Towards a Synthetic Tissue Model of the Lower Urinary Tract
Alexander Preis
1
a
, Christina Merkl
1
, Paula Miralles
1
, Svenja Heer
1
, Elisabeth Benke
1
b
,
Sebastian Reitelshöfer
1
c
, Sina Martin
1
d
, Ralf Rieker
2
and Jörg Franke
1
e
1
Institute for Factory Automation and Production Systems, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany
2
Institute of Pathology, Universitätsklinikum Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany
ralf.rieker@uk-erlangen.de
Keywords: Anatomical Model, Urology, Lower Urinary Tract, Urinary Bladder, Urethra, Artificial Urine.
Abstract: The development of medical devices often depends on in vivo studies to validate the proper functioning of
the products. These trials provide ethical as well as economic challenges, which can be partially addressed by
the usage of realistic synthetic tissue models that replicate human anatomy and the corresponding properties
of the biological tissue. In this work, a silicone-based model with a fiber structure and a PVA-based model
that exhibits fabrication-induced anisotropy are presented in the special context of the lower urinary tract. The
analysis of the materials in the uniaxial tensile test shows the anisotropic and viscoelastic properties of the
materials. Furthermore, the anatomical model of the lower urinary tract shows expected deformation in
simulation as well as in the real silicone model. Additionally, a suitable artificial urine according to ISO 20696
is shown for use with the model. First experiments to change the pH of the artificial urine are successfully
conducted.
1 INTRODUCTION
In the development of medical devices, a large
proportion of the costs occur due to elaborate in vivo
studies on animals and humans (J. A. DiMasi et al.,
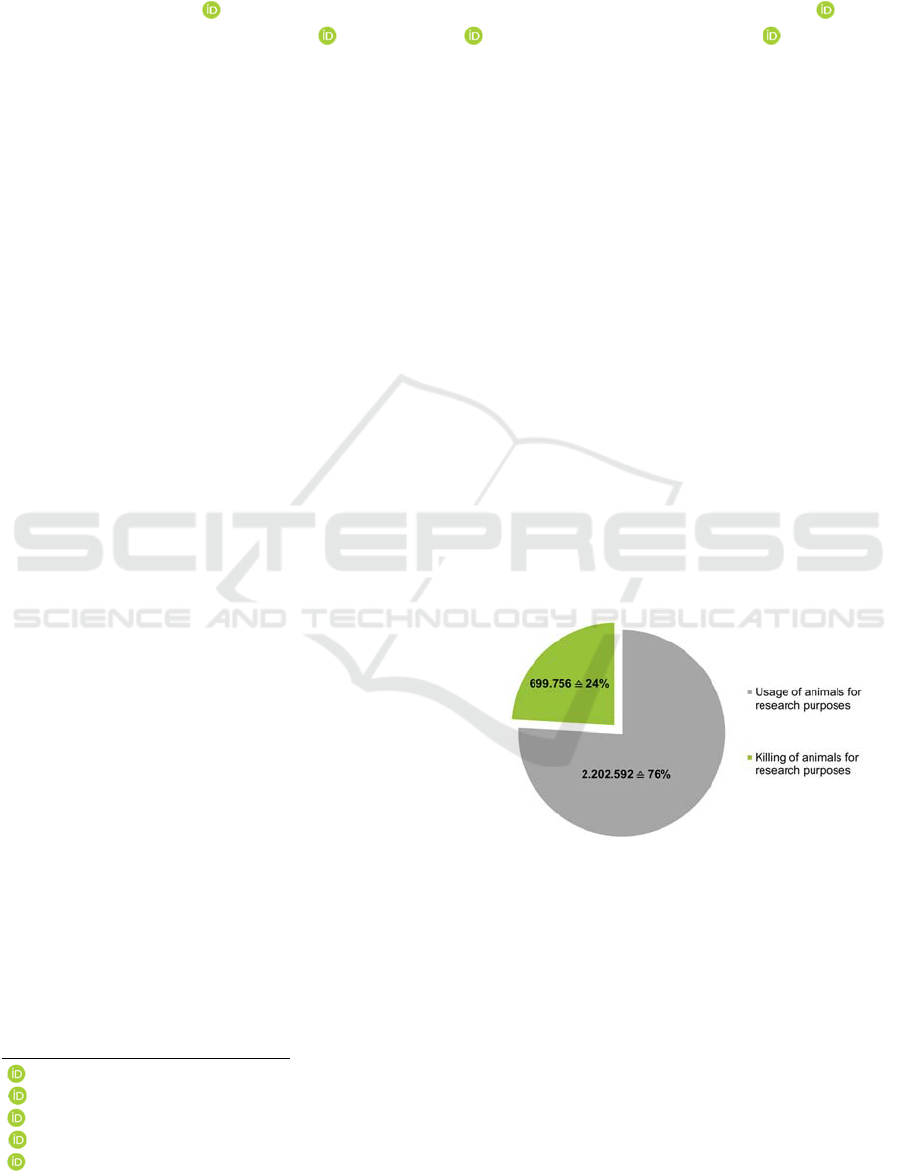
2016). As shown in Figure 1, approximately 2.8
million animals were used for research purposes in
Germany alone in 2014 (DFG, 2021).
