
Contrast Set Mining for Actionable Insights into Associations
Between Sleep and Glucose in a Normoglycemic Population
Hoang Huyen Nhung
1
and Zilu Liang
1,2 a
1
Ubiquitous and Personal Computing Lab, Kyoto University of Advanced Science (KUAS), 621-8555 Kyoto, Japan
2
Institute of Industrial Science, The University of Tokyo, 113-8654 Tokyo, Japan
Keywords: Data Mining, Personal Informatics, Ubiquitous Computing, Contrast Set Mining.
Abstract: Prior studies have suggested potential associations between poor sleep and glucose dysregulation among
diabetic patients. However, little is known about the relationship between sleep and glucose regulation in
healthy populations. In this study, we proposed a data mining pipeline based on contrast set mining to identify
significant associations between sleep and glucose in a dataset collected from a normoglycemic population in
free-living environments. Unlike traditional correlation analysis, our approach does not assume a linear
relationship between sleep and glucose and can potentially discover associations when a pair of metrics fall
within certain value ranges. The data mining result highlights the total sleep time as an important sleep metric
associated with glucose regulation the next day, which is characterised by rules with high lift and confidence.
Furthermore, the result suggests that having a higher time ratio in normal glucose range was associated with
better sleep continuity at night. These results may provide insights that people can immediately act on for
better sleep and better glucose control. Future research may leverage the proposed data mining protocol to
develop healthy behaviour recommender systems.
1 INTRODUCTION
Sleep is an essential part of human daily life. There is
consensus that adults need 7 or more hours of sleep
(Watson et al., 2015). Sleep deprivation was reported
to account for a wide spectrum of health problems,
including glycaemic disturbances and the
development of diabetes (Jiawei et al., 2017; Lou et
al., 2015; Xiao et al., 2014; Zuraikat et al., 2020).
Modern lifestyle has led to significant changes in
human sleep patterns, such as delayed sleep onset and
decreased total sleep time. Furthermore, modern
abundance has also caused significant shifts in
people’s eating habits and has consequently led to a
surge in chronic metabolic diseases such as diabetes,
which is characterised by glucose dysregulation. A
few prior studies have demonstrated a strong
connection between sleep quality and glucose
homeostasis, especially in people with health
conditions (Cauter et al., 1991 Sep; Gottlieb et al.,
2005; Kothari et al., 2021; Lou et al., 2015; Wang et
al., 2017). Nonetheless, those studies were limited by
the methods available for data collection and analysis,
a
https://orcid.org/0000-0002-2328-5016
and the healthy population is often underrepresented
or completely excluded.
With a variety of consumer wearable technologies
being developed, researchers are now able to
perform longitudinal measurements of sleep and
glucose in a more naturalistic environment.
Multidimensional sleep structure can be measured
using a Fitbit wristband, and 24-hour interstitial
glucose can be monitored using a CGM system such
as the FreeStyle Libre. Data collected with modern
wearable technologies make it possible to examine
the reciprocal relationships between sleep and
glucose at a finer resolution. Different from prior
studies that primarily consider the daily aggregates of
sleep and glucose data, our study introduces a
circadian perspective. To be more specific, we are
interested in how sleep metrics in the previous night
associates with glucose during the day and how the
glucose level in the daytime associates with the
subsequent night-time sleep. To the best of our
knowledge, this is the first study investigating the
associations between sleep and glucose using
ecologically valid and high-resolution data. The
contribution of this study is as follow:
522
Nhung, H. and Liang, Z.
Contrast Set Mining for Actionable Insights into Associations Between Sleep and Glucose in a Normoglycemic Population.
DOI: 10.5220/0011783600003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 5: HEALTHINF, pages 522-529
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
We designed a data mining pipeline based on
the contrast set mining algorithm STUCCO
(Bay & Pazzani, 2001) instead of traditional
statistical methods. Different from correlation
analysis or linear regression, our method allows
the identification of associations that only
manifest when the metrics are within certain
value ranges.
The data mining pipeline generates interesting
rules and hypotheses that may help inform the
design of future studies to deepen our
understanding of the relationships between
sleep and glucose regulation.
2 RELATED WORKS
Existing studies have found a link between sleep
quality and glucose regulation in both diabetic and
healthy populations. However, the relationship
between sleep and interstitial glucose is complex,
which makes it difficult to quantify the connection.
Multiple approaches have been proposed to examine
this correspondence within and between persons.
