
Data Augmentation, Multimodality, Subject and Activity Specificity
Improve Wearable Electrocardiogram Denoising with Autoencoders
Jo
˜
ao Areias Saraiva
1,2,3 a
, Mariana Abreu
1,3 b
, Ana Sofia Carmo
1,3 c
, Ana Fred
1,3 d
and Hugo Pl
´
acido da Silva
1,3 e
1
Department of Bioengineering, Instituto Superior T
´
ecnico, Univeristy of Lisbon, Portugal
2
Department of Computer Science and Engineering, Instituto Superior T
´
ecnico, Univeristy of Lisbon, Portugal
3
Pattern and Image Analysis Group, Instituto de Telecomunicac¸
˜
oes, Portugal
Keywords:
Biosignal Denoising, Electrocardiogram, Accelerometry, Ambulatory Wearables, Data Augmentation.
Abstract:
Event detection based on biosignals continuously acquired by wearable devices has become an emergent
topic. Particularly, real-time event detection with the electrocardiogram (ECG) has been explored to monitor
heart conditions and epileptic seizures in the ambulatory. However, ECG acquired in the ambulatory is much
more prone to noise and artifacts, due to the dynamic nature of these environments. Therefore, real-time and
robust ECG denoising methods are crucial if event detection is meant to succeed. Denoising autoencoders
(DAEs) are studied as robust and fast methods to attenuate ECG noise and artifacts. ECG data augmentation
techniques are shown to effectively improve the performance of such a deep learning method. Activity and
subject specific models are shown to output better ECG denoised estimates, than non-specific ones. And using
accelerometry (ACC) as noise reference exemplifies how biosignal multimodality improves ECG attenuation
of muscle and motion artifacts. Therefore, this work establishes effective design techniques to be considered
when engineering ECG deep learning models.
1 INTRODUCTION
Portable electrocardiography for the ambulatory is
currently a necessity to monitor patients outside the
hospital (Bansal and Joshi, 2018; Bayoumy et al.,
2021). At the same time, automation of that moni-
toring has become an emerging topic (Prieto-Avalos
et al., 2022), since human professional monitoring
is infeasible at all times. Events of interest to be
detected in real-time are, for instance, atrial fibril-
lation (Abu-Alrub et al., 2022), heart failure (Chen
et al., 2021), epileptic seizures (Vandecasteele et al.,
2021), and falls (Butt et al., 2021). Even for non-
medical use, commercial wearable devices to continu-
ously record the electrocardiogram (ECG), have been
marketed for fitness purposes (e.g., Fitbit
1
), or even
a
https://orcid.org/0000-0003-3715-0304
b
https://orcid.org/0000-0002-9340-6610
c
https://orcid.org/0000-0001-7954-3718
d
https://orcid.org/0000-0003-1320-5024
e
https://orcid.org/0000-0001-6764-8432
1
fitbit.com; accessed in Nov 2022
for everyday check-ups (e.g., Withings Move
2
).
However, the ambulatory recording of ECG
presents its challenges, primarily because the environ-
ment outside the hospital is dynamic and uncontrolled
(Rodrigues et al., 2017). Therefore, and adding up to
the fact that wearable hardware is usually less robust
than clinical-grade instruments, the ECG acquired in
the ambulatory is more likely to become contami-
nated with noise and artifacts (Chatterjee et al., 2020).
A highly distorted ECG signal will interfere with the
ability of any event detection algorithm to correctly
interpret it, and, consequently, event detection will
fail (Mohd Apandi et al., 2020). If that would be the
case, and if the clinical team is counting on the wear-
able to make informed decisions, those will become
conditioned, and the patient’s clinical condition can
become compromised. Therefore, before event de-
tection takes place, real-time robust ECG denoising
constitutes an important preprocessing step.
Denoising autoencoders (DAEs) have been widely
proposed to denoise the ECG (Arsene, 2020; Nur-
maini et al., 2020; Xiong et al., 2015; Chiang et al.,
2
withings.com/withings-move; accessed in Nov 2022
Saraiva, J., Abreu, M., Carmo, A., Fred, A. and Plácido da Silva, H.
Data Augmentation, Multimodality, Subject and Activity Specificity Improve Wearable Electrocardiogram Denoising with Autoencoders.
DOI: 10.5220/0011883400003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 4: BIOSIGNALS, pages 133-145
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
133

2019; Reljin et al., 2020). These works have achieved
great performances, however usually synthetic or real
noise time series are retrospectively added to the clean
ECG, which simplifies the DAE task to undoing an
additive operation. In contrast, this work proposes
a framework to train DAEs to map noisy ECG ac-
quired with wearable electrodes to the simultaneously
acquired ECG with gel electrodes.
We found out that, in this way, the denoising task
becomes much more difficult, for the goal is no longer
to merely undo a linear operation, but rather to atten-
uate non-stationary noise processes that may interact
with each other in non-linear ways. Hence, addition-
ally, design techniques were studied to improve DAE
performance, namely data augmentation, activity and
subject specificity, and multimodality. It is hypothe-
sised that these are four crucial design techniques that
should always be followed when engineering ECG
deep learning models:
Data Augmentation. Deep learning (DL) models
must be trained with a high number of labelled ex-
amples (Goodfellow et al., 2016). On the one hand,
the number of examples needs to be large enough
so that the model is able to generalise well. On the
other hand, the train examples need to be represen-
tative of the heterogeneity the model will be tested
against, otherwise it may lead to overfitting; but they
also cannot be too heterogeneous, otherwise it may
lead to underfitting. Overfitting and underfitting phe-
nomena must be avoided when engineering machine
learning (ML) models, and they can be prevented with
a high number of examples balanced in heterogene-
ity. A common problem in training these models is,
therefore, to access large datasets, correctly labelled,
with balanced heterogeneity, particularly for biosig-
nal datasets. A popular approach to solve this is to ex-
pand the datasets using data augmentation techniques
(Pan et al., 2020; Huerta et al., 2021).
Activity-Specific Models. The ECG acquired while
subjects execute different daily life activities is prone
to contain very specific noise processes, resulting
from the specific form of motion those activities in-
troduce. It is hypothesised that these noise processes
are somewhat similar for each activity. If so, DAEs
should, in theory, be better at denoising ECG of a
given activity if trained only with ECGs of that ac-
tivity. These are called activity-specific models.
Subject-Specific Models. Subject variability is also
responsible for different ECG waveforms and noise
processes (Ashley and Niebauer, 2004), related with
physiological and anatomical variance, or the individ-
ual way a subject executes an activity, or even the
way they wear the device. Hence, subject-specific
P
Q
R
S
T
Atrial
Depolarization
Ventricular
Depolarization
Ventricular
Repolarization
Figure 1: Illustration of a typical ECG waveform. QRS
complex (in blue) refers to the Q, R and S curves together.
DAEs are hypothesised to perform better than subject-
independent ones.
Multimodality. Employment of multiple biosignal
modalities, such as respiration (An et al., 2022),
and accelerometry (ACC) (Raya and Sison, 2002;
Abecassis et al., 2018), has shown to be of added
value; in a denosing task this is often coined a noise-
reference. In this work, it is hypothesised that ACC
contains useful motion information that can be used
to attenuate motion artifacts present on the ECG.
The remainder of the article is organised as fol-
lows. Section 2 introduces the background on chest
ECG, the common noise processes it presents, how
its overall quality can be mathematically evaluated,
and how chest ACC can serve as a complementary
way to document the torso physiology. Section 3 de-
scribes how chest ECG and ACC were experimentally
acquired with volunteers performing everyday tasks.
Section 4 introduces the DAE architecture used to test
these hypotheses and its general training process. Fi-
nally, the remaining sections discuss the impact of the
techniques introduced before: data augmentation in
Section 5.1, activity specificity in Section 5.2, sub-
ject specificity in Section 5.3, and multimodality in
the training process in Section 5.4. Conclusions and
applications are discussed in Section 6.
2 WEARABLE
ELECTROCARDIOGRAMS
The ECG measures the electrical activity of the heart
at the body surface. The recorded biosignal corre-
sponds to changes in the polarisation of cardiac mus-
cle tissue, which is responsible for the coordinated
contraction of the heart. Cardiomyocytes are depo-
larised by the action potentials generated by a spe-
cialised conducting system, giving rise to the differ-
ent phases of the cardiac cycle. Each cardiac cycle is
comprised of three stages: the atrial contraction, the
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
134

inter-ventricular propagation, and the ventricular con-
traction (Ashley and Niebauer, 2004). In the absence
of cardiac dysfunctions, the electrocardiogram (ECG)
signal presents a characteristic pattern, depicted in
Figure 1, which comprises five main waves – P, Q,
R, S and T – that are produced by those three stages.
