundi-
math et al., 2022). Other works measures such as the
fractal dimension (Bhowmik et al., 2018), the rela-
tive position of the nucleus within cytoplasm (Mari-
arputham and Stephen, 2015), roughness index, the
standard deviation of radial distance and Fourier de-
scriptors (Zhang et al., 2014). Diniz et al. (2021b)
uses the CRIC base (Rezende et al., 2021) and tra-
ditional ML techniques to classify pre-neoplastic le-
506
Terra, D., Lisboa, A., Rezende, M., Carneiro, C. and Bianchi, A.
Shape-based Features Investigation for Preneoplastic Lesions on Cervical Cancer Diagnosis.
DOI: 10.5220/0011900800003417
In Proceedings of the 18th International Joint Conference on Computer Vision, Imaging and Computer Graphics Theory and Applications (VISIGRAPP 2023) - Volume 4: VISAPP, pages
506-513
ISBN: 978-989-758-634-7; ISSN: 2184-4321
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

sions using cell nuclei shape and texture features.
Classification without segmentation is leveraged by
deep learning approaches (Dong et al., 2020; Ra-
haman et al., 2021). The work proposed by Diniz
et al. (2021a) achieved a high recall rate on detect-
ing cellular lesions using an ensemble of deep neural
networks tested with the CRIC dataset images.
This work proposes an investigation of the influ-
ence of morphological cell attributes during the clas-
sification of cervical cell lesions. The extracted fea-
tures are related to the size, shape, and edge con-
tours calculated for the nucleus, the cytoplasm, and
both components. Our solution is based on tradi-
tional machine learning (ML) techniques to classify
cervical cells with or without (pre)neoplastic lesions.
We evaluated a binary classifier (normal/abnormal), a
ternary classifier (normal cell/low-grade lesions/high-
grade lesions), and an identifier for the 6 (six) classes
of the Bethesda System for cytological diagnosis.
To the best of our knowledge, this is the first ap-
proach to investigate the adequacy or limitation of
these attributes in automated diagnosis. The main
contributions of our investigation are:
• Exploring the potential of shape-based features on
discrimination of cervical cells lesions;
• Verify the effectiveness of Elliptic Fourier De-
scriptors (EFD) in this classification process;
• Evaluate the proposed solution on real images of
conventional cytology;
• Analyzing the results of the shape-based classifi-
cation at the level of cells (both nucleus and cyto-
plasm), only nuclei and cytoplasms.
The next section discusses our proposal in detail.
Section 3 presents experiments and results. Finally,
Section 4 reviews the proposed solution’s results.
2 METHODOLOGY
This section presents the materials and methods con-
sidered. Section 2.1 presents the database used in ex-
periments. Section 2.2 describes the extracted fea-
tures and the feature selection procedure. Section 2.3
explains the computational model built for the exper-
iments.
2.1 Dataset
In this work, we use the CRIC Cervix-Seg database
of conventional cytology (Rezende et al., 2021). The
database contains 3,224 segmented cellular nuclei
and cytoplasm from 400 real Pap smear images.
Figure 1: CRIC Cervix-Seg example for nuclei and cyto-
plasm segmentation.
Classification and segmentation of cells were per-
formed according to Bethesda nomenclature and car-
ried out manually by experienced cytopathologists
from the Center for Recognition and Inspection of
Cells (CRIC) (see Figure 1).
The Cervix-Seg collection includes six (6) classes:
(a) negative for intraepithelial lesion or malignancy
(NILM); (b) atypical squamous cells of undetermined
significance, possibly non-neoplastic (ASC-US); (c)
low-grade squamous intraepithelial lesion (LSIL); (d)
atypical squamous cells which cannot exclude high-
grade lesions (ASC-H); (e) high-grade squamous in-
traepithelial lesion (HSIL); and (f) squamous cell car-
cinoma (SCC).
Table 1 presents the classification groups consid-
ered for computational experiments. Our model was
built to label cells considering the binary classifica-
tion (normal and abnormal), the ternary classification
(normal cells, low-grade lesions, and high-grade le-
sions), and the classification based on the Bethesda
nomenclature (6 classes).
Table 1: Three classification categories with the number of
class samples.
Binary Ternary Bethesda Nº of samples
Normal NILM 862
Abnormal
Low grade
ASC-US 286
LSIL 536
High grade
ASC-H 598
HSIL 874
SCC 77
Total: 3,233
For the binary categorization, the abnormal cells
comprise all Bethesda labels except NILM (nor-
mal). Another possible classification is used to group
Bethesda categories into 3 (three) classes: normal
Shape-based Features Investigation for Preneoplastic Lesions on Cervical Cancer Diagnosis
507

cells (NILM), low-grade lesions (ASC-US and LSIL),
and high-grade lesions (ASC-H, HSIL, and SCC).
Low and high-grade groupings become important due
to different treatment protocols. For low-grade le-
sions, the follow-up requires a repeat screening. In
the case of high-grade lesions cells, patients should
undergo colposcopy and/or biopsy (Sung et al., 2021).