Often previously unknown weaknesses of the
tested medical device become apparent in the course
of the in vivo test phases, which leads to further
iterations, further animal testing and means an
economic loss for the companies (I. S. Yoo et al.,
2020). In addition, a successful animal test study does
not necessarily indicate the suitability of the product
for use in humans, as there are sometimes significant
differences both anatomically and physiologically
(M. Viceconti et al., 2016). To overcome the ethical
as well as economic challenges of animal testing,
realistic synthetic tissue models that replicate human
a
https://orcid.org/0000-0003-3469-5982
b
https://orcid.org/0000-0002-6610-4430
c
https://orcid.org/0000-0002-4472-0208
d
https://orcid.org/0000-0002-2146-8265
e
https://orcid.org/0000-0003-0700-2028
anatomy and the properties of the biological tissues
are an obvious choice.
Figure 1: In Germany, approximately 2.8 million animals
were used for research purposes in 2014 (DFG, 2021).
In this work, first steps towards a synthetic in-
vitro and in-silico tissue model of the lower urinary
tract are presented that will be used for the testing of
intraurethral artificial urinary spincters like the one
presented in (A. Preis et al., 2022).
190
Preis, A., Merkl, C., Miralles, P., Heer, S., Benke, E., Reitelshöfer, S., Martin, S., Rieker, R. and Franke, J.
Towards a Synthetic Tissue Model of the Lower Urinary Tract.
DOI: 10.5220/0011780500003414
In Proceedings of the 16th Inter national Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 1: BIODEVICES, pages 190-197
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

1.1 Structure of Biological Tissues
Biological tissues often have unique properties
compared to many engineering materials. They are
capable of load-dependent adaptation and repair
when damaged, which means, that they can adapt to
changing mechanical requirements (remodeling).
Most biological tissues are complex composite
materials with inhomogeneous and anisotropic
properties. As a result, the mechanical properties vary
from point to point within a tissue and the response to
forces acting in different loading directions can be
distinct. As an example, the values for the strength
and stiffness of bone differ both between various
bones and at individual points within the same bone.
Furthermore, biological tissues are viscoelastic to a
large extent, so the rate of load application and the
creep and relaxation processes that occur are also
relevant to the analysis of mechanical properties. (Y.
C. Fung, 1993)
The mechanical properties of biological tissues,
which are mainly composed of cells and an
extracellular matrix consisting of fibers and ground
substance, are largely determined by the collagen and
elastin fiber content. The properties of the tissues are
optimized by different compositions and orientations
according to the specific application. (Y. C. Fung,
1993)
The primary mechanical function of collagen
fibers is to resist axial tension. Because of their large
length-to-diameter ratio, they are susceptible to
buckling and not suited to withstand compressive
loads. When a fiber is pulled, its length increases, as
with a mechanical spring, and the energy applied to
stretch the fiber is stored. The release of this energy
subsequently returns the fiber to its unstretched
configuration. The individual fibrils of collagen are
surrounded by a gel-like ground substance, which is
largely composed of water and contributes to the
viscoelastic material behavior with relatively high
tensile and low compressive strength of the collagen
fibers. Among the non-collagenous tissue
components, elastin is another important fibrous
protein with material properties comparable to those
of rubber. The elastic elastin fibers are highly
extensible, and their elongation is reversible even
under high stress. They behave elastically with low
stiffness up to an elongation of about 200 %, followed
by a short range where the stiffness increases sharply
until failure. In summary, elastin fibers exhibit elastic
material properties with low Young's modulus, while
collagen fibers exhibit viscoelastic material behavior
with higher Young's modulus. (N. Özkaya et al.,
2012)
1.2 Mechanics of Biological Tissues
Viscoelastic materials like biological tissues exhibit
both elastic and viscous behavior. In the following,
elasticity, viscosity, and viscoelasticity are briefly
explained for better understanding.
Elasticity
Elasticity describes the ability of a material or body
to reverse a change in shape caused by an external
force through its own internal force, which is also
referred to as the restoring force. If a specimen with
an original length L
0
is loaded longitudinally with a
force that deforms the specimen to length L, the strain
ε is defined according to (1):
𝜀=
𝐿−𝐿
𝐿
=
∆𝐿
𝐿
(1)
with:
𝜀 strain
𝐿 current length
𝐿
0
initial length
∆𝐿 change in length
Stress σ and strain are related by Hooke's law (2)
and are proportional to each other. The
proportionality factor is a material constant which is
called Young's modulus 𝐸.