A clinical-grade ECG would be acquired by at-
taching two to ten wet electrodes on the chest and
limbs, each providing a different view angle and di-
rection – a lead – from which it acquires the heart de-
polarisation. However, ambulatory and wearable de-
vices usually do not acquire signals with twelve leads,
but rather with just one or two on the chest, abdomen
or left upper limbs. Also, wearables often use dry
electrodes, whereas in clinical units wet electrodes are
used (Bansal and Joshi, 2018).
From the ECG time series, it is usually derived an-
other one called the R-R peak interval (RRI) time se-
ries. This corresponds to the time difference between
each consecutive pair of R peaks. From the RRI, the
heart rate variability (HRV) features are commonly
extracted, which is a set of statistics, rich in physio-
logical information, that can serve as biomarkers for
the detection of events of interest (Behbahani et al.,
2013). Since the computation of the RRI relies on
the accurate identification of the R peaks, it is cru-
cial for event detection algorithms that ECG segments
present enough quality for the identification of all R
peaks. Even for highly contaminated ECG segments,
they can still be of value for event detection if the R
peaks are identifiable (Munoz-Minjares et al., 2021).
2.1 Noise and Artifacts Present in ECG
Electrodes capture any electrical potentials, whatever
they are, and do not distinguish between what truly
is our signal of interest and everything else that is
not. So, when acquiring ECG, specially with dry
electrodes, the ECG trace is susceptible to numerous
noise and artifact sources. Background noise, η, and
the signal of interest, y, are usually additive, which
means that both components superimpose each other
and may become indistinguishable in the acquired
time series, x, that is, x[n] = y[n] + η[n]. Next are
introduced the common noise processes described in
the literature (Semmlow and Griffel, 2014).
Power-line Interference (PLI). Offsets due to
electrical couplings between external electromagnetic
fields and the human body (50Hz in Europe and 60Hz
in the US). The most traditional and simple method to
remove PLI is to apply a notch filter (Kutz, 2010).
Baseline Wander (BW). Pressure on the electrodes
during acquisition will cause deformation of the
skin and, consequently, variations in skin impedance,
which, in turn, will create an offset potential in the
acquired signal. Also, chest and diaphragm move-
ment due to respiration, gastrointestinal movements,
and normal gating are sufficient to induce BW. Res-
piration movements of the rib cage produce a wander
on the base axis of the recorded ECG trace, that is, the
baseline moves up and down, rather than maintaining
constant. This can cause T waves to be higher than
R waves, which may end up being detected as false
R peaks (Kutz, 2010). The BW spectrum is usually
lower than 0.5–1 Hz, hence the most traditional way
to remove it being with a highpass filter. However, if
it presents spiky or trendy structures, losing its quasi-
sinusoidal morphology, a highpass filter can come as
a naive strategy (Mohaddes et al., 2020).
Myogenic Noise (EMG). Generated by skeletal
muscle activity. EMG typically ranges from 10 Hz to
5 kHz, whereas the ECG typically ranges from 0.05
Hz to 100 Hz (Kutz, 2010). Hence, at least up to
a point, both biosignals can be separated in the fre-
quency domain. Traditionally, bandpass filters are
used to attenuate EMG noise; however EMG is a
nonstationary and nonlinear biopotential (Mohaddes
et al., 2020), so this is often an insufficient effort.
Electrodermal Noise (EDA). Accumulation of
sweat under the electrodes changes the skin
impedance, in turn changing the skin electrical po-
tential. This noisy offset varies in a pressure, temper-
ature, hydration, and time dependent manner, hence
the superimposed drifts can be difficult to remove
from low-frequency ECG components (Kutz, 2010).
Motion Artifacts (MAs). In daily life activities,
limbs and trunk movement and normal gating can cre-
ate artifacts in the ECG that look like physiological
features, although they are not. This kind of move-
ments can be seen as a nonstationary and nonlinear
process (Mohaddes et al., 2020). Dry electrodes are
particularly prone to MAs, because upon motion elec-
trodes often stop touching the skin for a few mo-
ments, creating an air gap, which translates into an
increased capacitance in the interface. Conversely,
the gel present in wet electrodes helps to minimise
impedance variations caused by MAs (Kutz, 2010).
2.2 ECG Signal Quality Indexes
Two ECG signal quality indexes (SQIs) (Clifford
et al., 2012; Li et al., 2007; Li et al., 2014) can be
used to empirically evaluate the ECG quality. The
first is the kurtosis signal quality index (kSQI):
Data Augmentation, Multimodality, Subject and Activity Specificity Improve Wearable Electrocardiogram Denoising with Autoencoders
135

Figure 2: Biosignal acquisition setup. Left: chest electrocardiogram acquired with dry electrodes embedded in the band
textile (Band-ECG) and chest electrocardiogram acquired with gel electrodes (Gel-ECG) placement. Right: Body location of
all modalities.
kSQI =
E{(y −
¯
y)
4
}
σ
4
y
, (1)
where y is any ECG signal,
¯
y its mean, σ
y
its stan-
dard deviation, and E{·} is the expected value, i.e.,
the average. It evaluates the absence of noise in gen-
eral, and segments with kSQI ≤ 5 present high levels
of noise and unsatisfactory quality. The second SQI
is the R-quantity signal quality index (qSQI), which
quantifies the agreement of any two R-peak detection
algorithms with the ratio:
qSQI =
2R
R
1
+ R
2
, (2)
where R
1
and R
2
are the number of R peaks detected
by each of the chosen algorithms, and R is the number
of R peaks detected in common by both algorithms,
assumed to be the true number of R peaks. ECG seg-
ments with qSQI > 0.9 can be considered with sat-
isfactory quality. In this work, the R-peaks detectors
used to compute qSQI were the Hamilton algorithm
(Hamilton, 2002) and the Christov algorithm (Chris-
tov, 2004), as suggested in (Saraiva, 2022a).
2.3 Chest and Torso Movement
Chest ACC can be acquired to complement the ECG.
ACC sensors convert motion into electrical voltage
based on the gauge effect, capacitive, or piezoelectric
physical phenomena. The recorded signals are usu-
ally measured in meters per squared second (m.s
−2
)
or g-force units (1g ≈ 9.81m.s
−2
) (Kavanagh and
Menz, 2008). Usually, three ACC channels are ac-
quired, one per each dimension of the physical space,
which allow us to extract translational and rotational
information about the torso movement.
3 EXPERIMENTAL DATASET
ACQUISITION
An acquisition protocol was devised to collect human
biosignals in dynamic environments, which was ap-
proved by the Ethics Committee of Instituto Superior
T
´
ecnico, with the reference 22/2022. A set of 17 sub-
jects volunteered to participate in our biosignal acqui-
sition sessions. The cohort comprehends 59% male
and 41% female Caucasian subjects. The median age
is 24 years old, with the younger subject having 18
and the older subject having 57 years old. At the time
of acquisition, eleven subjects had recovered from a
COVID-19 infection in the previous six months. No
subject had a history of cardiac disease, implanted de-
vices, pain or difficulty in breathing. Every subject
participated voluntarily in this study, having signed an
informed consent, that authorises the use and sharing
of all data for research.
3.1 Hardware Setup
A chestband device was crafted out of a Scien-
tISST board
3
for the purpose of this study. Scien-
tISST boards are general-purpose biosignal acquisi-
tion boards, which can be modified according to re-
search needs. Four sensors were soldered to four ana-
logue channels of the board: two ECG sensors, a 3-
axis ACC sensor, and a respiration sensor. All sen-
sors acquired the respective biosignals at a sampling
frequency of 300 Hz.