2.2 Shape-based Features
We used 200 features for each cell. The same 98 mea-
surements applied to the nucleus were calculated for
the cytoplasm. The remaining 4 comprise the two cel-
lular components. Features are related to the size,
shape, and edge contour of the nucleus (N) and the
cytoplasm (C), and some ratios between N and C:
• Size: area, bounding box, convex hull, perimeter,
equivalent diameter (circumference), minor and
major axis;
• Shape: circularity, compacity, eccentricity, con-
vexity, solidity, elongation, fractal dimension;
• Contour: roughness index, entropy, kurtosis, and
other statistics of normalized radial distance (from
the centroid to edge points). Also, the first 20 co-
efficients of the elliptic Fourier series (Kuhl and
Giardina, 1982);
• N/C relations: nucleus relative position (within
the cell), nucleus to cytoplasm ratios for the area,
perimeter, bounding box, and convex hull.
The Box Counting method was used to calculate
the fractal dimension (FD) for the cell components
(N and C) (Konatar et al., 2020). As known, the more
irregular the regions, the higher the FD value.
Elliptic Fourier coefficients are also related to
edge contour irregularities in the frequency spectrum.
The EFD method is based on the string code (con-
nectivity 8) extracted from contour points of a region
(Kuhl and Giardina, 1982). We use the first 20 EFD
coefficients for later feature selection.
Roughness index and standard deviation of radial
distance were used for cervical cells by Zhang et al.
(2014). We calculated these features as described by
Po-Hsiang Tsui et al. (2010), as they are commonly
used in breast tumor detection.
2.2.1 Feature Selection
To improve performance and get some intuition about
the interpretability of the model, we select the most
relevant features for each estimator shown in Figure
2.
At most, 30 attributes were chosen from 200 using
two methods: mutual information (MI) and simulta-
neous perturbation stochastic approximation (SPFSR)
(Akman et al., 2023).
The MI is a filter method based on the statistical
measure related to the joint entropy of the variables.
The SPFSR is based on stochastic simultaneous per-
turbation approximation. The SPFSR as a wrapper-
based proposal can be used with any classifier or re-
gression to optimize a suitable performance metric. It
is a multivariate approach that considers the interac-
tions between features so that redundancies decrease
their scores.
2.3 Computational Model
Figure 2 presents the proposed model. The procedure
starts from the two .csv files. One file contains la-
bels for each cell/image along with the set of contour
points (x, y) of the nucleus (Figure 2, f1). The other
file is analogous to that of the nucleus for the cell’s
cytoplasm (Figure 2, f2). These points are the contour
of the manual segmentation made by cytopathologists
and used here to reconstruct the cells’ masks for the
nucleus and cytoplasm. From masks, all described
features are calculated (Figure 2, fx).
Before classification, a selection of the most im-
portant features was considered for each model esti-
mator (Figure 2, fs). The filtered set of features is used
as input to the model.
Cells were classified according to Table 1 in two
ways: with independent classifiers for 2, 3, and 6
classes (Figure 2, by estimators a1, a2, and a3, re-
spectively ) and with a hierarchical classifier (Figure
2, by estimators b1, b2, b3.1, and b3.2). Our solution
implements a hierarchical classification as proposed
by Diniz et al. (2021c).
A hierarchical categorization operates in levels.
The first level defines a binary classifier to distinguish
normal and abnormal cells (Figure 2, b1). Cells iden-
tified as abnormal are reclassified as low- or high-
grade lesions at the second level (Figure 2, b2). Fi-
nally, two third-level classifiers must identify specific
lesions according to Bethesda nomenclature (Figure
2, b3.1 and b3.2). In b3.1, low-grade lesions will be
differentiated as ASC-US or LSIL. In estimator b3.2,
cells with more severe anomalies will be categorized
into ASC-H, HSIL, or SCC.
The class predictions of both approaches are eval-
uated in the end through evaluation metrics (Figure 2,
ev). We employ commonly used metrics for classifi-
cation as mentioned by Jiang et al. (2022): accuracy
(Acc), precision (P), recall (R), specificity (Spec), and
F1-score (F1).