𝜎=𝐸∙𝜀
(2)
with:
𝜀 strain
𝐸 Young’s modulus
𝜎 stress
In models, elastic behavior is usually represented
by linear elastic springs. They can deform reversibly
when a load is applied and return to their original
shape when the load is removed. If a material behaves
linearly elastic, the stress is linearly proportional to
the strain. In elastic materials, the mechanical
properties are independent of time. When externally
loaded, they deform instantaneously and return to
their original shape almost immediately when
unloaded. The fibrous portion of biological tissues
can be simplified in such a way. (N. Özkaya et al.,
2012)
Viscosity
Viscosity provides information about the flow
behavior of a material. The dynamic viscosity η
describes the internal friction of fluids and represents
the resistance to a forced, irreversible change of
location of its volume elements. For a better
illustration, one can imagine that the fluid is located
Towards a Synthetic Tissue Model of the Lower Urinary Tract
191

between two plates oriented parallel to each other and
adheres to both plates. If the upper plate is moved
with a velocity 𝑣, the fluid layer in the immediate
vicinity also moves with the velocity 𝑣 due to
adhesion. Since the lower plate has not moved, the
adjacent fluid layer is at rest. The velocity within the
fluid increases from the lower plate at rest to the
moving upper plate. In a laminar flow, the velocity
gradient 𝑑𝑣/𝑑𝑦 arises, which is also often
abbreviated as 𝛾
. For ideal fluids, Newton's law
applies to calculate the resulting shear stress τ
according to (3):
𝜏= 𝜂
𝑑𝑣
𝑑
𝑦
= 𝜂 𝛾
(3)
with:
𝜏 shear stress
𝜂 dynamic viscosity
𝛾
velocity gradient
The dynamic viscosity 𝜂 acts as a constant of
proportionality. Thus, in contrast to elastic solids, the
stresses do not depend on the deformation per se, but
on the rate of deformation. In models, purely viscous
behavior is therefore usually represented by a
Newtonian damper. In this model, the resulting stress
at a constant viscosity depends only on the
deformation rate and the strain rate. The ground
substances portion of biological tissues can be
simplified as purely viscous. (J. de Vicente, 2012)
Viscoelasticity
Because of their composite structure, biological
tissues exhibit both elastic and viscous properties.
They therefore exhibit creep and relaxation processes
when subjected to loading and unloading. The
response of viscoelastic materials depends on the rate
at which the load is changed. As a result, the stress-
strain diagram of a viscoelastic material also depends
on the rate at which the load is applied to the material.
Its behavior can be described by characteristic
material functions, which are determined in special
experiments, first and foremost the creep and
relaxation test. Here, the response of a material to a
constant stress applied at time 𝑡
0
and removed later at
time 𝑡
1
is observed. Such a stress immediately causes
a strain 𝜀 in a linearly elastic material at time 𝑡
0
. This
constant strain remains in the material until time 𝑡
1
.
If the applied stress is removed at time 𝑡
1
, the linear
elastic material immediately and completely recovers
from the deformation. To the same constant loading
condition, a viscoelastic material responds with strain
that gradually increases between times 𝑡
0
and 𝑡
1
. At
time 𝑡
1
, when the load is removed, a gradual recovery
begins.
To represent viscoelastic behavior, models that
have springs as well as dampers are used as basic
elements. One of the best-known models is the
Kelvin-Voigt model, which is based on a parallel
connection of the two basic elements. Due to the
parallel connection, the strain of the damper equals
that of the spring. Using Hooke's law for the elastic
spring and Newton's law for the damper, the first
order differential equation shown in (4) is obtained
(Y. C. Fung, 1993):
𝜎= 𝜎
+𝜎
=𝐸 𝜀+𝜂𝜀 (4)
with:
𝜎 total stress
𝜎
𝐻
stress of the spring
𝜎
𝑁
stress of the damper
𝐸 Young's modulus
𝜀 strain of the spring
𝜂 dynamic viscosity
𝜀
deformation rate
The deformation of a damper placed parallel to a
spring, as in the Kelvin-Voigt model, is limited by the
response of the spring to the applied forces. The
damper cannot deform continuously in this
arrangement. Therefore, the Kelvin-Voigt model
represents a viscoelastic solid behavior. (N. Özkaya
et al., 2012)
Another well-known model for simulating
viscoelastic behavior is the Maxwell model. Like the
Kelvin-Voigt model, it consists of a spring and a
damper. However, these are not connected in parallel,
but in series. From the arrangement it follows that the
total stress of the system must be equal to the stress
in the spring and to the stress in the damper. The total
strain of the system results from the individual strains
of the spring and the damper, as described in (5):
𝜀= 𝜀
+𝜀
=𝐸 𝜎+𝜂𝜎 =𝐸𝜂𝜀
(5)
with:
𝜀 total strain
𝜀
𝐻
strain of the spring
𝜀
𝑁
strain of the damper
𝐸 Young's modulus
𝜎 total stress
𝜂 dynamic viscosity
𝜀
deformation rate
In the case of the Maxwell model, a force
application leads to deformation of both the spring
and the damper. The deformation of the spring is
finite, whereas the damper deforms as long as the
force is applied to the system. Therefore, the overall
behavior of the Maxwell model resembles a fluid
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
192

rather than a solid and is referred to as a viscoelastic
fluid model. (N. Özkaya et al., 2012)
However, the Kelvin-Voigt and Maxwell models
alone are not capable of representing the real behavior
of many viscoelastic materials but can be used to
create more complex viscoelasticity models. A well-
known one is the Zener model, which is also referred
to as the standard linear solid model and can be
described by two equivalent representations: the first
consists of a series connection of a Kelvin model with
a spring, and the second one consists of a parallel
connection of a Maxwell model with a spring. It is
used to represent the viscoelastic behavior of some
biological materials, such as cartilage. Similarly,
there is also the three-element fluid model used in the
study of blood. In this one, the additional spring is
replaced by a damper. Also, other models can be
created by combining any number of Maxwell and/or
Kelvin-Voigt bodies to represent the behavior of
other materials. (N. Özkaya et al., 2012)
2 MATERIALS AND METHODS
In the present work, two possible materials for a
synthetic in-vitro tissue model of the lower urinary
tract are presented. Additionally, a suited anatomical
geometry and a recipe for artificial urine, which can
be used to test urinary stone formation, are shown.