The ScientISST board, the sensors and all associ-
ated hardware were embodied into a textile chestband
from Polar
4
, similar to the ones our group uses to
monitor patients with epilepsy at the hospital (Carmo
3
scientisst.com/sense; accessed in Dec 2022
4
polar.com/en/products/accessories/polar-soft-strap;
accessed in Dec 2022
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
136
ECG
ACC
DAE
BW FILTERINGNORMALIZATION
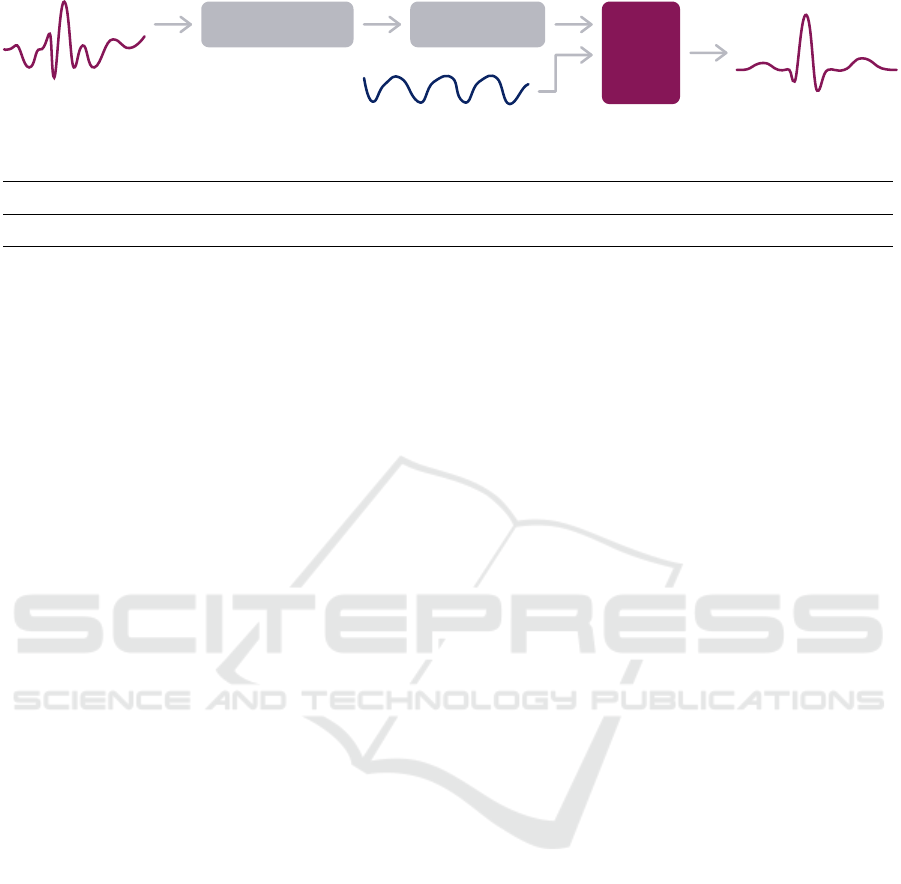
Figure 3: Proposed ECG denoising pipeline. Inputs: Noisy ECG and correspondent ACC. Output: Denoised ECG estimate.
Table 1: Useful recorded duration (in minutes) in each session.
A B C D E F G H I J K L M N O P Q R
45.2 27.6 46.1 39.9 31.7 36.1 41.1 31.05 36.1 40.4 41.1 44.4 16.4 17.6 45.4 15.0 47.8 33.1
et al., 2022). One ECG sensor was connected to the
dry conductive plastic electrodes of the chestband,
hence from herein it will be referred to as Band-ECG.
The other ECG sensor was connected to disposable
wet electrodes, hence it will be referred to as Gel-
ECG. Two of the wet electrodes were placed con-
tralateraly on the chest (Lead I), mirroring the textile
electrodes. The ground electrode was placed on the
left iliac crest. Since the activities performed in these
acquisitions involved high-amplitude movement and
high sweat release, the Gel-ECG wet electrodes were
secured in place with Transpore surgical tape, con-
trarily to the wearable dry electrodes. As will be-
come clear ahead, the Gel-ECG signal will be used
as ground-truth of the Band-ECG signal. Hence, both
were placed to capture identical Lead I ECG, as de-
picted in Figure 2.
3.2 Preprocessing
Both ECG sensors included an analogue [0.5, 40] Hz
passband filter. Two median filters were applied to
the Band-ECG time series, to estimate BW, the out-
put of which was subtracted from the original ECG,
resulting in BW-free time series. This preprocessing
has been validated in previous works (Xia et al., 2018;
Saraiva, 2022b), for robust real-time BW denoising of
ECG acquired in nonstationary and uncontrolled en-
vironments. Hence, the following experiments on the
Band-ECG start from with this preprocessed time se-
ries, as illustrated in Figure 3.
The Gel-ECG time series were enhanced with a
FIR filter of [1.2, 40] Hz passing band and order of
250. This is because the Gel-ECG channel will serve
as ground-truth of Lead I ECG of each subject.
No filtering was applied to the ACC time series,
as conveyed in Figure 3.
3.3 Acquisition Protocol
In each session, the volunteers were asked to orderly
perform the following activities:
1. Lift: To repeatedly lift a heavy object;
2. Greetings: To repeatedly handshake and to wave;
3. Gesticulate: To gesticulate while talking;
4. Jumps: To jump repeatedly;
5. Walk-Before: To walk outside before running;
6. Run: To run outside;
7. Walk-After: To walk outside after running.
This protocol was considered to be illustrative of
daily life activities that are prone to hinder ECG qual-
ity. These activities either due to excessive sweat re-
lease, associated motion, or both, usually lead to noise
and artifacts in the recorded biosignals.
For different reasons, not all subjects performed
all activities, and the running and walking durations
were different for each subject. Table 1 shows the use-
ful recorded duration of each session. One of the sub-
jects volunteered for two sessions on different days,
hence there are a total of 18 sessions, identified from
A to R. In each session, a median of 55 useful minutes
were recorded, summing up to a cohort total of 10.58
hours. By ”useful” it should be understood ”after dis-
carding the periods in which no activity was being ex-
ecuted”.
3.4 Initial Quality Assessment
Cohort median kSQI of Band-ECG channels was 3.5
times lower than that of Gel-ECG signals, convey-
ing that more noise was present on the wearable-
alike ECG. Particularly, Lift, Jumps, Run, and Walk-
After segments showed unacceptable kSQI below 5.
Moreover, the median R peak detection agreement of
two detectors (qSQI) was 1.00 when using Gel-ECG
for all activities, except Jumps (0.98), whereas using
Band-ECG signals was 0.96 on average. In particular,
Run segments showed qSQI below 0.9 for Band-ECG
signals. Therefore, the ECG acquired with the chest
band presents as noisier and with poorer quality than
that acquired with gel electrodes, not necessarily just
because of the differences in hardware, but also due
to its resilience to the executed activities.
Data Augmentation, Multimodality, Subject and Activity Specificity Improve Wearable Electrocardiogram Denoising with Autoencoders
137
4 DENOISING AUTOENCODER
DAEs were designed to attenuate EMG noise, EDA
noise, MAs, and any other type of process that devi-
ates the Band-ECG waveform from that of the Gel-
ECG. Unlike other denoising methods, DAEs allevi-
ate the need to characterise the noise processes, and
instead they estimate how a version of the signal with-
out noise would look like (Goodfellow et al., 2016).
This is because these models undergo a training pro-
cess with the goal of learning an implicit representa-
tion of the ECG trace without noise. This represen-
tation is an internal state of neural network’s weights
and biases (the model’s parameters) that, when ap-
plied to a noisy ECG segment, output a denoised ver-
sion of it. These parameters are learned from large
datasets of input (noisy) and output (clean) pairs.