VISAPP 2023 - 18th International Conference on Computer Vision Theory and Applications
508
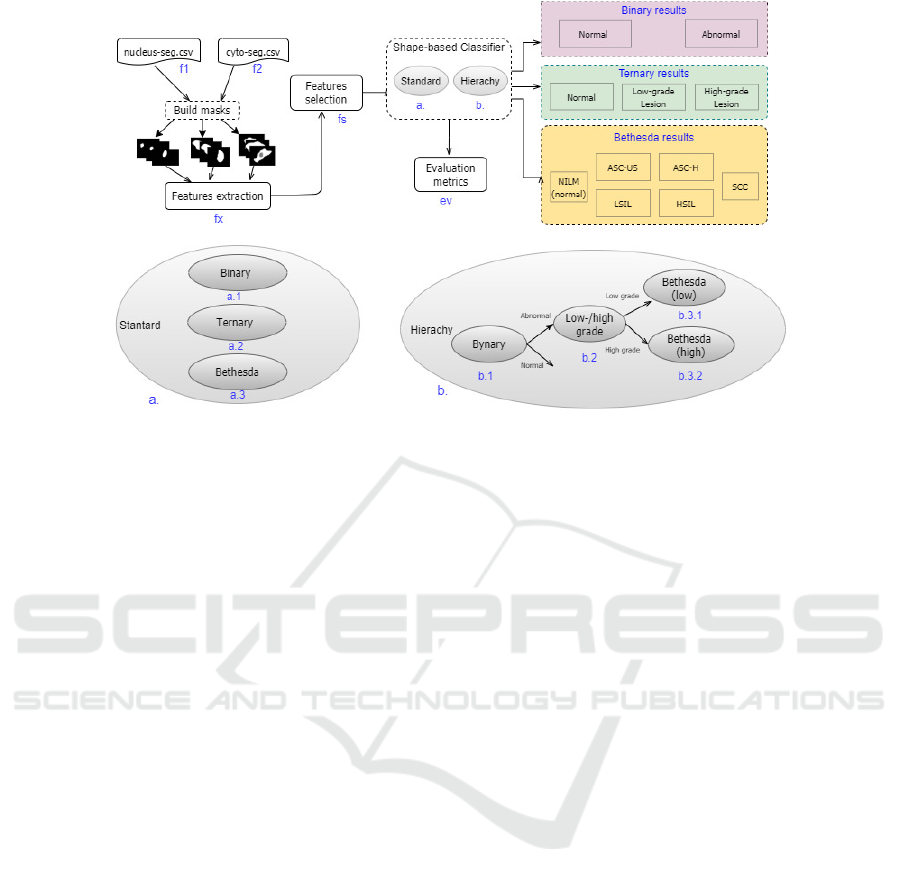
Figure 2: Shape-based diagnostic solution: CRIC-Seg files (f1, f2), attribute extraction (fx) and selection (fs), standard
classification (estimators a.1, a.2 and a.3) and hierarchical classification (b1, b2, b3.1 and b3.2 estimators).
2.3.1 Learning Algorithms
Each classifier component of Figure 2 is a traditional
machine learning (ML) algorithm: Support Vector
Machine (SVM), Random Forest (RF), and eXtreme
Gradient Boosting (XGBoost).
An SVM outputs an ordered sorted map based on
the training data using a subset of those points in
the decision function (called support vectors). Such
points define the best margins to separate samples in
classes in an n-dimensional space (Geron, 2022).
RF is a bagging method based on decision trees
(DC). It introduces randomness in selecting subsam-
ples and features from the data to build the trees. Re-
sults have a decreasing variation of the error (Geron,
2022).
XGBoost is another ensemble model based on
gradient boosting. Predictions are adjusted sequen-
tially after each weak estimator (for example, a shal-
low DC). The method improves performance, overfit-
ting, and other flexibilities (Chen and Guestrin, 2016).
2.3.2 Oversampling
While the random over-sampler technique dupli-
cates some of the original samples, other techniques
build ’synthetic’ samples based on original examples
(Chawla et al., 2004). The Synthetic Minority Over-
sampling Technique (SMOTE) and the Borderline-
SMOTE are some of these methods (Chawla et al.,
2002; Han et al., 2005). They operate in the feature
space rather than at the data level (i.e., the image).
In the SMOTE method, oversampling is done by
taking an original sample from the minority class and
introducing a new sample considering any of its near-
est k neighbors through interpolation. The difference
between the Borderline-SMOTE method and the orig-
inal SMOTE is that the former restricts the original
samples of the minority class. Selected samples in
Borderline-SMOTE are those at the borderline be-
tween the minority class and the majority classes.
3 RESULTS AND DISCUSSIONS
Experiments were written in Python (version 3.9.1)
using well-known libraries for ML and data ma-
nipulation/visualization, such as scikit-learn, SciPy,
and scikit-image. Other modules were employed
for specific tasks. For data augmentation tech-
niques (SMOTE and Borderline-SMOTE) we ap-
ply the imbalanced-learn module (https://imbalanced-
learn.org/stable/install.html). The pyEFD package
was used to calculate the Elliptic Fourier coeffi-
cients (https://pyefd.readthedocs.io/en/latest/). The
implementation of the SPFSR method used is avail-
able at (https://github.com/akmand/spFSR). Codes
for the experiments described here are avail-
able at https://github.com/danielaterra/shape-based-
CervicalCellsClassifier.
In all model executions, we used 10-fold cross-
validation with the data augmentation techniques de-
fined in Section 2.3.2. Data augmentation was ap-
plied within each fold of the training data to equal the
number of instances with those of the majority class.
The results of the experiment show that the results of
SMOTE were slightly superior to those of Borderline-
Shape-based Features Investigation for Preneoplastic Lesions on Cervical Cancer Diagnosis
509
SMOTE. The evaluation metrics presented below are
the results of experiments using SMOTE.
3.1 Features Selection Procedure
Calling pyEFD method to retrieve the first 20 EFD
coefficients results in 40 values: 20 to x variations and
20 to y. All EFD coefficients plus the other features
described in Section 2.2 were calculated from each
mask of the nucleus and cytoplasm (see Figure 2). At
most 30 features were selected from the total: 200
(when considering the nucleus and cytoplasm) or 98
for experiments applied to only one of these cellular
structures.
As mentioned in Section 2.2.1 two FS techniques
were used: MI and the SPFSR.
Table 2: Feature selection methods: binary results using MI
and SPFSR.