Factors such as total sleeping time, sleep
structure, time going to bed, age, and eating habits are
widely known to have a strong influence on blood
glucose levels (Frank et al., 1995; Reutrakul et al.,
2013; Tasali et al., 2008). In a study (Cauter et al.,
1991), eight normal men were supervised for a total
of 53 hours (8h of night sleep, 28h of non-sleep
period, and 8h of daytime sleep). Although glucose
was infused into the body at a constant rate, plasma
glucose levels went through large fluctuations
throughout the study. Interestingly, during sleep
deprivation, glucose levels rise gradually to reach the
maximum at roughly the same time as during regular
sleep, then decrease to daytime levels.
Many studies repeatedly report the association of
sleep dept, sleep curtailment, and reduced of sleep
hygiene contribute to the increase of insulin
sensitivity (SI). Even one night of partial sleep
deprivation may decrease glucose tolerance and
insulin sensitivity significantly (Donga et al., 2010).
(Tasali et al., 2008) paid attention to slow-wave sleep
(SWS) when his team tried to reduce SWS proportion
but sustain total sleep time. His findings suggest that
low levels of SWS, as occurs in the elderly and obese
patients, can decrease insulin sensitivity by 25%,
reaching the reported value for high-risk of diabetes.
In the case of sleep debt, the product of insulin
sensitivity and acute insulin response to intravenous
glucose (AIRg) was decreased by an average of about
40% as compared to the fully rested state, indicating
a high risk of diabetes (Xiang et al., 2006).
Despite evidence had been found to prove the
connection, they were reported as weak and did not
reach statistical significance. In the study focusing on
women only, no impact of progressive sleep
curtailment over 4 nights was reported on measures
of glucose tolerance and SI (Bosy-Westphal et al.,
2008). Similarly, (Zielinski et al., 2008) assessed the
impact of 8 weeks sleep curtailment on glucose
tolerance in self-reported older long sleepers (≥8.5
h/night), compared to a control group; throught
OGTT, the authors observed no effect of sleep
restriction on glucose tolerance. Sleep hygiene is
more challenging to measure as it is more difficult to
define than sleep duration.
Just as sleep affects glucose levels, glucose levels
may as well impact sleep quality. For example, one
study found that people with prediabetes have a
higher rate of suffering from poor sleep than people
with normal glucose (Iyegha et al., 2019). Diabetic
patients display shorter sleep duration and worse
sleep quality, demonstrated by both self-report and
objective measures (Yoda et al., 2015).
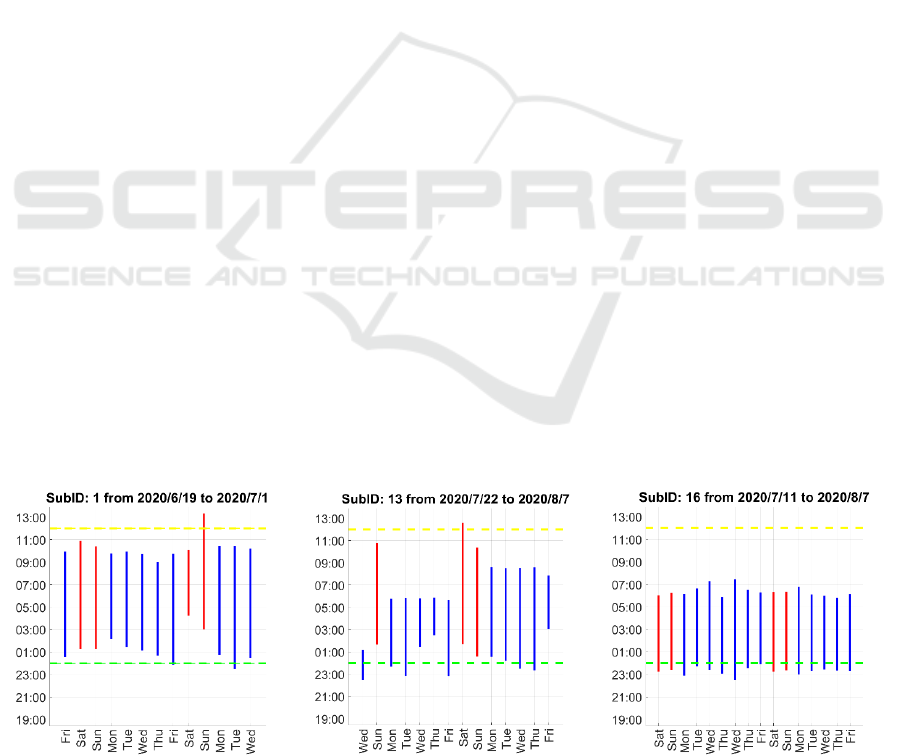
Figure 1: sleep hours of some of our subject indicated by vertical lines (blue lines for weekdays and red lines for weekends).