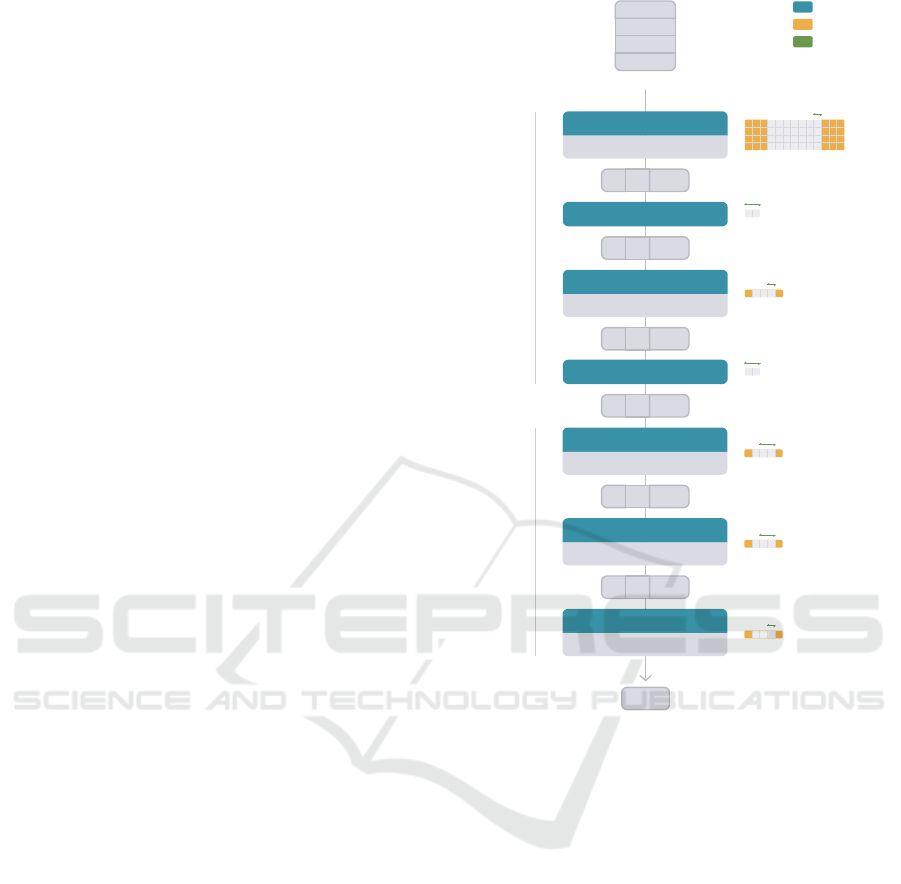
4.1 Architecture
As aforementioned in Section 1, it is hypothesised
that the motion information of the torso, present in the
ACC time series, is correlated, causally or not, with
the MAs present in the ECG time series, and that this
information can be used to attenuate them. Hence,
the DAE inputs are (4, 300) matrices, where 4 is the
number of segments (1 ECG + 3 ACC), and 300 is
the number of samples of each, which corresponds to
1 second. The ECG segment is timely synchronised
with the ACC segment. Figure 4 illustrates these ma-
trices, as well as the complete network architecture,
which was inspired in (Abecassis et al., 2018). The
network architecture is divided in two modules: en-
coder and decoder.
The encoder task is to represent the input seg-
ments in a latent space, that isolates noise and arti-
facts from the ECG process. In our approach, the en-
coder module has four layers. The input passes first
by a 2D convolutional layer that extracts 8 features
with a kernel of size (4, 7). In this process, the kernel
strides (or shifts) every 1 sample. Additionally, three
samples of padding are added to each input. The ac-
tivation function of this layer is the hyperbolic tan-
gent. Then, a maximum pooling layer of kernel size
(2, 1), which strides by two, selects the maximum of
each pair of values in each feature, reducing the fea-
ture length from 300 to 150. A second 2D convo-
lutional layer extracts 4 features using a kernel size
(3, 1), which strides by one. One value of padding is
added to each input feature. The same activation func-
tion is used of this layer. A second maximum pooling
layer, equivalent to the one before, selects the maxi-
mum of each pair of values in each feature, reducing
the feature length from 150 to 75. Therefore, the la-
2
1
2
3
1
1
1
2
1
2
7
4
3
1
2D CONVOLUTIONAL
tanh
1
300
2D T-CONVOLUTIONAL
tanh
2D T-CONVOLUTIONAL
tanh
2D T-CONVOLUTIONAL
tanh
3
1
1
1
3
1
1
2
3
1
1
2
8 1 300
MAX POOLING
8 1 150
2D CONVOLUTIONAL
tanh
4 1 150
MAX POOLING
4 1 75
8 1 150
8 1 300
ECG
4
300
ECG
ACC X
ACC Y
ACC Z
ENCODER
DECODER
Padding
Layer
Stride
Figure 4: Proposed DAE architecture. Kernel sizes on the
right side of each layer. Tensor sizes in (C, H, W) format.
tent space has a size of 4 features of 75 points each,
i.e., downsampling occurs from 300 to 75 points.
The decoder task is to recover the segment to the
original space, potentially outputting a denoised ver-
sion of the input. The decoder module has three 2D
transpose convolutional layers, that reverse the en-
coder process. The first layer takes 4 features and out-
puts 8, using a kernel of size (3, 1). It strides by two
and outputs with (1, 0) padding, increasing the feature
length from 75 to 150. The second layer also outputs
8 features, using a kernel of size (3, 1). It strides by
two and outputs with (1, 0) padding, increasing the
feature length from 150 to 300. These two layers are
activated by the hyperbolic tangent. The last layer en-
compasses the 8 features into 1, using a kernel of size
(3, 1), and has no activation function. Its output is the
potentially denoised ECG segment.
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
138
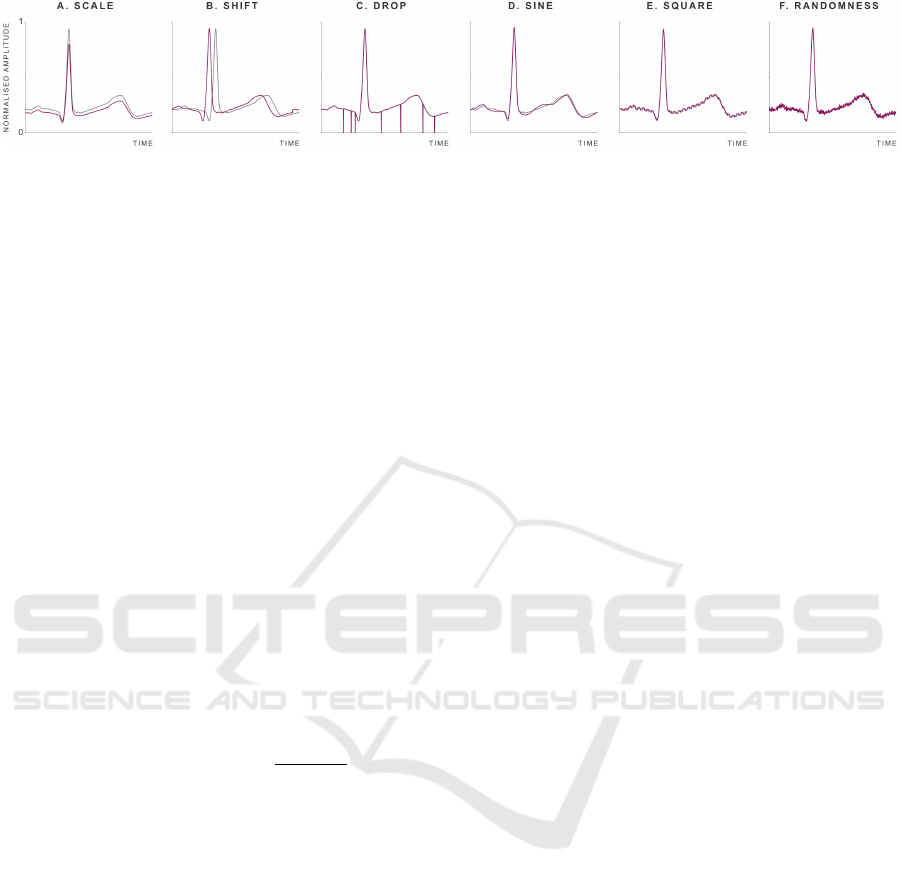
Figure 5: Examples of derived ECG segments when applying data augmentation techniques, in pink. Original time series in
grey. Scale M = 0.85. Shift D = 0.05. Drop p = 0.02. Sine A = 0.02. Square A = 0.01. Randomness A = 0.01. Illustrative
heartbeat segment of 700 ms.
4.2 Training and Evaluation
The DAEs were trained using the Adam optimizer
(Kingma and Ba, 2014). Each network was allowed
to be trained for as many epochs as needed until con-
vergence was reached. Convergence was defined by
the validation loss not decreasing for 20 epochs. The
output loss of each iteration was computed using the
mean squared error (MSE) against the targets, which
were the corresponding Gel-ECG segments. Essen-
tially, given an object (Band-ECG, ACC) the network
should output a segment with minimal error against
the pair target (Gel-ECG). In each experiment, the ex-
amples (object, target) were divided in 20%, trimmed
from the centre of each time series, for the test dataset,
and the remaining 80% of segments would constitute
the train dataset. The train:validation ratio was 8:2.
The denoised estimates,
ˆ
y, were compared with
their timely corresponding Gel-ECG segment, y, us-
ing the normalized mean squared error (NMSE):
NMSE (dB)
:
= 10 ·log
10
||y −
ˆ
y||
2
2
||y −
¯
y||
2
2
. (3)
This metric is similar to the MSE, in the sense
that the estimation squared errors are in the numer-
ator, and the normalisation factor in the denominator.