Acc P R Espec F1
MI
SVM 0.94 0.969 0.954 0.91 0.961
RF 0.94 0.969 0.964 0.91 0.966
XGB 0.95 0.971 0.968 0.91 0.969
SPFSR
SVM 0.94 0.969 0.950 0.91 0.959
RF 0.94 0.968 0.963 0.90 0.965
XGB 0.94 0.967 0.961 0.90 0.964
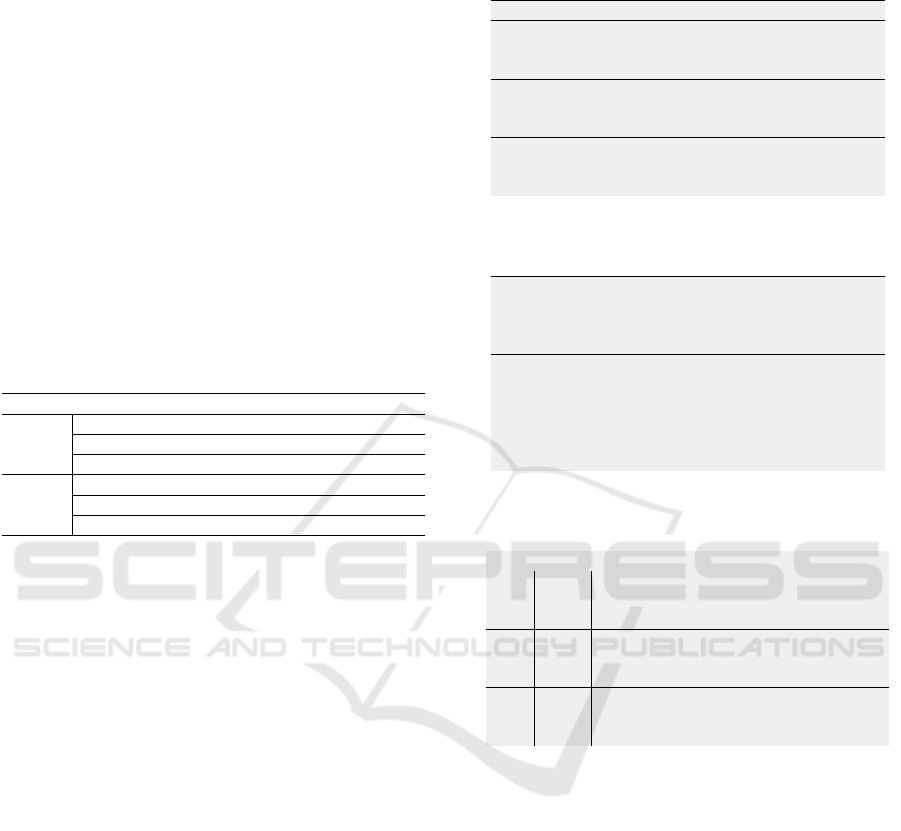
Figure 3 presents SPFSR features selected for the
normal/abnormal classification. The scores suggest
the most discriminatory attributes: 1) area of the
convex hull and the edges entropy of the nuclei; 2)
compacity and area of the cytoplasm (the larger, the
less circular or irregular); and (3) cells’ nucleus-to-
cytoplasm ratio (N/C).
Table 2 shows the classification metrics of the bi-
nary prediction using both methods. As the values
were similar, hereafter the best prediction is shown.
3.2 Experiments
We performed 3 (three) tests with the proposed solu-
tions using a different set of features: 1) shape fea-
tures selected considering nucleus, cytoplasm, and
both (N/C); 2) nucleus shape features (N); and 3) cy-
toplasm shape features(C). The first tests perform the
classification using the standard solution.
3.2.1 Standard Classification
Table 3 presents evaluation metrics for the nor-
mal/abnormal classification using attributes for the
cell (N/C), the nucleus (N), and the cytoplasm (C).
Results for detections of normal cells, low-grade or
high-grade lesions are shown in Table 4. Table 5
presents the results for the 6 (six) classes detection.
Table 3: Binary classification: from cells, nuclei, and cyto-
plasms.
Acc P R Spec F1
SVM 0.942 0.971 0.951 0.917 0.960
RF 0.952 0.970 0.966 0.912 0.967
1.
N/C
XGB 0.954 0.971 0.968 0.917 0.969
SVM 0.877 0.952 0.880 0.870 0.912
RF 0.874 0.949 0.878 0.863 0.910
2.
N
XGB 0.884 0.937 0.906 0.822 0.919
SVM 0.901 0.970 0.894 0.922 0.927
RF 0.835 0.939 0.830 0.849 0.870
3.
C
XGB 0.884 0.944 0.897 0.847 0.916
Table 4: Ternary classification: from cells, nuclei, and cy-
toplasms.
Acc P R Spec F1
SVM 0.936 0.939 0.936 0.968 0.935
RF 0.938 0.941 0.938 0.968 0.936
1.
N/C
XGB 0.941 0.943 0.941 0.970 0.939
SVM 0.695 0.708 0.695 0.843 0.689
RF 0.713 0.724 0.713 0.852 0.709
2.
N
XGB 0.711 0.720 0.711 0.848 0.707
SVM 0.915 0.917 0.915 0.957 0.913
RF 0.846 0.849 0.846 0.926 0.844
3.
C
XGB 0.884 0.888 0.884 0.943 0.882
Table 5: Bethesda classification: cells, nuclei, and cyto-
plasms.