2.1 Silicone-Based Model
For the silicone-based model, the RTV-2 silicone
"Elastosil P7670 A/B" with a 10 wt% silicone oil
(AK 100) content is used (Wacker Chemie AG). To
create the anisotropy, additional ‘fibers’ without
silicone oil are embedded in a matrix of silicone
according to the previous formula. The silicone is
mixed in a vacuum stirrer to avoid air inclusion and
subsequently cast into sheets with a thickness of
2 mm and fully cured. The resulting tissue model has
a fiber volume content of around 24 %.
2.2 PVA-Based Model
For the PVA-based model, a solution consisting of 10
wt% PVA powder (MW: 133000 g/mol, degree of
hydrolysis: 99 %, Polysiences Inc.) in distilled water
is prepared. Both components are mixed in a sealed
vessel at a constant temperature of 100 °C for a
duration of 6 h. The finished, transparent solution is
poured into sheets of 2 mm thickness, analogously to
the silicone. Any air pockets are removed by vacuum
and the plate is cooled to room temperature.
Afterwards it is frozen for at least 16 hours. After the
first freezing cycle (consisting of freezing and
complete thawing to room temperature), the
previously viscous mass has a gelatinous consistency
and a whitish color. After two freezing cycles, two
opposite ends of the sheet are fixed in a jig, the sheet
is stretched by 80 % of its original length and eight
more freezing cycles are performed to create the
anisotropy. The material is packed airtight to keep the
samples from drying out.
2.3 Material Characterization
To determine the mechanical properties of the tissue
models, uniaxial tensile tests (Z 2.5/TN1S,
ZwickRoell GmbH & Co. KG) are performed with
the uniform base materials as well as along and
perpendicular to the fiber orientation and strain
direction of the anisotropic tissue models. For this
purpose, specimen geometry S3A of the DIN 53504
standard is used at strain rates of 200 and
800 mm/min. The significance of the results is then
statistically analyzed.
2.4 Lower Urinary Tract
The lower urinary tract consists of the urinary bladder
(vesica urinaria) and urethra. They work together as
a functional unit and perform the tasks of storing and
emptying urine. In both sexes, the vesica urinaria is
located in the lesser pelvis just behind the symphysis.
In women, it lies in front of the vagina and in front of
and below the uterus; in men, it lies in front of the
rectum. The bladder of an adult has a capacity of
about 400 to 500 ml and is emptied to less than 50 ml
during micturition. Depending on the state of filling,
it is bowl-shaped flattened or spherical. The thickness
of the bladder wall varies according to the volume of
urine, decreasing accordingly as it expands. The wall
thickness ranges from 1 to 5 mm, and can also reach
up to 10 mm. The transition from the vesica urinaria
to the urethra is called the bladder neck. The female
urethra is straight and short, the male urethra passes
through the penis and is longer and has several
curves. The female urethra considered for this work
is about 40 mm long and has a diameter of about 8
mm. (D. Schultz-Lampel et al., 2012; M. Schünke et
al., 2022)
The anatomical model of the urinary bladder was
created using Autodesk Inventor (Autodesk Inc.).
Afterwards the deformation during normal
micturition with an abdominal pressure of 20 cmH
2
O
and a detrusor pressure of 30 cmH
2
O as well as
different flow rates with a cumulated intravesical
Towards a Synthetic Tissue Model of the Lower Urinary Tract
193
pressure of 25, 50 and 80 cmH
2
O were simulated
using the multiphysics simulation software Ansys
(Ansys Inc.).