The normalisation factor is the squared errors of the
signal’s mean constant function. Hence, semantically,
the NMSE tells us how good the denoised estimate is
in comparison with the signal’s mean. If the NMSE is
negative, the denoised estimate error is smaller than
the signal’s mean error – which is the desirable. Con-
trarily, if the NMSE is positive, then the signal’s mean
predicts the true signal better than the denoised esti-
mate – which is the undesirable.
Additionally, the ECG SQIs previously described
in Section 2.2 were used to compare the quality of
Band-ECG segments before and after denoising.
5 RESULTS AND DISCUSSION
This section addresses the research questions raised
in Section 1, in the same order they were introduced.
The ECG process can be thought of comprehending
three main sources of variability:
• Environmental: Electrode variability, pressure,
hydration, temperature, and skin conductance in-
troduce variability to the ECG trace. This is ad-
dressed, not only, but primarily, in Subsection 5.1.
• Activity: If the subject is at rest or performing an
activity of some pattern, it introduces variability
to the ECG. This is explored in Subsection 5.2.
• Subject: The subject’s physiology and own way
of wearing the chestband introduce variability to
the ECG trace. This is explored in Subsection 5.3.
5.1 Impact of Data Augmentation
The techniques recently suggested in (Nonaka and
Seita, 2022) were implemented to augment the num-
ber of ECG and ACC segments of each activity. Given
a biosignal segment, similar segments were derived,
without significantly altering its original natural mor-
phology, by applying the following operations:
• Scale: Contraction or dillation, in amplitude, by a
multiplier, M (Figure 5A).
• Shift: Left or right translation, in time, by D ×
number of samples (Figure 5B).
• Drop: Multiplication of each sample by zero with
probability p (Figure 5C).
• Sine: Addition of a sinusoidal wave of random
frequency, f , and amplitude A (Figure 5D).
• Square: Addition of a square pulse of random
frequency, f , and amplitude A (Figure 5E).
• Randomness: Addition of gaussian noise of am-
plitude A (Figure 5F).
Parameter M, in Scale, should be between [0.25,1[
for contraction or between ]1, 4] for dilation. Param-
eter D, in Shift, should be between ]0, 1[. The max-
imum displacement is achieved when D = 0.5. The
Data Augmentation, Multimodality, Subject and Activity Specificity Improve Wearable Electrocardiogram Denoising with Autoencoders
139

Table 2: Average test losses of DAEs trained with datasets augmented ”Multiplier” times, grouped by augmentation technique.
Values in ×10
−3
.
Multiplier Scale Shift Drop Sine Square Randomness All
10 178.23 111.43 140.82 91.943 81.306 82.924 42.534
50 101.40 93.354 84.532 62.463 41.050 43.263 26.135
100 57.343 49.935 61.930 34.928 22.856 27.050 14.938
shift direction (left or right) is random. Parameter p,
in Drop, should be between ]0, 1[, since it is a proba-
bility. Parameter A should be between ]0, 1] in Sine,
]0, 0.02] in Square and Randomness, so that the nat-
ural biosignal morphology does not get significantly
altered. Frequency f is random between [0.001, 0.02]
in Sine, and between [0.001, 0.1] in Square. As il-
lustrated in Figure 5, the derived segments (in pink)
could have very well been acquired in a real scenario,
due to environmental variability.
Universal DAE. On a first basis, a DAE to be used
for all subjects and for all activities was trained only
with real examples. There are 37821 examples in the
dataset, which correspond to the total number of sec-
onds reported in Table 1. Herein this and the other
models presented in this subsection will be termed
universal DAEs. The model was trained for 262
epochs, in batches of 64 examples, and initial learn-
ing rate of 0.0005. An average test loss of 0.5305 was
achieved, which is not satisfactory, since the biosig-
nals were normalised in amplitude between 0 and 1.
Different Augmentation Multipliers. The dataset
of real examples was then linearly augmented 10
times with each of the six described techniques, re-
sulting in six augmented datasets, each containing
416031 examples, which corresponds to eleven times
the number of real examples. Six DAEs were trained
and tested with each of these augmented datasets, in
the same conditions as before, and the average test
losses are reported in Table 2. The test loss de-
creased when using each of these augmented datasets.
The same experiment was repeated with datasets aug-
mented 50 times with the same techniques, and test
losses further decreased for every model. Repeating
the experiment with datasets augmented 100 times
also further reduced the test losses of every model,
to one order of magnitude lower than that of the uni-
versal DAE.
Different Augmentation Techniques. Neural net-
works by nature lack explainability, however, in an
attempt to understand these results, one might at-
tribute the success of Sine, Square, and Random-
ness techniques (Table 2) to the fact that they actu-
ally generate different examples in a nonlinear way,
that cannot be traced back to the original examples,
unless the augmentative process that was added is
kept stored, therefore increasing environmental vari-
ability in a nonlinear way. Conversely, Scale, Shift,
and Drop techniques apply linear transformations or
nullify some samples. Scale contracts or expands
the biosignals’ amplitude, which occurs in real envi-
ronments, but most of the waveform characteristics
are still the same. Shift translates segments in time,
which could be achieved by nothing more than seg-
menting the biosignals in a different way. For this
reason, from the next subsection forward only Sine,
Square, and Randomness techniques are employed.
It might seem paradoxical to increase environmental
variability, however the dramatic increase in the num-
ber of examples allows the models to implicitly cap-
ture these environmental differences and to discrimi-
nate them from the ECG pattern, consequently lead-
ing to better generalisations and to avoid underfitting.
All Augmentation Techniques. Another experi-
ment was conducted by augmenting the original
dataset all-together with the six techniques, 10, 50 and
100 times. These datasets contained approximately
more than 2.6, 13.2, 26.5 million examples, respec-
tively. The test losses can be found in the last col-
umn of Table 2. It can be concluded that the test
loss decreases by increasing the dataset size. How-
ever, for the dataset augmented 100 times, the test
loss (0.0149) is only 0.0079 lower than the best result
achieved with a dataset generated by a unique tech-
nique (Square, 0.0229), hinting that more examples
do not necessarily lead to significant improvements.
Comparisons aside, a test loss of 0.0149 may still not
be satisfactory, as shall be discussed later. In this case,
since segments are normalised in amplitude between
0 and 1, such a loss represents a 1.5% deviation from
the targets, which in artifacts can still appear to be
quite noisy. Activity and subject specificity strategies
are explored ahead to improve the denoising perfor-
mance. Meanwhile, the impact of data augmentation
will continue to be addressed.
5.2 Impact of Activity Specificity
The full dataset was divided by activity, including
Baseline when the subjects were at rest in the begin-
ning of the session, into smaller datasets, that is, eight
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
140

datasets with the number of examples indicated in Ta-
ble 3. Each of these activity-specific datasets was also
augmented 50 and 100 times, using Sine, Square, and
Randomness. For each of these, a DAE was trained
for at most 150 epochs, in the same conditions as be-
fore. The test losses are given in Table 4.
Table 3: Total useful duration, in seconds, of each activity.
Lift Greetings Gesticulate Jumps Walk-B Run Walk-A
1262 767 1469 132 2970 26953 2697
When no data augmentation is applied, and only
real examples are used to train the activity-specific
DAEs, the average test losses are two orders of mag-
nitude lower than that of the best universal DAE.
When the datasets of each activity are augmented,
the average test losses are one order of magnitude
lower than the universal DAEs trained with an aug-
mented dataset. Concretely, the average test loss is
2.5 times smaller for Run DAEs and 12 times smaller
for Baseline DAEs. Gesticulate and Greetings mod-
els achieved the lower test losses. Therefore, activity-
specific DAEs perform better than non-specific ones,
at least up to the point of subject variability in task
execution and the environmental differences.
5.3 Impact of Subject Specificity
Assuming each subject’s dataset is representative
enough to capture the different ways they can exe-
cute an activity, the full dataset was divided by sub-
ject and by activity into smaller datasets, so that only
environmental conditions could influence the denois-
ing process. Hence, A datasets per subject, being A
the number of activities recorded with each subject.
Each of these datasets was augmented 100 times, us-
ing Sine, Square, and Randomness techniques. For
each of these, a DAE was trained in the same condi-
tions as before. The test losses are reported in the first
row of Table 5.