Contrast Set Mining for Actionable Insights into Associations Between Sleep and Glucose in a Normoglycemic Population
523
3 PROPOSED DATA MINING
PIPELINE
3.1 Dataset
We used a public dataset which contains sleep and
glucose readings recorded between June and August
2020 (Bertrand et al., 2021). In total there are 228
days of data collected from 12 healthy subjects who
had no glucose dysregulation. Half of the subjects
were female, and the average age was 32.7 years. The
sleep patterns varied a lot across participants, as
illustrated in Figure 1.
Currently, several technologies are available to
quantitatively assess all-day interstitial glucose
concentration. In this study, the glucose readings
were recorded using the FreeStyle Libre 2 system
which is a coin-size continuous glucose monitoring
(CGM) device attached at the back of the upper arms.
Sleep data were measured with the accelerometer and
photoplethysmography (PPG) embedded in the Fitbit
Charge 3 worn on the non-dominant wrists. Both the
FreeStyle Libre and the Fitbit devices were proven to
be able to generate reasonable valid measurements in
free-living conditions (Li & Bao, 2018; Liang &
Chapa-Martell, 2019).
Table 1: Features constructed from sleep and glucose data.
Feature Denotation
Sleep Total Sleep Time
(hours)
TST
Wake After Sleep
Onset
WASO
Number of wakes ≥ 5
minutes
awake5minCnt
Slee
p
Efficienc
y(
%
)
SE
Dee
p
slee
p
/TST
(
%
)
dee
p
Ratio
Rem sleep/TST (%) remRatio
Glucose Mean (mg/dL) mean
Maximum
(
m
g
/dL
)
max
Minimum
(
m
g
/dL
)
min
Mean glucose level
outside range (mg/dL)
mge
Mean glucose level
inside range (mg/dL)
mgn
Standard Deviation
(
m
g
/dL
)
sd
Coefficient of
variation (%)
cv
Time spent in range
(%)
tir
Low glucose index LBGI
Hi
g
h
g
lucose index HBGI
J-index
(
m
g
2/dL2
)
j_
index
3.2 Data Preprocessing
The data preprocessing protocol includes segmenting
the CGM data, deriving sleep and CGM features,
categorizing numeric features, synchronizing sleep
and CGM features, and removing missing data.
The dataset is not in the appropriate format to
which the rule induction algorithms may be applied.
Therefore, the next step in the pre-processing
protocol is to categorise the numeric features into
intervals before feeding them into a mining
algorithm. Discretization helps improve the
performance of contrast set mining algorithms and
improve the interpretability of the results. The
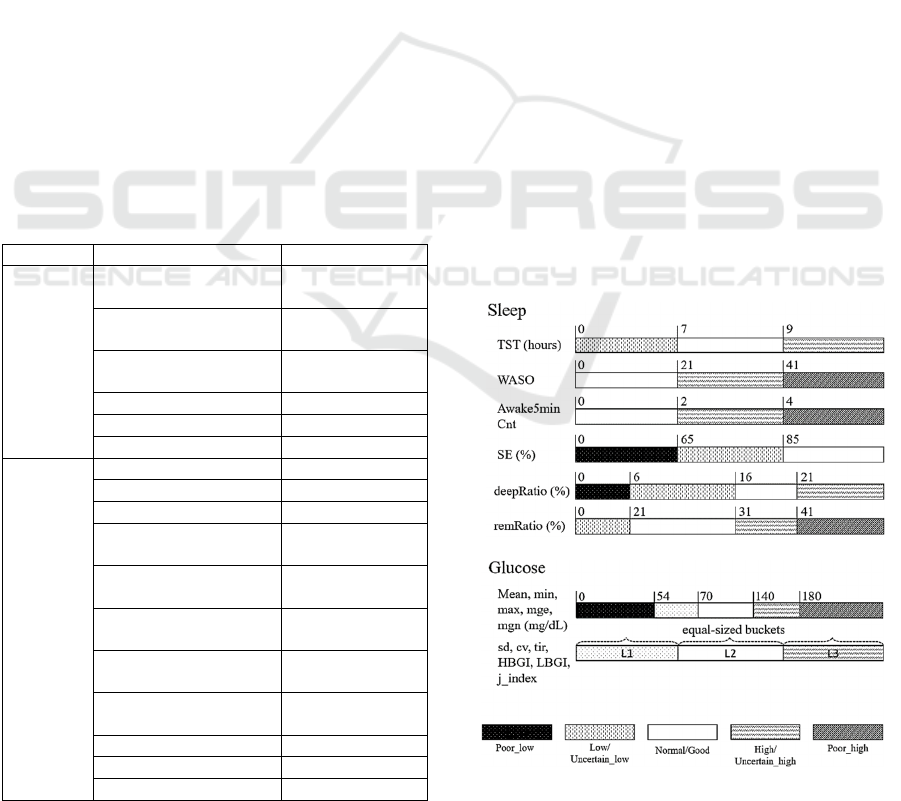
features were discretized using the methods shown in
Figure 2. We mainly focused on features that have
well-defined clinical cut-offs. For sleep features, we
used medical cut-offs for young adults (18-25 years)
reported by (Ohayon et al., 2017). The National Sleep
Foundation concluded that sleep quality was a matter
of sleep continuity and sleep architecture. The
optimal sleep architecture for good sleep quality for
adults was agreed to be <5% stage 1 sleep, <81%
stage 2 sleep, 16-20% slow wave sleep (SWS), and
21-30% rapid eye movement (REM) sleep. However,
stage 1 and stage 2 were combined as light sleep in
Fitbit device, therefore the cut-off limits for this
parameter remain unknown. Consequently, we did
not include light sleep ratio in the sleep feature set.