Acc P R Spec F1
SVM 0.632 0.693 0.632 0.927 0.640
RF 0.658 0.700 0.658 0.931 0.663
1.
N/C
XGB 0.682 0.698 0.682 0.935 0.682
SVM 0.442 0.482 0.442 0.888 0.437
RF 0.475 0.493 0.475 0.892 0.469
2.
N
XGB 0.490 0.489 0.490 0.893 0.479
SVM 0.620 0.664 0.6202 0.924 0.623
RF 0.557 0.601 0.5577 0.910 0.563
3.
C
XGB 0.619 0.634 0.6190 0.921 0.617
3.2.2 Hierarchical Classification
As observed in Table 5 the model proved to be un-
feasible for a Bethesda diagnosis. Trying to improve
and analyze the results we implemented the hierar-
chical solution as depicted in Section 2.3. Tables 6-8
exhibit the results for the 2, 3, and 6 classes, respec-
tively. (Small differences in the results of tables 3 and
6 are due to the use of a 10-stratified fold considering
binary and Bethesda labels, respectively.)
Experiments confirmed that the hierarchical solu-
tion did not solve class confusion for adequate predic-
tion of specific lesions according to Bethesda System.
Figure 4 shows the results of the hierarchical classifier
in a confusion matrix. Note that most errors occurred
within the low-grade (ASCUS/LSIL) and high-grade
(ASCH/HSIL) lesion categories. Likewise, Carci-
noma diagnoses were more frequently confused with
ASCH and HSIL.
VISAPP 2023 - 18th International Conference on Computer Vision Theory and Applications
510
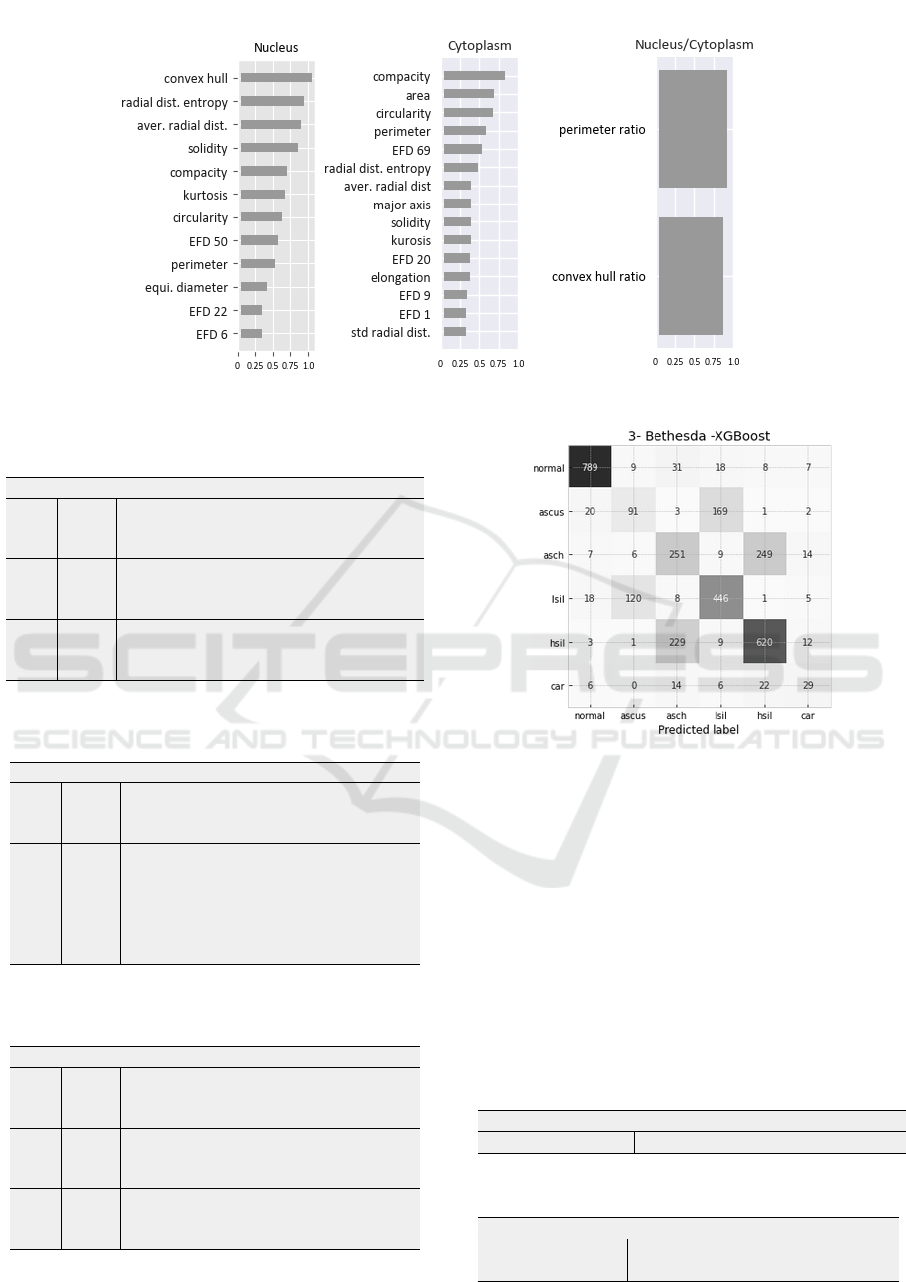
Figure 3: SPFSR relative scores: features selected to normal/abnormal classifier (Figure 2, a.1).