Inspecting the cohort median, when training is
specific of the subject, the average test loss is one or-
der of magnitude lower for all activities, except for
Jumps and Run, than that of the subject-independent
activity-specific models. Jumps and Run segments
continue to be the most challenging to denoise, simi-
larly to Table 4. Nonetheless, for Run segments, a me-
dian 0.119% deviation from the target is more accept-
able than that achieved with a subject-independent
DAE. And for some subjects, the Run test loss was
one order of magnitude lower than that of subject-
independent models, such as in session B (0.000429)
and session O (0.000304) (not shown). Moreover,
Baseline’s loss also decreased one order of magni-
tude, and, similar to Table 4, it is the lowest test loss,
which is expected since subjects were at rest in these
periods. Therefore, subject-specific DAEs perform
better than non-specific ones, at least up to the point
of environmental differences.
5.4 Importance of Motion Information
One might question if the ACC segments, given along
with the noisy ECG, actually contribute to the de-
noising process, or if the optimiser is minimising the
loss between the noisy and target ECGs by train-
ing the networks to ignore the ACC inputs. The
trained weights immediately after the input layer were
checked and no evidence was found that, up to this
level, the ACC inputs were being nullified. However,
a much more fine inspection would have to take place
in the remaining trained parameters to answer if and
how this would occur. But we do not need to know
how the networks use the ACC inputs in the denois-
ing process, we simply need to know if they do. To
overcome such a cumbersome study, the same activ-
ity and subject specific DAEs were retrained with no
ACC information.
In this experiment, per each object, three time
series were still fed to the network along with the
noisy ECG – otherwise the architecture would have
to change – but these time series were fabricated and
were not the real ACC ones. To synthesise ACC,
time series of the same length were generated with
Gaussian noise of zero mean and unit standard devi-
ation, N (0, 1). The same example pairs of objects
and targets were used, however the ACC segments of
each object were replaced by the Gaussian time series,
which has no real motion information. The dataset
was augmented in the same way, and the models were
trained under the same conditions. The median test
losses can be found in the last row of Table 5. The test
MSE in every activity is similar and in the same order
of magnitude as the universal DAE, except for Base-
line segments, meaning these models do not output
satisfactory denoised estimates if the ACC-dedicated
segments are not actually the chest ACC time series.
It is understandable that Baseline models show lower
MSE due to the absence of motion in the input seg-
ments during this period. Therefore, the ACC time
series contain motion information regarding the chest
that is useful to denoise ECG.
5.5 Final Design
The performance of the designed activity and sub-
ject specific models were validated in a real scenario,
Data Augmentation, Multimodality, Subject and Activity Specificity Improve Wearable Electrocardiogram Denoising with Autoencoders
141
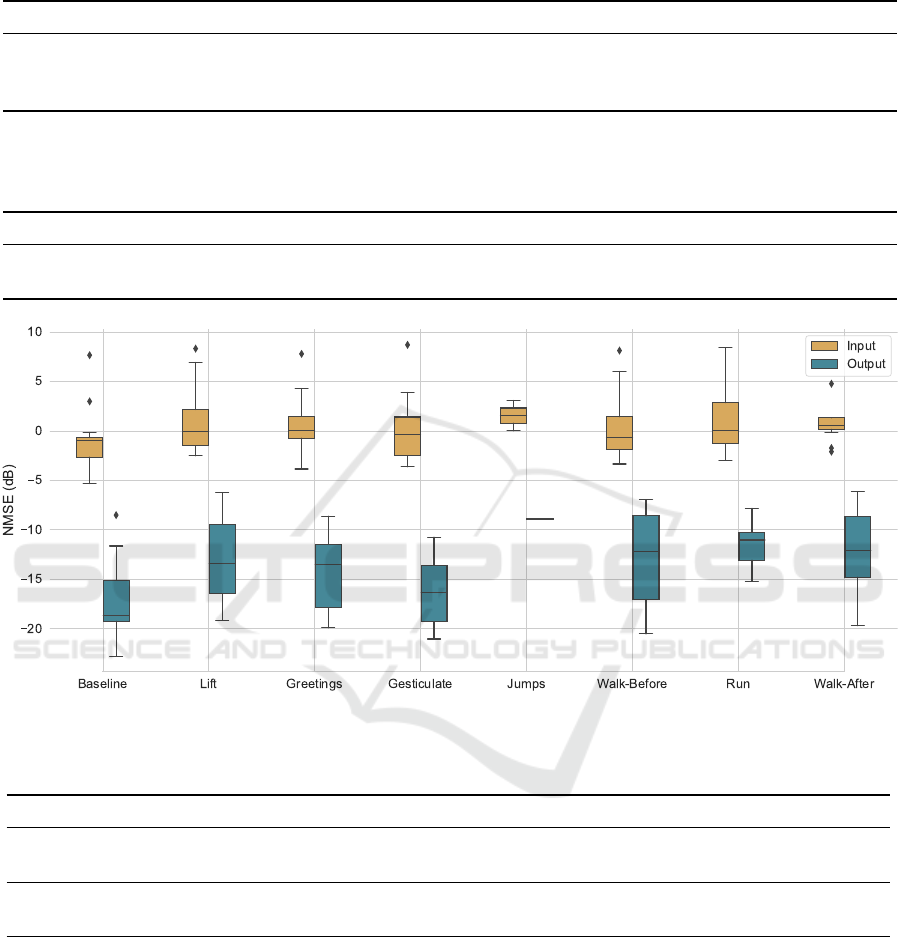
Table 4: Average test losses of DAEs trained with activity-specific datasets, grouped by augmentation multiplier. Values in
×10
−3
.
Multiplier Baseline Lift Greetings Gesticulate Jumps Walk-Before Run Walk-After
0 1.660 2.903 4.153 1.723 3.394
1
3.395 6.381 3.743
50 1.343 2.530 2.034 1.430 3.249 2.991 6.283 2.901
100 1.005 2.000 1.487 1.037 3.027 2.543 5.918 2.396
1
Trained in batches of 16 examples, because there were not enough examples.
Table 5: Cohort median of average test loss of DAEs trained with activity-specific and subject-specific datasets, grouped by
activity. Former row: With real and augmented ACC; Later row: With no real ACC. Values in ×10
−3
.
Baseline Lift Greetings Gesticulate Jumps Walk-Before Run Walk-After
With ACC 0.172 0.442 0.286 0.211 1.264 0.553 1.198 0.706
No ACC 28.134 908.421 631.102 120.807 937.195 720.910 944.261 852.044
Figure 6: Improvement in NMSE of subject and activity specific DAEs tested only with real examples.
Table 6: Comparing ECG signal quality indexes (SQIs) before and after denoising steps, grouped by activity. Cohort Quartile
1 (Q1) and Quartile (Q3) values, in the format ”Q1–Q3”. Values in red are below satisfactory the specific SQI threshold.
Baseline Lift Greetings Gesticulate Jumps Walk-B Run Walk-A
kSQI Input 9.1–14.2 1.4–5.1 3.7–7.5 3.7–12.4 3.4–4.2 1.5–8.1 0.8–2.9 0.9–2.4
kSQI Output 12.4–22.1 10.0–16.5 12.4–20.1 10.1–19.5 11.1-11.3 10.9–15.8 11.3–14.5 9.2–17.3
qSQI Input 0.98–1.00 0.91–0.96 0.95–1.00 0.96–1.00 0.78–0.92 0.94–1.00 0.86–0.99 0.94–1.00
qSQI Output 1.00–1.00 0.94–1.00 0.97–1.00 1.00–1.00 0.97–0.98 0.97–1.00 0.95–1.00 0.98–1.00
where the networks are not tested with augmented
examples. With the same trained parameters, each
DAE was tested with the respective non-augmented
test set, i.e., only with the real examples segmented
from the original time series. For instance, the Run
DAE of session F was tested with a time series of
4.28 minutes, whereas the Lift DAE was tested with
only 29 seconds (cohort durations not shown). The
NMSEs of these inputs and output denoised estimates
against the Gel-ECG are compared in Figure 6. The
denoised median NMSE decreased below −10 dB for
all activities, and Gesticulate NMSE decreased below
−15 dB. Gesticulate, Greetings and Lift segments ob-
tained the higher improvements, respectively, −15.9,
−13.6 and −13.3 dB. Run and Jumps segments ob-
tained the lower improvements, although still valu-
able, of respectively, −11.0 and −10.4 dB. This indi-
cates that the denoised estimations approximate better
the Gel-ECG than the signal’s mean.