It is important to properly handle the missing data
and ensure the timestamp matches correctly between
Figure 2: Discretization of sleep and glucose level metrics.
HEALTHINF 2023 - 16th International Conference on Health Informatics
524
the sleep features and the glucose features. Rows that
did not meet the required conditions were removed.
Criteria for removing data are nights that lack glucose
data, non-consecutive nights (isolated nights without
any data recorded the day before or after), features
that are dominated by one component (appear >90%
number of nights). After pre-processing, the cleaned
dataset contains 74 days of data from 8 subjects.
3.3 Contrast Set Mining Algorithm
Contrast set mining is a data mining technique that
helps identify contrast patterns between two groups.
The output of contrast set mining are logical rules
with 1 or n antecedents on the left-hand-side and the
consequent on the right-hand-side.
A typical contrast set mining is the Search and
Testing for Understandable Consistent Contrast
(STUCCO) algorithm developed in (Bay & Pazzani,
2001). The STUCCO algorithm follows a basic
pipeline of identifying a basic idea, controlling the
error, filtering the results, evaluating the results, then
drawing conclusions based on the problem at hand.
For this study, we expect to generate some rules as:
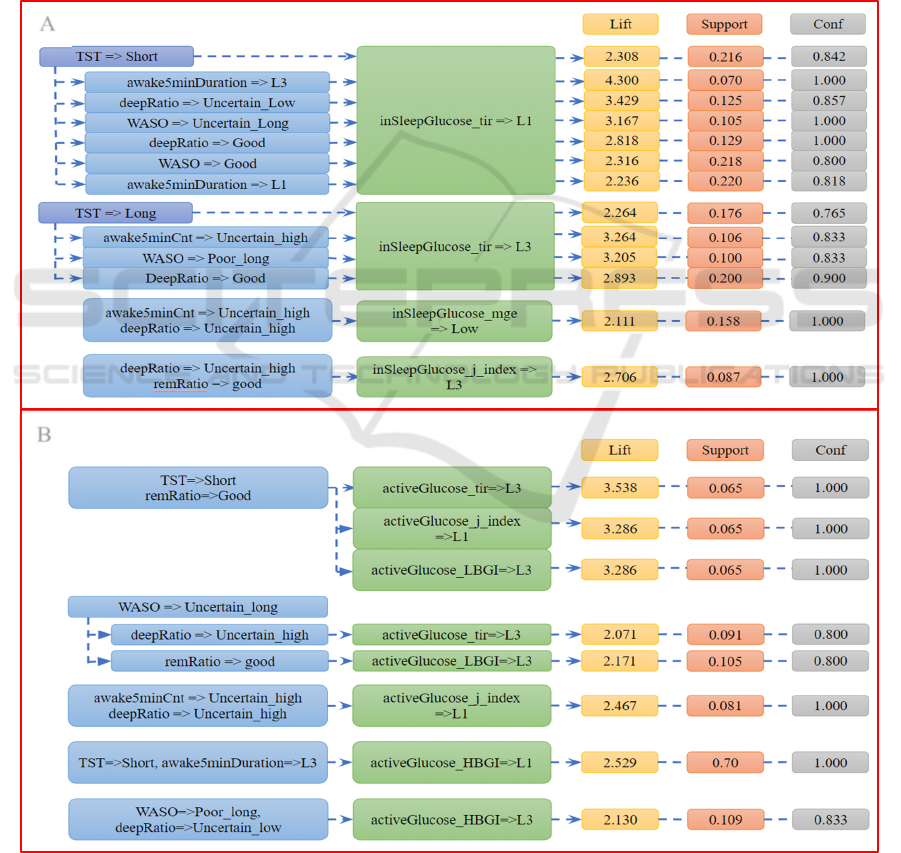
TST=low ∩deepRatio=poor → Glucose_tir=low
Figure 3: Contrast sets were distributed into two groups. Group A reveals the relationship between sleep metrics and
interstitial glucose during nocturnal sleep. Group B reveals the relationship between sleep metrics and interstitial glucose the
following day.