Table 6: Binary (hierarchical) classification: from cells, nu-
clei, and cytoplasms.
Acc P R Spec F1
SVM 0.944 0.969 0.955 0.916 0.962
RF 0.961 0.968 0.978 0.913 0.973
1.
N/C
XGB 0.960 0.969 0.977 0.915 0.973
SVM 0.879 0.950 0.881 0.872 0.914
RF 0.879 0.950 0.881 0.874 0.914
2.
N
XGB 0.887 0.933 0.911 0.822 0.922
SVM 0.9122 0.968 0.910 0.917 0.938
RF 0.8413 0.933 0.843 0.835 0.886
3.
C
XGB 0.8797 0.930 0.903 0.813 0.916
Table 7: Ternary (hierarchical) classification: from cells,
nuclei, and cytoplasms.
Acc P R Spec F1
SVM 0.928 0.929 0.928 0.964 0.928
RF 0.945 0.945 0.945 0.971 0.945
1.
N/C
XGB 0.944 0.944 0.944 0.971 0.944
SVM 0.688 0.695 0.688 0.842 0.683
RF 0.706 0.712 0.706 0.851 0.702
2.
N
XGB 0.701 0.701 0.701 0.843 0.700
SVM 0.897 0.903 0.897 0.949 0.897
RF 0.836 0.847 0.836 0.921 0.831
3.
C
XGB 0.885 0.886 0.885 0.944 0.885
Table 8: Bethesda (hierarchical) classification: from cells,
nuclei, and cytoplasms.
Acc P R Spec F1
SVM 0.631 0.665 0.631 0.926 0.642
RF 0.685 0.698 0.685 0.935 0.689
1.
N/C
XGB 0.688 0.684 0.688 0.935 0.685
SVM 0.425 0.458 0.425 0.885 0.416
RF 0.479 0.473 0.479 0.893 0.469
2.
N
XGB 0.490 0.479 0.490 0.893 0.483
SVM 0.617 0.647 0.617 0.923 0.623
RF 0.593 0.596 0.593 0.915 0.585
3.
C
XGB 0.629 0.622 0.629 0.923 0.625
Figure 4: Confusion matrix of a Bethesda classification for
N/C features using Random Forest.
3.2.3 Fourier Coefficients Results
Table 9 shows the results of running the model us-
ing only EFD features as cell shape descriptors. We
confirm the irrelevance of these descriptors by again
running the solution with all other features except the
EFD coefficients. The results are shown in Table 10.
As shown in Figure 3, EFD did not well appear to ex-
plain cervix lesions as received low scores by feature
selectors (e.g., SPFSR).
Table 9: EFD-based classification results: 30 descriptors
from cells (binary).
Acc P R Spec F1
1. N/C (XGBoost) 0.78 0.86 0.84 0.61 0.85
Table 10: Classification without EFD: from cells (binary).
Type Acc P R Spec F1
binary 0.95 0.97 0.96 0.91 0.96
1. N/C
Ternary 0.94 0.94 0.94 0.97 0.94
Shape-based Features Investigation for Preneoplastic Lesions on Cervical Cancer Diagnosis
511
3.3 Discussions
As Diniz et al. (2021c) pointed out, the recog-
nized correlation of cervical lesions with toxicolog-
ical changes in the nucleus allows an analysis based
only on this components to classify the degree of le-
sions. However, the results presented for a classifica-
tion based on morphology suggest the influence of the
cytoplasm in the diagnosis. Furthermore, we observe
that:
• Morphology attributes, as proposed in this
work, can assist a cytopathologist’s final diag-
nosis for binary (normal/abnormal) and ternary
(normal/low-/high grade) classifications. The F1-
score values from cell tests (N/C) are above 92%
(see Tables 3, 4, 6, and 7);
• The confusion matrix in Figure 4 confirms that
most errors fall within subcategories of low/high-
grade lesions. Despite the failure, it is worth re-
membering that the same clinical procedure must
be applied in cases of ASC-US and LSIL (low
grade) and in cases of ASC-H and HSIL (high
grade).
• The nucleus/cytoplasm ratio attributes for the
area, perimeter, and convex hull received high
scores in the feature selection procedures. These
features always contributed to the prediction re-
sults for cells (N/C) (see Tables 3, 4, 6 and 7).
4 CONCLUSION
This is the first work to validate a classification based
only on morphological attributes. A model for classi-
fying cervical cell lesions was evaluated according to
Bethesda System’s diagnostic classes. We extract 200
features related to the size, shape, and edge contour of
each cell from a total of 3,233 samples from a real Pa-
panicolaou image dataset (CRIC Cervix-Seg). As can
be seen in Tables 9 and 10 the discrimination test re-
sults confirm that Elliptic Fourier Descriptors (EFD)
as features showed a result lower than expected.
Table 11 presents our shape-based solution re-
sults compared to other works (Diniz et al., 2021b,a)
for cervix cell diagnosis. Both solutions employed
only cellular nuclei from image patches of the CRIC
dataset, and both performed texture analysis.