The first block of Table 6 shows that segments
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
142
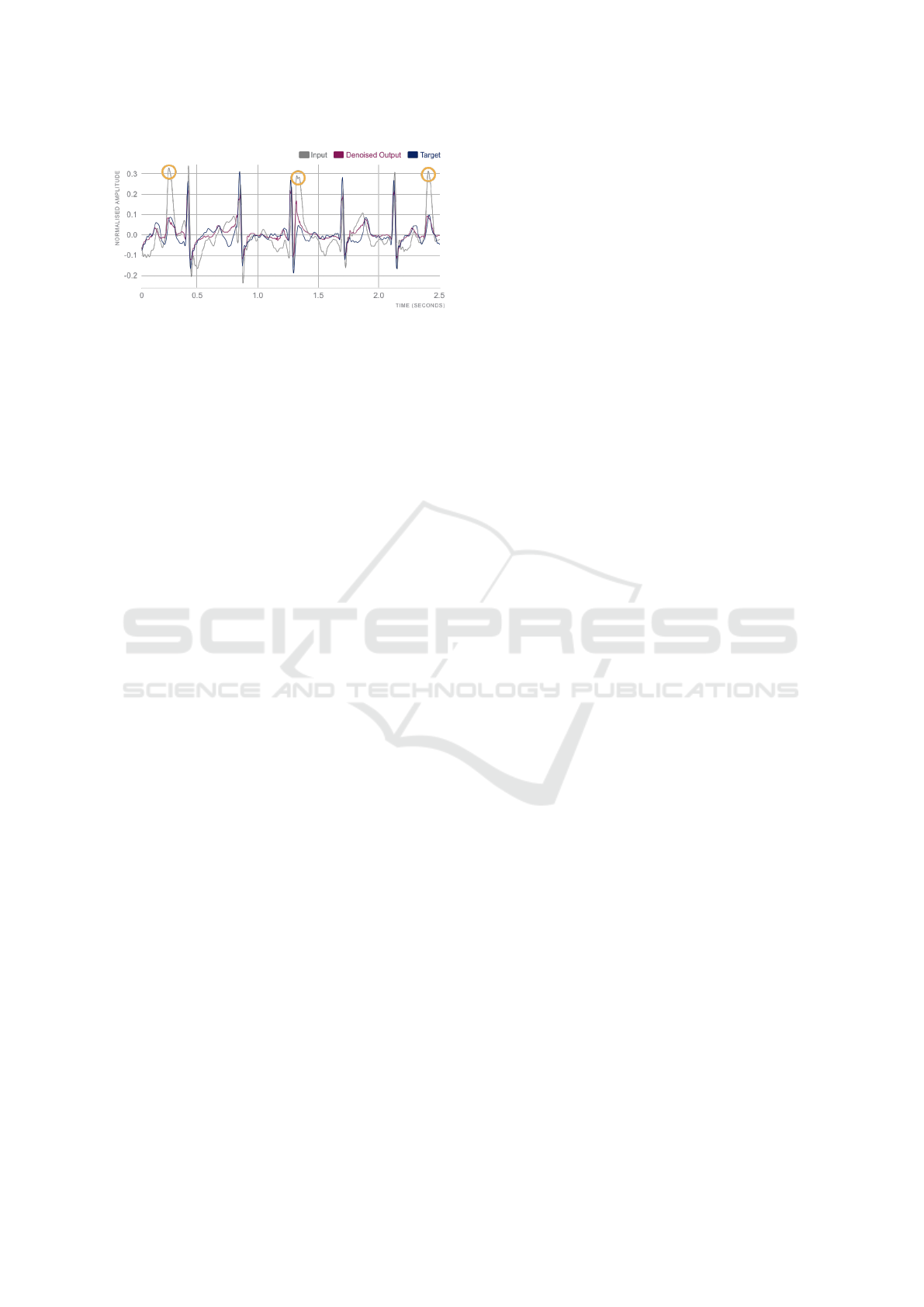
Figure 7: Example of Walk-Before DAE of session Q. Po-
tentially false R peaks highlighted in yellow.
from all activities, except Baseline, showed unaccept-
able kSQI before denoising (in red). After DAE de-
noising all interquartile kSQI increased. The median
kSQI was higher than 11 for all activities after de-
noising, demonstrating there was less noise present in
the Band-ECG segments after denoising. The second
block of Table 6 shows that the R peaks agreement
by two algorithms was below 0.9 in some Jumps and
Run input segments (in red). After subject and ac-
tivity denoising, the R peaks of these segments were
more salient and unequivocally identified by the two
different algorithms. Nearly all Baseline and Gestic-
ulate segments showed 1.00 agreement. Therefore,
removal of BW is not sufficient to make better R peak
detections, and the DAE denoising process most con-
tributed to accurate detections. As aforementioned,
particularly in highly noisy environments, it is very
important that R peaks can still be accurately detected
in order to extract HRV features. Figure 7 showcases
an example of how multiple high-amplitude peaks
were attenuated in walking activities. Such high-
amplitude peaks could very well be mistakenly iden-
tified as R peaks. Moreover, in this example, Q and S
waves were well pronounced in all heartbeats; and P
and T waves were well corrected in most heartbeats,
both in amplitude and in time, to approximate those
of the Gel-ECG (target).
6 CONCLUSIONS
The training process of an ECG-based DAE architec-
ture was studied. The best results for the test dataset
were achieved when DAEs were trained specifically
for each subject and each activity, with augmented
datasets, and using chest ACC as noise-reference.
Therefore, subject and activity specificity, data aug-
mentation, and multimodality, together prove as ef-
fecting design techniques to take into consideration
when engineering ECG deep learning models.
It should be noted that it is data augmentation
that possibilities the design of these specific models,
otherwise there would not be enough subject-specific
and activity-specific examples for each subject, gath-
ered from only 55 minutes (median) of multi-activity
sessions. Hence, data augmentation enables subject-
specific models even from short duration acquisitions.
Moreover, the proposed denoising method has two
main advantages. Firstly, the ECG can be blindly seg-
mented in real-time in 1-second segments, producing
outputs with no ringing effects, therefore dispensing
the need for R peak computation to segment the ECG
by heartbeats. Secondly, the proposed DAE outputs
denoised estimates in polynomial time, since the 2D
convolution operation is majored at O(N
4
), where N
is the segment number of samples, hence it is feasible
for online denoising in wearables.
ACKNOWLEDGEMENTS
This work was partially funded by the IST re-
search grant BL88/2022, under the scope of project
1018P.06071.1.01.01 ”CardioLeather”, by the
IT research grant BI16/2021, under the project
PCIF/SSO/0163/2019 ”SafeFire”, and by the
Fundac¸
˜
ao para a Ci
ˆ
encia e Tecnologia (FCT) /
Minist
´
erio da Ci
ˆ
encia, Tecnologia e Ensino Supe-
rior (MCTES) research grants 2021.08297.BD and
2022.12369.BD, through national funds and when
applicable co-funded by EU funds.
REFERENCES
Abecassis, L., Ho, M., Tit-Lartey, O., Hwang, W., Gath-
mann, T., Lum, Z., Loong, N. Y., and Moo, J. (2018).
Machine Learning based Denoising of Electrocardio-
gram Signals from a Wearable ECG Monitor. BSc.
Report, Imperial College London, Department of Bio-
engineering.
Abu-Alrub, S., Strik, M., Ramirez, F. D., Moussaoui,
N., Racine, H. P., Marchand, H., Buliard, S.,
Ha
¨
ıssaguerre, M., Ploux, S., and Bordachar, P. (2022).
Smartwatch Electrocardiograms for Automated and
Manual Diagnosis of Atrial Fibrillation: A Compar-
ative Analysis of Three Models. Front Cardiovasc
Med, 9:836375.
An, X., Liu, Y., Zhao, Y., Lu, S., Stylios, G. K., and Liu, Q.
(2022). Adaptive Motion Artifact Reduction in Wear-
able ECG Measurements Using Impedance Pneumog-
raphy Signal. Sensors, 22(15):5493.
Arsene, C. (2020). Design of Deep Convolutional Neural
Network Architectures for Denoising Electrocardio-
graphic Signals. In 2020 IEEE Conference on Com-
putational Intelligence in Bioinformatics and Compu-
tational Biology (CIBCB), pages 1–8.
Data Augmentation, Multimodality, Subject and Activity Specificity Improve Wearable Electrocardiogram Denoising with Autoencoders
143

Ashley, E. A. and Niebauer, J. (2004). Conquering the
ECG. Remedica.
Bansal, A. and Joshi, R. (2018). Portable out-of-hospital
electrocardiography: A review of current technolo-
gies. Journal of Arrhythmia, 34(2):129–138.