A
B
Contrast Set Mining for Actionable Insights into Associations Between Sleep and Glucose in a Normoglycemic Population
525
Given a dataset D, let Y = {Y
1
, Y
2
, … ,Y
n
} be a set
of consequences and X = {X
1
, X
2
, … , X
n
} be a set of
antecedents that consist of 1 or more attributes.
Support, confidence, and lift are some of the measures
widely used to assess the quality of the generated
rules. Support for X1 → Y1 is the percentage of
example in D containing X1∪Y1. Confidence is the
ratio of example X1 in total X that contributes to Y1.
Lift is the threshold defined by the user to find the
contrast set. Long rules with too many features in the
antecedents are often hard to interpret and could be
redundant. Therefore, we limit the length of the rules
to 2 features. We consider a contrast set as validate if
it meets the requirements: support ≥ 0.02, confident ≥
0.75, and lift ≥ 2. After contrast sets generation, rules
which share the same attribute were grouped together
(Figure 3 & 4) for better interpretation.
4 RESULTS
4.1 Case 1: Antecedence = Sleep of Day
N, Consequence = Glucose of Day N
The contrast set mining results show connection
between the sleep quality at night and the glucose
patterns the next day, as well as the sleep quality at
night and the concurrent glucose characteristics
during sleep. The identified rules are presented in
Figure 3. TST is a major factor that associates to
affect the TIR of glucose both in sleep and during
active periods. It is shown that the time the glucose
levels stayed within the normal range was shorter
when the participants slept less than 7 hours at night.
In contrast, people who had long sleep periods (> 9
hours) spent a longer time in normal glucose level
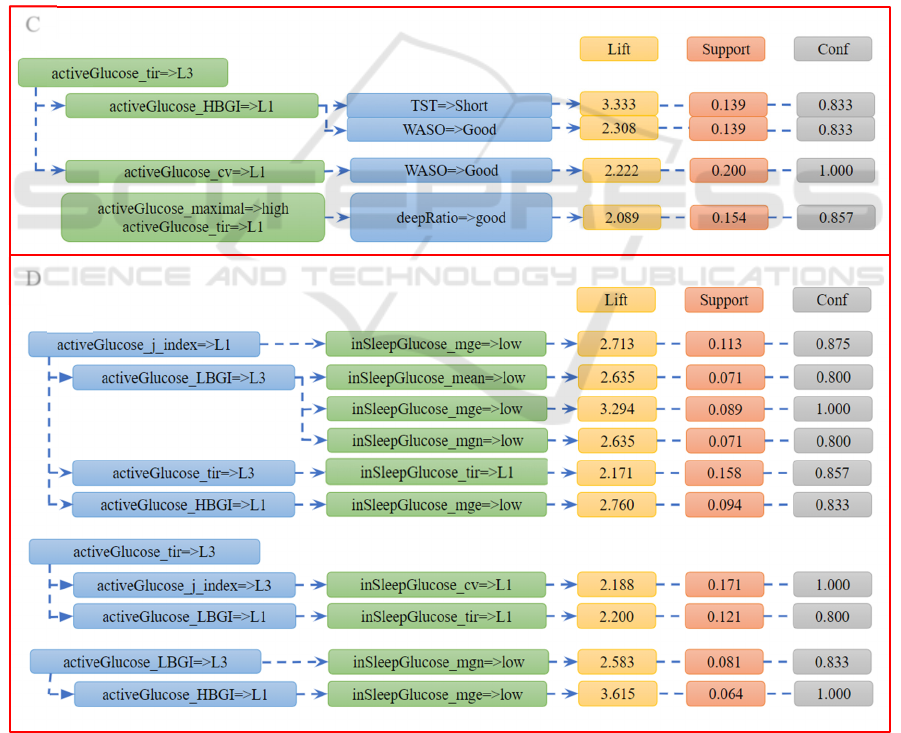
Figure 4: The contrast sets show how interstitial glucose during the day connected with the following night sleep (group C)
and interstitial glucose level during sleep (group D).