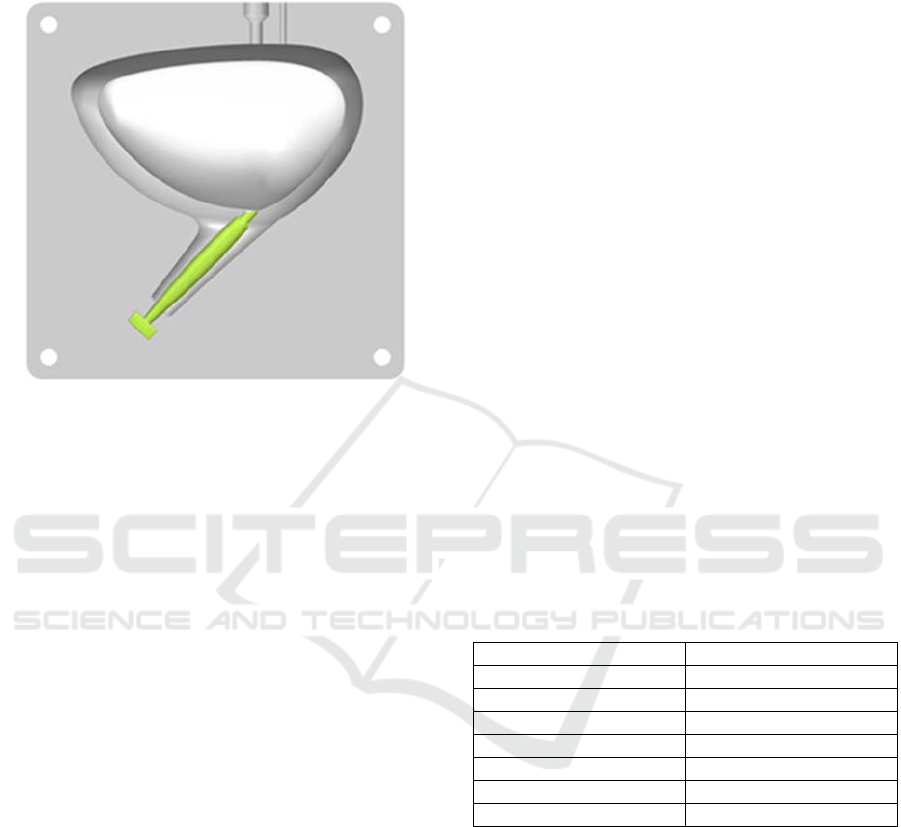
Figure 2: The assembled silicone mold consists of the rigid
outer part and inner urethra as well as the inner bladder part
made out of wax melting at low temperature.
To manufacture the in-vitro model of the urinary
bladder out of silicone, an additive manufactured
mold is utilized. While the outer part and the inner
urethra are made of rigid material, the inner bladder
is made out of wax, which allows the removal by
melting after the silicone has been cured. A slice of
the assembled mold is shown in Figure 2.
2.5 Artificial Urine
The formation of urinary stones is particularly
problematic with foreign bodies inserted in the
urinary tract, such as stents, catheters, or the above
mentioned intraurethral artificial urinary sphincter. A
maximum retention time of two to three months is
often recommended for such urinary tract implants. It
has been shown that after three or more months, more
than 75% of ureteral stents exhibit severe
encrustation, making removal of the implant with the
standard procedures partially impossible. (T.
Kawahara et al., 2012)
As in ‘normal’ urolithiasis, the formation of the
urinary stone here is favored by changes in pH.
Classically, infestation also begins by the adsorption
of proteins on the implants’ surface, which allows
subsequent accumulation of bacteria and eventually
biofilm formation. This leads to a local change in pH
and ultimately to mineral encrustation of the implant.
Mainly, the two different mechanisms homogeneous
and heterogeneous nucleation can be distinguished.
Homogeneous nucleation results in uric acid and
cystin stones caused by an oversaturation of the urine
precipitation of the corresponding crystals. On the
other hand, heterogeneous nucleation results in
calcium and infectious stones caused by detritus or
other crystal nuclei, which can be induced by
bacterial infection. (R. Hautmann & J. E. Gschwend,
2014)
The formation of the already mentioned three
most common main components of urinary stones has
different causes:
Oxalate stones are often idiopathic but can also
be caused by malnutrition or metabolic defects. Here,
both homogeneous and heterogeneous nucleation can
be considered.
Uric acid stone formation is increased by more
acidic urine since uric acid is poorly soluble in urine
with a pH value of less than 6. This can be caused by
malnutrition, disease or medication and leads to a
homogeneous nucleation.
Phosphate stones are mainly formed in urine
with higher pH values above 6.8. The cause of the
change in pH is usually an infectious disease of the
urinary tract which leads to a local change in pH and
therefore a homogeneous nucleation.
It is clearly shown, that changes in pH can have
an impact on the formation and type of urine stones.
(C. A. Wagner & N. Mohebbi, 2010; H.-U. Schmelz
et al., 2014; R. Hautmann & J. E. Gschwend, 2014)
Table 1: Composition of artificial urine (ISO 20696).