Our work suggests the dependence of other types
of attributes, such as the texture of the nucleus and
cytoplasm for discrimination of specific degrees of
lesions as the 6 (six) classes considered here from
Bethesda nomenclature. However, the proposed
morphological attributes play an important role in
Table 11: Comparison with the methods from the literature.
Method Nº classes Acc P R Spec F1
2 0.96 0.97 0.97 0.91 0.97
3 0.94 0.94 0.94 0.97 0.941.
6 0.68 0.68 0.68 0.93 0.68
2 0.95 0.95 0.95 0.95 0.95
3 0.96 0.96 0.96 0.97 0.962.
6 0.96 0.91 0.90 0.98 0.90
2 0.96 0.96 0.96 0.96 0.96
3 0.96 0.94 0.94 0.97 0.943.
6 0.95 0.85 0.85 0.97 0.85
1. Our proposal/ XGBoost,RF/ cells’morphology
2. Diniz et al. (2021b)/ RF / nuclei texture/shape
3. Diniz et al. (2021a)/ DNN/ cells’ image
binary (normal/abnormal) and ternary (normal/low
grade/high grade) classification. As shown in Ta-
ble 11, the results of our shape-based proposal for 2
(two) and 3 (three) classes were comparable to exist-
ing works using the CRIC dataset.
ACKNOWLEDGEMENTS
Daniela C. Terra acknowledges the support of
the Federal Institute of Minas Gerais (IFMG).
We also acknowledge Federal University of Ouro
Preto (UFOP), FAPEMIG [APQ-00751-19, APQ-
01306-22, APQ-01518-21, PPSUS-FAPEMIG/APQ-
03740-17]; CNPq [303266/2019-8, 305895/2019-
2, 308947/2020-7]; Pr
´
o-Reitoria de Pesquisa, P
´
os-
Graduac¸
˜
ao e Inovac¸
˜
ao - PROPPI/UFOP [19/2020,
23109.000928/2020-33, and 23109.000929/2020-
88]; CAPES, and Ministry of Health [905103/2020].
REFERENCES
Akman, D. V., Malekipirbazari, M., Yenice, Z. D., Yeo, A.,
Adhikari, N., Wong, Y. K., Abbasi, B., and Gumus,
A. T. (2023). k-best feature selection and ranking via
stochastic approximation. Expert Systems with Appli-
cations, 213:118864.
Bhowmik, M. K., Roy, S. D., Nath, N., and Datta, A.
(2018). Nucleus region segmentation towards cervical
cancer screening using AGMC-TU Pap-smear dataset.
In ACM Int. Conf. Proceeding Series, pages 44–53,
New York, New York, USA. ACM Press.
Chankong, T., Theera-Umpon, N., and Auephanwiriyakul,
S. (2014). Automatic cervical cell segmentation and
classification in Pap smears. Computer Methods and
Programs in Biomedicine, 113(2):539–556.
Chawla, N. V., Bowyer, K. W., Hall, L. O., and Kegelmeyer,
W. P. (2002). SMOTE: synthetic minority over-
sampling technique. Journ. of Art. Intel. Research,
16:321–357.
VISAPP 2023 - 18th International Conference on Computer Vision Theory and Applications
512

Chawla, N. V., Japkowicz, N., and Kotcz, A. (2004). Edi-
torial: Special issue on learning from imbalanced data
sets. SIGKDD Explor. Newsl., 6(1):1–6.
Chen, T. and Guestrin, C. (2016). XGBoost: A Scalable
Tree Boosting System. In Proceedings of the 22nd
ACM SIGKDD Int. Conf. on Knowledge Discovery
and Data Mining, pages 785–794, New York, NY,
USA. ACM.
Das, M. (2021). WHO launches strategy to accelerate
elimination of cervical cancer. The Lancet Oncology,
22(1):20–21.
Diniz, D. N., Rezende, M. T., Bianchi, A. G., Carneiro,
C. M., Luz, E. J., Moreira, G. J., Ushizima, D. M.,
de Medeiros, F. N., and Souza, M. J. (2021a). A deep
learning ensemble method to assist cytopathologists
in pap test image classification. Journal of Imaging,
7(7).
Diniz, D. N., Rezende, M. T., Bianchi, A. G. C., Carneiro,
C. M., Ushizima, D. M., de Medeiros, F. N. S., and
Souza, M. J. F. (2021b). A Hierarchical Feature-Based
Methodology to Perform Cervical Cancer Classifica-
tion. Applied Sciences, 11(9):2–19.
Diniz, D. N., Vitor, R. F., Bianchi, A. G. C., Delabrida,
S., Carneiro, C. M., Ushizima, D. M., de Medeiros,
F. N. S., and Souza, M. J. F. (2021c). An ensemble
method for nuclei detection of overlapping cervical
cells. Expert Systems with Applications, 185:115642.
Dong, N., Zhao, L., Wu, C., and Chang, J. (2020). Inception
v3 based cervical cell classification combined with ar-
tificially extracted features. Applied Soft Computing,
93:106311.
Geron, A. (2022). Hands-on machine learning with Scikit-
Learn, Keras, and TensorFlow. O’Reilly Media, Inc.