C
D
HEALTHINF 2023 - 16th International Conference on Health Informatics
526

range throughout the night. Under the confounding
effect of other sleep metrics, TST was also associated
with the TIR of glucose level during active time the
next day. For example, short TST combined with a
good ratio of REM sleep were associated with higher
TIR the next day.
In addition to sleep duration, sleep continuity and
sleep structure as characterised by the ratio of sleep
stages, were also found to be associated with glucose
regulation during sleep and during active time the
next day. Long awake5minDuration strengthens the
association between short TST and low TIR during
sleep, with the lift increased from 2.31 to 4.30.
However, the same combination was associated with
a low level of HBGI the next day. Long WASO,
together with the confounding effect of sleep
structure, were associated with higher LBGI and
HBGI the next day, indicating impaired glucose
regulation.
The associations between sleep structure (i.e., the
ratio of deep sleep and REM sleep) and glucose
regulation were complex and were often confounded
by sleep duration and sleep continuity.
4.2 Case 2: Antecedent = Glucose
of Day N, Consequent = Sleep
of Day N+1
There is a vicious cycle between sleep and glucose
levels when one affects the other and the impact
repeats days and nights. While finding the rules
between glucose levels at day and sleep metrics at
nights, we found that a long time in range for glucose
level helps to keep sleep WASO within good range
(less than 20 minutes of awakening per night). People
with long tir glucose are highly likely to sleep less
than 7 hours the following night. In addition, long
time in range link with good WASO while short time
in range link with good deep sleep ratio.
It is noticeable that the relationship between
active glucose and in-sleep glucose are more complex
and extremely difficult to interpret. Interestingly, all
the right-hand-side components belong to the “low”
section of the features and the rules are mainly
associated with glucose mge and mgn. From figure 4,
there is a contrast set with 2 completely opposite
components: if active glucose time in range is high,
then in sleep glucose time in range is low. Several
studies have suggested multiple pathways in the
possible connection between sleep quality and the
fluctuation of glycaemic variability. But whether
daytime glucose impacts in-sleep glucose remains a
research gap that needs further examination.
5 DISCUSSIONS
In this study, we analysed the potential connections
between sleep and glucose in the series of consecutive
days. Our finding revealed that there are bi-direction
relationships between TST and glucose TIR. Total
sleep time is the feature that dominated most of the
contrast sets. Short sleep time was associated with the
fluctuation of in-sleep glucose, whereas long sleep
time was associated with more stable glucose
regulation. On the flip side, an association was found
between glucose fluctuation and sleep continuity,
which is consistent with previous findings (Griggs et
al., 2022). Our result indicated that long TIR and low
HBGI correlate to shorter WASO. This echoes
findings in prior studies that long WASO occurred on
days with high J index, high HBGI, and less time in
hypoglycemia (Griggs et al., 2020).
This study has several limitations that should be
addressed in future studies. First, the size of the
dataset is limited. Future studies should collect data
from a larger cohort and potentially cover a wide
spectrum of demographic characteristics.
Furthermore, not only nocturnal sleep, but also
napping may be linked to glucose regulation, as
suggested in previous lab-based studies (Kothari et
al., 2021). Since napping is a common habit of the
young population and people in tropical areas, this
line of research is likely to generate new insights.
Another promising aspect for future examination is
the impact within and between personal variations in
sleep patterns. With a large dataset, the impact of
interpersonal differences should be studied when
each person has different lifestyles and circadian
rhythms. Finally, with the abundance of rules found
by STUCCO, it is necessary for a post-mining method
to select quality rules.
6 CONCLUSIONS
We have designed a data mining pipeline featuring
the contrast set mining algorithm STUCCO to
identify reciprocal associations between sleep and
glucose levels. The finding highlights the total sleep
time as an important sleep metric associated with
glucose regulation the next day, which is
characterised by rules with high lift and confidence.
Reversely, associations were also found between the
glucose fluctuation in wake time and the continuity of
the subsequent sleep at night. Compared to sleep-
>glucose relation, glucose has a weaker association
with nocturnal sleep as the lift of the identified rules
Contrast Set Mining for Actionable Insights into Associations Between Sleep and Glucose in a Normoglycemic Population
527

was lower. These findings added to the existing
knowledge looking at the glucose profile in the
normoglycemic population and helped generate
actionable insights for the holistic management of
sleep and metabolic health. With a better method to
remove abundant rules and interpret information, this
contrast set mining algorithm can be applied to a
recommendation system for adjusting human
behaviour.