CH
4
N
2
O 25.0
g
N
aCl 9.0
g
N
a
2
HPO
4
2.5 g
KH
2
PO
4
2.5
g
N
H
4
Cl 3.0
g
C
4
H
7
N
3
O 2.0 g
N
a
2
SO
3
(h
y
drated) 3.0
g
H
2
O1.0 l
Five-fold artificial urine concentrate according to
ISO 20696 was acquired from Synthetic Urine e.K.
and mixed with distilled water. The composition is
detailed in Table 1. Hydrochloric acid (37 %) and
sodium hydroxide (20 %) acquired from Algin
Chemie e.K. were used to evaluate the titration curve
of the artificial urine for future urinary stone
formation experiments with changed pH.
3 RESULTS
The results of the uniaxial tension tests of the
preliminary tissue models as well as the anatomical
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
194

model and the titration curve of the artificial urine are
presented in this chapter.
3.1 Properties of the Tissue Models
Figure 3 shows the distribution of elongation at break
and tensile strength of the measurements at different
test speeds. In case of the silicone-based model, there
are 12 valid measurements for the test speed of 200
mm/min and 16 at 800 mm/min. For the PVA-based
samples, the number is lower with 8 valid
measurements at a test speed of 200 mm/min and 6 at
800 mm/min.
Figure 3: Significant strain rate dependent differences for
the materials along fiber direction are highlighted in color.
The statistical analysis of the results shows that
for the elongation at break of both models and the
tensile strength of the silicone-based tissue model,
significant changes in the parameters occur as a
function of the test speed. These are highlighted in
color in the figure.
Figure 4 shows the distribution of elongation at
break and tensile strength of the measurements with
loading along and across the preferential direction.
The silicone-based model has 16 valid measurements
for the test across and 15 along the fiber direction. For
the PVA-based specimens, the number is 26 valid
measurements across to and 22 along the direction of
strain during freezing.
Figure 4: Significant loading direction dependent
differences for the materials are highlighted in color.
The statistical analysis of the results shows that
for both the elongation at break and the tensile
strength of both tissue models significant changes of
the parameters occur as a function of the loading
direction and thus, as expected, an anisotropy exists.
The significant changes are highlighted in color in the
figure.
3.2 Discussion of the Tissue Models
The silicone-based tissue model shows a significant
increase in values with higher test speed in terms of
both elongation at break and tensile strength. Thus,
although the stress-strain curve is similar for both
strain rates, the tissue model exhibits some
viscoelasticity. In addition, significant anisotropy
with respect to mechanical properties can also be
shown for the model. This is consistent with the
theory on fiber composites, according to which the
material takes the main load when loaded in fiber
direction. (Y. C. Fung, 1993) Due to the high
durability of the silicone, the silicone-based tissue
model is also well suited for long-term storage and
thus is first used for the fabrication of the anatomical
lower urinary tract model.
The PVA-based tissue model also shows
significant differences in elongation at break
depending on the test speed, with the maximum
elongation decreasing with increasing loading speed.
Although the strength remains the same and shows no
significant difference, the decreasing elongation at
break still results in a higher slope of the stress-strain
curve. The anisotropy test shows significantly higher
values for all the material properties investigated for
a loading direction corresponding to the loading
direction during the freezing and thawing cycles. This
is due to the fact that by straining during thermal
treatment, the orientation of the PVA-rich phases can
be affected (J. L. Holloway et al., 2013). However,
the model shows poor long-term stability with
changing mechanical properties over time due to the
evaporation of water (C. K. McGarry et al., 2020).
3.3 Evaluation of the Anatomical
Model
In Figure 5, the simulation of the anatomical model
of the lower urinary tract during normal micturition is
shown. A consistent abdominal pressure of 20
cmH
2
O and an increasing detrusor pressure up to 30
cmH
2
O was applied, resulting in a maximum
intravesical pressure of 50 cmH
2
O. As visible in the
figure, the desired bowl shaped deformation is
created.