Bayoumy, K., Gaber, M., Elshafeey, A., Mhaimeed, O.,
Dineen, E. H., Marvel, F. A., Martin, S. S., Muse,
E. D., Turakhia, M. P., Tarakji, K. G., and Elshazly,
M. B. (2021). Smart wearable devices in cardiovascu-
lar care: Where we are and how to move forward. Nat
Rev Cardiol, 18(8):581–599.
Behbahani, S., Dabanloo, N. J., Nasrabadi, A. M., Teixeira,
C. A., and Dourado, A. (2013). Pre-ictal heart rate
variability assessment of epileptic seizures by means
of linear and non-linear analyses. Anatolian Journal
of Cardiology, 13(8):797–803.
Butt, F. S., La Blunda, L., Wagner, M. F., Sch
¨
afer, J.,
Medina-Bulo, I., and G
´
omez-Ullate, D. (2021). Fall
Detection from Electrocardiogram (ECG) Signals and
Classification by Deep Transfer Learning. Informa-
tion, 12(2):63.
Carmo, A. S., Abreu, M., Fred, A. L. N., and da Silva, H. P.
(2022). EpiBOX: An Automated Platform for Long-
Term Biosignal Collection. Front. Neuroinform., 16.
Chatterjee, S., Thakur, R. S., Yadav, R. N., Gupta, L., and
Raghuvanshi, D. K. (2020). Review of noise removal
techniques in ECG signals. IET Signal Processing,
14(9):569–590.
Chen, L., Yu, H., Huang, Y., and Jin, H. (2021). ECG
Signal-Enabled Automatic Diagnosis Technology of
Heart Failure. J Healthc Eng, 2021:5802722.
Chiang, H.-T., Hsieh, Y.-Y., Fu, S.-W., Hung, K.-H., Tsao,
Y., and Chien, S.-Y. (2019). Noise Reduction in ECG
Signals Using Fully Convolutional Denoising Autoen-
coders. IEEE Access, 7:60806–60813.
Christov, I. I. (2004). Real time electrocardiogram QRS de-
tection using combined adaptive threshold. BioMedi-
cal Engineering OnLine, 3(1):28.
Clifford, G. D., Behar, J., Li, Q., and Rezek, I. (2012).
Signal quality indices and data fusion for determining
clinical acceptability of electrocardiograms. Physiol.
Meas., 33(9):1419–1433.
Goodfellow, I., Bengio, Y., and Courville, A. (2016). Deep
Learning. Adaptive Computation and Machine Learn-
ing. The MIT Press, Cambridge, Massachusetts.
Hamilton, P. (2002). Open source ECG analysis. In Com-
puters in Cardiology, pages 101–104.
Huerta, A., Martinez-Rodrigo, A., Rieta, J., Alcaraz, R.,
and IEEE (2021). ECG Quality Assessment via Deep
Learning and Data Augmentation. In 2021 CINC.
Kavanagh, J. J. and Menz, H. B. (2008). Accelerometry: A
technique for quantifying movement patterns during
walking. Gait & Posture, 28(1):1–15.
Kingma, D. P. and Ba, J. (2014). Adam: A Method for
Stochastic Optimization.
Kutz, M. (2010). Biomedical Engineering and Design
Handbook, Volumes I and II (2nd Edition). McGraw-
Hill Professional Publishing, New York, USA.
Li, Q., Mark, R. G., and Clifford, G. D. (2007). Robust heart
rate estimation from multiple asynchronous noisy
sources using signal quality indices and a Kalman fil-
ter. Physiol. Meas., 29(1):15–32.
Li, Q., Rajagopalan, C., and Clifford, G. D. (2014). A
machine learning approach to multi-level ECG signal
quality classification. Comp. Methods Prog. Biomed.,
117(3):435–447.
Mohaddes, F., da Silva, R. L., Akbulut, F. P., Zhou, Y., Tan-
neeru, A., Lobaton, E., Lee, B., and Misra, V. (2020).
A Pipeline for Adaptive Filtering and Transformation
of Noisy Left-Arm ECG to Its Surrogate Chest Signal.
Electronics, 9(5):866.
Mohd Apandi, Z. F., Ikeura, R., Hayakawa, S., and Tsut-
sumi, S. (2020). An Analysis of the Effects of Noisy
Electrocardiogram Signal on Heartbeat Detection Per-
formance. Bioengineering (Basel), 7(2):53.
Munoz-Minjares, J. U., Lopez-Ramirez, M., Vazquez-
Olguin, M., Lastre-Dominguez, C., and Shmaliy, Y. S.
(2021). Outliers detection for accurate HRV-seizure
baseline estimation using modern numerical. Biomed-
ical Signal Processing and Control, 67.
Nonaka, N. and Seita, J. (2022). RandECG: Data Augmen-
tation for Deep Neural Network Based ECG Classi-
fication. In Takama, Y., Matsumura, N., Yada, K.,
Matsushita, M., Katagami, D., Abe, A., Kashima, H.,
Hiraoka, T., Uchiya, T., and Rzepka, R., editors, Ad-
vances in Art. Intel., volume 1423, pages 178–189.
Nurmaini, S., Darmawahyuni, A., Sakti Mukti, A. N., Rach-
matullah, M. N., Firdaus, F., and Tutuko, B. (2020).
Deep Learning-Based Stacked Denoising and Autoen-
coder for ECG Heartbeat Classification. Electronics,
9(1):135.
Pan, Q., Li, X., and Fang, L. (2020). Data Augmentation
for Deep Learning-Based ECG Analysis. In Feature
Engineering and Computational Intelligence in ECG
Monitoring, pages 91–111. Springer, Singapore.
Prieto-Avalos, G., Cruz-Ramos, N. A., Alor-Hern
´
andez,
G., S
´
anchez-Cervantes, J. L., Rodr
´
ıguez-Mazahua, L.,
and Guarneros-Nolasco, L. R. (2022). Wearable De-
vices for Physical Monitoring of Heart: A Review.
Biosensors, 12(5):292.
Raya, M. and Sison, L. (2002). Adaptive noise cancelling of
motion artifact in stress ECG signals using accelerom-
eter. In Proc. of the 2nd Joint 24th Ann. Conf. and
the Ann. Fall Meeting of the Biomedical Eng. Soc.
(IEMBS), volume 2, pages 1756–1757 vol.2.
Reljin, N., Lazaro, J., Hossain, M. B., Noh, Y. S., Cho,
C. H., and Chon, K. H. (2020). Using the Re-
dundant Convolutional Encoder–Decoder to Denoise
QRS Complexes in ECG Signals Recorded with an
Armband Wearable Device. Sensors, 20(16):4611.
Rodrigues, J., Belo, D., and Gamboa, H. (2017). Noise
detection on ECG based on agglomerative clustering
of morphological features. Computers in Biology and
Medicine, 87:322–334.
Saraiva, J. (nov-2022a). Deep Residual Learning for
Epileptic Seizure Prediction and Tools to Expedite
Biosignal Research. MSc. Thesis, Department of
Computer Science and Engineering, Instituto Superior
T
´
ecnico, Universidade de Lisboa, Lisbon.
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
144

Saraiva, J. (nov-2022b). Denoising and Artifact Re-
moval of Ambulatory Electrocardiogram for Patient
Continuous-Monitoring. MSc. Thesis, Department of
Bioengineering, Instituto Superior T
´
ecnico, Universi-
dade de Lisboa, Lisbon.
Semmlow, J. L. and Griffel, B. (2014). Biosignal and Med-
ical Image Processing. CRC Press, 3rd ed. edition.
Vandecasteele, K., Cooman, T. D., Chatzichristos, C.,
Cleeren, E., Swinnen, L., Ortiz, J. M., Huffel, S. V.,
D
¨
umpelmann, M., Schulze-Bonhage, A., Vos, M. D.,
Paesschen, W. V., and Hunyadi, B. (2021). The power
of ECG in multimodal patient-specific seizure moni-
toring: Added value to an EEG-based detector using
limited channels. Epilepsia, 62(10).
Xia, Y., Zhang, H., Xu, L., Gao, Z., Zhang, H., Liu, H.,
and Li, S. (2018). An Automatic Cardiac Arrhyth-
mia Classification System With Wearable Electrocar-
diogram. IEEE Access, 6:16529–16538.
Xiong, P., Wang, H., Liu, M., and Liu, X. (2015). De-
noising Autoencoder for Eletrocardiogram Signal En-
hancement. Journal of Medical Imaging and Health
Informatics, 5(8):1804–1810.
Data Augmentation, Multimodality, Subject and Activity Specificity Improve Wearable Electrocardiogram Denoising with Autoencoders
145