ACKNOWLEDGEMENTS
This study was supported by JSPS KAKENHI Grant
Number 21K17670.
REFERENCES
Bay, S. D., & Pazzani, M. J. (2001). Detecting Group
Differences: Mining Contrast Sets. Data Mining and
Knowledge Discovery, 5, 213–246.https://doi.org/
10.1023/A:1011429418057
Bertrand, L., Cleyet-Marrel, N., & Liang, Z. (2021).
Recognizing Eating Activities in Free-Living
Environment Using Consumer Wearable Devices.
Eng. Proc. , 6, 58.
Bosy-Westphal, A., Hinrichs, S., Jauch-Chara, K., Hitze,
B., Later, W., Wilms, B., . . . Muller, M. J. (2008).
Influence of partial sleep deprivation on energy balance
and insulin sensitivity in healthy women. Obesity facts,
1(5),266–273. https://doi.org/10.1159/000158874
Cauter, E. V., Blackman, J. D., Roland, D., Spire, J. P.,
Refetoff, S., & Polonsky, K. S. (1991). Modulation of
glucose regulation and insulin secretion by circadian
rhythmicity and sleep. J Clin Invest., 88(3), 934-942.
https://doi.org/10.1172/JCI115396
Donga, E., Dijk, M. v., Dijk, J. G. v., Biermasz, N. R.,
Lammers, G.-J., Kralingen, K. W. v., . . . Romijn, J. A.
(2010). A single night of partial sleep deprivation
induces insulin resistance in multiple metabolic
pathways in healthy subjects. The Journal of clinical
endocrinology and metabolism, 95(6), 2963–2968.
Frank, S. A., Roland, D. C., Sturis, J., Byrne, M. M.,
Refetoff, S., Polonsky, K. S., & Van Cauter, E. (1995).
Effects of aging on glucose regulation during
wakefulness and sleep. The American journal of
physiology, 269(6 Pt1)(E1006–E1016). https://doi.org/
10.1152/ajpendo.1995.269.6.E1006
Gottlieb, D. J., Punjabi, N. M., Newman, A. B., Resnick, H.
E., Redline, S., Baldwin, C. M., & Nieto, F. J. (2005).
Association of sleep time with diabetes mellitus and
impaired glucose tolerance. Archives of internal
medicine,165(8),863–867.
https://doi.org/10.1001/archinte.165.8.863
Griggs, S., Barbato, E., Hernandez, E., Gupta, D.,
Margevicius, S., Grey, M., & Jr., R. L. H. (2022).
Glucose and unstructured physical activity coupling
during sleep and wake in young adults with type 1
diabetes. Scientific reports,12(1)(5790). https://doi.org/
10.1038/s41598-022-09728-2
Griggs, S., Redeker, N. S., Jeon, S., & Grey, M. (2020).
Daily variations in sleep and glucose in adolescents
with type 1 diabetes. Pediatric diabetes, 21(8), 1493–
1501. https://doi.org/10.1111/pedi.13117
Iyegha, I. D., Chieh, A. Y., Bryant, B. M., & Li, L. (2019).
Associations between poor sleep and glucose
intolerance in prediabetes. Psychoneuroendocrinology,
110(104444). https://doi.org/10.1016/j.psyneuen.2019.
104444
Jiawei, Y., Xiaoling, J., Zhilei, S., Shuzhen, L., Hao, H.,
Peiyun, L., . . . Liegang, L. (2017). Relationship of Sleep
Duration With All-Cause Mortality and Cardiovascular
Events: A Systematic Review and Dose-Response Meta-
Analysis of Prospective Cohort Studies. Journal
of the American Heart Association,6(9)(e005947).
https://doi.org/10.1161/JAHA.117.005947
Kothari, V., Cardona, Z., Chirakalwasan, N.,
Anothaisintawee, T., & Reutrakul, S. (2021). Sleep
interventions and glucose metabolism: systematic review
and meta-analysis. Sleep medicine, 78, 24-35.
https://doi.org/10.1016/j.sleep.2020.11.035
Li, M., & Bao, Y. (2018). Methods for Interpreting
Continuous Glucose Monitoring Graphs. In W. Jia (Ed.),
Continuous Glucose Monitoring. https://doi.org/
10.1007/978-981-10-7074-7_5
Liang, Z. (2022). Mining associations between glycemic
variability in awake-time and in-sleep among non-
diabetic adults. Front Med Technol. https://doi.org/
10.3389/fmedt.2022.1026830
Liang, Z., & Chapa-Martell, M. A. (2018). Validity of
consumer activity wristbands and wearable EEG for
measuring overall sleep parameters and sleep structure in
free-living conditions. Journal of Healthcare Informatics
Research, 1-27.