Han, H., Wang, W.-Y., and Mao, B.-H. (2005). Borderline-
SMOTE: a new over-sampling method in imbalanced
data sets learning. In Int. conf. on intelligent comput-
ing, pages 878–887. Springer.
Jantzen, J., Norup, J., Dounias, G., and Bjerregaard, B.
(2005). Pap-smear Benchmark Data For Pattern Clas-
sification. Proc. NiSIS 2005, Albufeira, Portugal,
pages 1–9.
Jiang, H., Zhou, Y., Lin, Y., Chan, R. C., Liu, J., and Chen,
H. (2022). Deep learning for computational cytology:
A survey. Medical Image Analysis, page 102691.
Konatar, I., Popovic, T., and Popovic, N. (2020). Box-
Counting Method in Python for Fractal Analysis of
Biomedical Images. 2020 24th Int. Conf. on Informa-
tion Technology, IT 2020, (February).
Kuhl, F. P. and Giardina, C. R. (1982). Elliptic fourier fea-
tures of a closed contour. Computer Graphics and Im-
age Processing, 18(3):236–258.
Li, X., Xu, Z., Shen, X., Zhou, Y., Xiao, B., and Li, T.-
Q. (2021). Detection of Cervical Cancer Cells in
Whole Slide Images Using Deformable and Global
Context Aware Faster RCNN-FPN. Current Oncol-
ogy, 28(5):3585–3601.
Mariarputham, E. J. and Stephen, A. (2015). Nominated
Texture Based Cervical Cancer Classification. Com-
putational and Mathematical Methods in Medicine,
2015:1–10.
Marinakis, Y., Dounias, G., and Jantzen, J. (2009). Pap
smear diagnosis using a hybrid intelligent scheme fo-
cusing on genetic algorithm based feature selection
and nearest neighbor classification. Computers in Bi-
ology and Medicine, 39(1):69–78.
Nayar, R. and Wilbur, D. C. (2015). The bethesda system for
reporting cervical cytology: Definitions, criteria, and
explanatory notes. Springer International Publishing.
Po-Hsiang Tsui, Yin-Yin Liao, Chien-Cheng Chang, Wen-
Hung Kuo, King-Jen Chang, and Chih-Kuang Yeh
(2010). Classification of Benign and Malignant Breast
Tumors by 2-D Analysis Based on Contour Descrip-
tion and Scatterer Characterization. IEEE Trans. on
Med. Imaging, 29(2):513–522.
Rahaman, M. M., Li, C., Yao, Y., Kulwa, F., Wu, X., Li, X.,
and Wang, Q. (2021). DeepCervix: A deep learning-
based framework for the classification of cervical cells
using hybrid deep feature fusion techniques. Comput-
ers in Biology and Medicine, 136:104649.
Rezende, M. T., Silva, R., Bernardo, F. d. O., Tobias,
A. H., Oliveira, P. H., Machado, T. M., Costa, C. S.,
Medeiros, F. N., Ushizima, D. M., Carneiro, C. M.,
and Bianchi, A. G. (2021). Cric searchable image
database as a public platform for conventional pap
smear cytology data. Scientific Data, 8(1):151.
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjo-
mataram, I., Jemal, A., and Bray, F. (2021). Global
cancer statistics 2020: Globocan estimates of in-
cidence and mortality worldwide for 36 cancers in
185 countries. CA: a cancer journal for clinicians,
71(3):209–249.
Teixeira, J. B. A., Rezende, M. T., Diniz, D. N., Carneiro,
C. M., Luz, E. J. d. S., Souza, M. J. F., Ushizima,
D. M., de Medeiros, F. N. S., and Bianchi, A. G. C.
(2022). Segmentac¸
˜
ao autom
´
atica de n
´
ucleos cervicais
em imagens de Papanicolaou. In Anais do XXII Simp.
Bras. de Computac¸
˜
ao Aplicada
`
a Sa
´
ude, pages 346–
357. Soc. Bras. de Computac¸
˜
ao.
Umadi, A., Nagarajan, K., Venkatesha, J. B., Ganesh, A.,
and George, K. (2020). Automated Segmentation of
Overlapping Cells in Cervical Cytology Images Using
Deep Learning. In 2020 IEEE 17th India Council Int.
Conf.e, INDICON 2020, pages 1–7. IEEE.
Williams, A. (2021). Cervical cancer: what’s new in
squamous cell neoplasia. Diagnostic Histopathology,
27(12):478–482.
Yakkundimath, R., Jadhav, V., Anami, B., and Malvade,
N. (2022). Co-occurrence histogram based ensem-
ble of classifiers for classification of cervical cancer
cells. Journal of Electronic Science and Technology,
20(3):100170.
Zhang, L., Kong, H., Ting Chin, C., Liu, S., Fan, X., Wang,
T., and Chen, S. (2014). Automation-assisted cervi-
cal cancer screening in manual liquid-based cytology
with hematoxylin and eosin staining. Cytometry Part
A, 85(3):214–230.
Zhao, Y., Fu, C., Xu, S., Cao, L., and Ma, H.-f.
(2022). LFANet: Lightweight feature attention net-
work for abnormal cell segmentation in cervical cy-
tology images. Computers in Biology and Medicine,
145:105500.
Shape-based Features Investigation for Preneoplastic Lesions on Cervical Cancer Diagnosis
513