Towards a Synthetic Tissue Model of the Lower Urinary Tract
195
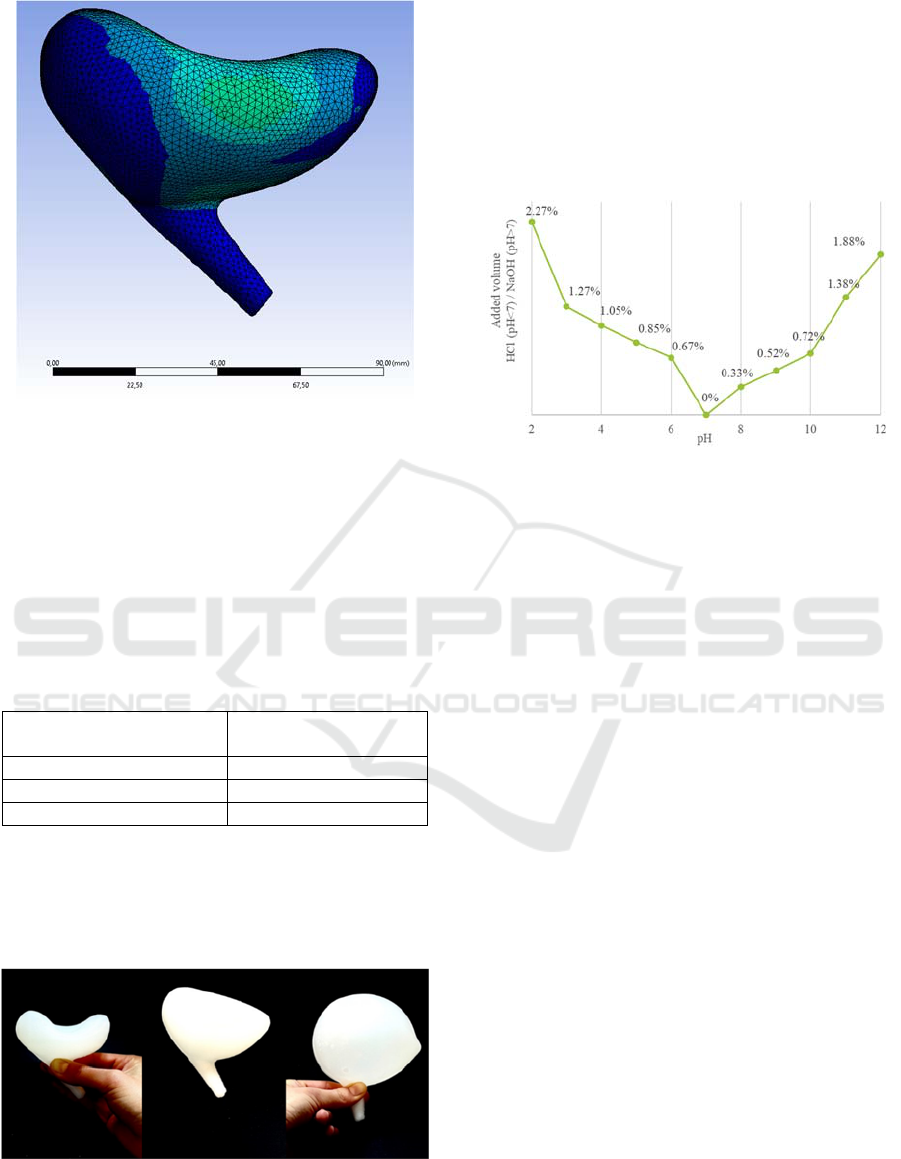
Figure 5: Deformation of the bladder after normal
micturition with a detrusor pressure of 30 cmH
2
O.
The flow rate was simulated utilizing different
intravesical pressures between 25 and 80 cmH
2
O. As
shown in Table 2, the resulting flow rates of 22.81,
28.87 and 34.97 ml/s are within the physiological
values of 20 to 35 ml/s (D. Schultz-Lampel et al.,
2012).
Table 2: The simulated flow rates caused by different
intravesical pressures are within the physiological range of
20 to 35 ml/s (D. Schultz-Lampel et al., 2012).
Intravesical pressure in
cmH
2
O
Flow rate in ml/s
25 22.81
50 28.87
80 34.97
The bladder model manufactured out of silicone
is shown in Figure 6. Here, different fill volumes of
50, 100, and 500 ml are shown. The resulting
deformations reflect the results of the simulation as
well as the one of the real urinary bladder.
Figure 6: The silicone bladder model filled with 50, 100,
and 500 ml (left to right) shows realistic deformation.
3.4 pH Change of the Artificial Urine
Figure 7 shows the titration curve of the artificial
urine with its pH decreased by adding 37 % HCl and
increased by addition of 20 % NaOH. When
comparing the pH decrease with the pH increase by a
pH difference of 5, it is noticeable that the amount of
HCl used is 0.39% higher by volume than for NaOH.
Figure 7: Comparison of titration curves for the addition of
37 % HCl (pH < 7) and 20 % NaOH (pH > 7) in volume
percent.
4 SUMMARY AND OUTLOOK
To avoid relying on samples of human or animal
origin, it is desirable to have a synthetic tissue model
that exhibits proper material behavior and is a
sufficient representation of the real human anatomy.
Silicone-based models with a fiber structure and
PVA-based models that exhibit fabrication-induced
anisotropy are both suitable for this purpose. The
analysis of the materials in the uniaxial tensile test at
200 mm/min and 800 mm/min strain rate shows the
viscoelasticity of the base materials of the models.
Due to the room temperature vulcanization, the
silicone can be easily mixed and poured into any
mold. Furthermore, by adjusting the base silicone as
well as silicone oil and fiber content in the model, the
mechanical properties of the material can be adjusted
in future work, allowing the targeted replication of
specific tissue types. The fiber orientation makes the
anisotropy of the model readily adjustable. It thus
offers the possibility of replicating tissue structures
that exhibit strong fiber orientation, such as skeletal
muscle. Because of this, the material was chosen to
create the in-vitro model of the lower urinary tract.