Liang, Z., & Chapa-Martell, M. A. (2019). Accuracy of Fitbit
wristbands in measuring sleep stage transitions and the
effect of user-specific factors. JMIR Mhealth Uhealth,
7(6), e13384. https://doi.org/10.2196/13384
Lou, P., Zhang, P., Zhang, L., Chen, P., Chang, G., Zhang,
N., . . . Qiao, C. (2015). Effects of sleep duration and
sleep quality on prevalence of type 2 diabetes mellitus: A
5-year follow-up study in China. Diabetes research and
clinical practice, 109 (1), 178–184.
https://doi.org/10.1016/j.diabres.2015.04.012
Ohayon, M., Wickwire, E. M., Hirshkowitz, M., & al., e.
(2017). National Sleep Foundation's sleep quality
recommendations: first report. Sleep Health, 3(1), 6-19.
Reutrakul, S., Hood, M. M., Crowley, S. J., Morgan, M. K.,
Teodori, M., Knutson, K. L., & Cauter, E. V. (2013).
Chronotype is independently associated with glycemic
control in type 2 diabetes. Diabetes care, 36(9),2523–
2529. https://doi.org/10.2337/dc12-2697
Tasali, E., Leproult, R., Ehrmann, D. A., & Cauter, E. V.
(2008). Slow-wave sleep and the risk of type 2 diabetes
in humans. Proceedings of the National Academy of
Sciences of the United States of America,
HEALTHINF 2023 - 16th International Conference on Health Informatics
528

Wang, H., Leng, J., Li, W., Wang, L., Zhang, C., Liu, H., . . .
Yang, X. (2017). Sleep duration and quality, and risk of
gestational diabetes mellitus in pregnant Chinese women.
Diabetic medicine : a journal of the British Diabetic
Association, 34(1), 44–50. https://doi.org/10.1111/
dme.13155
Watson, N. F., Badr, M. S., Belenky, G., Bliwise, D. L.,
Buxton, O. M., Buysse, D., . . . Tasali, E. (2015).
Recommended Amount of Sleep for a Healthy Adult: A
Joint Consensus Statement of the American Academy of
Sleep Medicine and Sleep Research Society. Sleep,
38(6):843-4.
Xiang, A. H., Peters, R. K., Kjos, S. L., Marroquin, A., Goico,
J., Ochoa, C., . . . Buchanan, T. A. (2006). Effect of
pioglitazone on pancreatic beta-cell function and diabetes
risk in Hispanic women with prior gestational diabetes.
Diabetes, 55(2), 517–522. https://doi.org/10.2337/
diabetes.55.02.06.db05-1066
Xiao, Q., Keadle, S. K., Hollenbeck, A. R., & Matthews, C.
E. (2014). Sleep duration and total and cause-specific
mortality in a large US cohort: interrelationships with
physical activity, sedentary behavior, and body mass
index. American journal of epidemiology, 180(10),997–
1006.
Yoda, K., Inaba, M., Hamamoto, K., Yoda, M., Tsuda, A.,
Mori, K., . . . Yamada, S. (2015). Association between
Poor Glycemic Control, Impaired Sleep Quality, and
Increased Arterial Thickening in Type 2 Diabetic
Patients. PLoS One, 10(4)(e0122521). https://doi.org/
10.1371/journal.pone.0122521
Zielinski, M. R., Kline, C. E., Kripke, D. F., Bogan, R. K., &
Youngstedt, S. D. (2008). No effect of 8-week time in
bed restriction on glucose tolerance in older long
sleepers. Journal of sleep research, 17(4), 412–419.
https://doi.org/10.1111/j.1365-2869.2008.00673.x
Zuraikat, F. M., Makarem, N., Redline, S., Aggarwal, B.,
Jelic, S., & St-Onge, M. P. (2020). Sleep Regularity and
Cardiometabolic Heath: Is Variability in Sleep Patterns a
Risk Factor for Excess Adiposity and Glycemic
Dysregulation?. Current diabetes reports ,, 20 (8), 38.
https://doi.org/10.1007/s11892-020-01324-w
Contrast Set Mining for Actionable Insights into Associations Between Sleep and Glucose in a Normoglycemic Population
529