The manufacturing process of the PVA-based
model is simple and the mechanical properties can be
adjusted in many ways. This enables the modeling of
a wide range of biological tissues. In addition,
anisotropic properties could be generated even for
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
196

more complex geometries, as this is dependent on the
load during the freezing cycles. In the case of the
human urinary bladder, the load could be applied by
inflation. However, since the mechanical properties
of the PVA-based model change over time due to the
evaporation of water, it is not suitable for long-term
storage.
Additionally, an anatomical model of the female
human lower urinary tract is presented. The model is
created with ease of manufacturing in mind.
Openings for ureters can be added after casting.
Simulation utilizing Ansys shows, that the
deformation of the created in-silico model during
micturition represents the normal bowl shaped
deformation of the real counterpart. The
manufactured in-vitro silicone model also shows the
fitting deformation during filling and micturition.
After implantation of an intraurethral artificial
urinary sphincter like the one presented by (A. Preis
et al., 2022) into the lower urinary tract model,
artificial urine using the recipe of ISO 20696 can be
used to test for possible urinary stone formation
caused by the implant. The titration curve of the urine
using hydrochloric acid and sodium hydroxide is
shown and will be used to modify the pH and thus
check for different urinary stone formation situations.
In future work, the results will be used as a
starting point to create a realistic mechatronic
urodynamic test bench, which can be used to test the
already presented purely mechanical intraurethral
artificial urinary sphincter. The main components that
need to be addressed are the material properties of the
bladder, the urethra and the method of
mechatronisation of the test bench to create the
wanted urodynamic conditions.
REFERENCES
C. A. Wagner, & N. Mohebbi (2010). Urinary pH and stone
formation. Journal of Nephrology. https://doi.org
/10.5167/uzh-45805
C. K. McGarry, L. J. Grattan, A. M. Ivory, F. Leek, G. P.
Liney, Y. Liu, P. M., R. Rai, A. P. Robinson, A. J. Shih,
B. Zeqiri, & C. H. Clark (2020). Tissue mimicking
materials for imaging and therapy phantoms: a review.
Physics in Medicine and Biology. https://doi.org/
10.1088/1361-6560/abbd17
D. Schultz-Lampel, M. Goepel, & A. Haferkamp. (2012).
Urodynamik (3., vollst. bearb. Aufl.). Springer
Medizin.
DFG. (2021). Tierversuche in der Forschung.
https://www.dfg.de/download/pdf/dfg_im_profil/gesch
aeftsstelle/publikationen/tierversuche_forschung.pdf
H.-U. Schmelz, C. Sparwasser, & W. Weidner. (2014).
Facharztwissen Urologie. Springer.
I. S. Yoo, A. Preis, & J. Franke (2020). Development of a
test bench for the urodynamic simulation of the lower
urinary tract. https://doi.org/10.1109/EMBC44109.
2020.9176198
J. A. DiMasi, H. G. Grabowski, & R. W. Hansen (2016).
Innovation in the pharmaceutical industry: New
estimates of R&D costs. Journal of Health Economics.
https://doi.org/10.1016/j.jhealeco.2016.01.012
J. de Vicente (Ed.). (2012). Viscoelasticity - From Theory
to Biological Applications. IntechOpen Limited.
https://doi.org/10.5772/3188
J. L. Holloway, A. M. Lowman, & G. R. Palmese (2013).
The role of crystallization and phase separation in the
formation of physically cross-linked PVA hydrogels.
Soft Matter. Advance online publication. https://
doi.org/10.1039/C2SM26763B
M. Schünke, E. Schulte, & U. Schumacher. (2022).
PROMETHEUS: LernAtlas Anatomie. Thieme.
M. Viceconti, A. Henney, & E. Morley-Fletcher (2016). In
silico clinical trials: how computer simulation will
transform the biomedical industry.
https://doi.org/10.13140/RG.2.1.2756.6164
N. Özkaya, M. Nordin, D. Goldsheyder, & D. Leger.
(2012). Fundamentals of Biomechanics. Springer.
A. Preis, J. Treviranus, E. Benke, S. Reitelshöfer, & J.
Franke (2022). Novel Concept for a Mechanical
Intraurethral Artificial Urinary Sphincter. Proceedings
of the 15th International Joint Conference on
Biomedical Engineering Systems and Technologies -
BIODEVICES. https://doi.org/10.5220/001088570000
3123
R. Hautmann, & J. E. Gschwend. (2014). Urologie.
Springer.
T. Kawahara, H. Ito, H. Terao, M. Yoshida, & J. Matsuzaki
(2012). Ureteral stent encrustation, incrustation, and
coloring: morbidity related to indwelling times. Journal
of Endourology. https://doi.org/10.1089/end.2011.0385
Y. C. Fung. (1993). Biomechanics: Mechanical Properties
of Living Tissues (2nd ed.). Springer.
Towards a Synthetic Tissue Model of the Lower Urinary Tract
197
