
A Portable System for Screening of Cervical Cancer
Paloma Cepeda Andrade and Sesh Commuri
University of Nevada, Reno, 1664 N Virginia St, Reno, NV, E.U.A.
Keywords: Cervical Cancer, Portable Colposcope, Specular Reflections, Segmentation.
Abstract: Cervical cancer is one of the most common cancers that affect women, with the highest incidence and
mortality rates occurring in low- and middle-income countries. Early detection is crucial for successful
treatment, but the need for expensive equipment, trained colposcopists, and clinical infrastructure has made
it difficult to eradicate this disease. To address such limitations, we propose the development of a portable,
low-cost colposcope that is easy to use, which uses image processing techniques to automate lesion detection
and provides a quantitative measure to evaluate progression of the disease or to measure treatment efficacy.
Through this paper, we present the development of a system that encompasses the above, and preliminary
results show that we can achieve a low-cost bioinformatics-based screening for early detection of cervical
cancer in a clinical setting.
1 INTRODUCTION
Cervical cancer is a preventable and curable disease.
Despite this, it is the fourth most common cancer
amongst women worldwide, with an estimated
604,000 new cases and 342,000 deaths reported in
2020 ((WHO), 2022). It has been reported that around
90% of these cases occurred in low- and middle-
income countries (Sung et al., 2021), where there is
limited access to healthcare, and it is difficult to carry
out screening and treatment for this disease.
Approximately 95% of cases of cervical cancer
are caused by the human papillomavirus (HPV)
(Franco et al., 2001). Prevention of this disease can
be made through vaccination against HPV, as well as
screening methods to identify cervical dysplasia
(abnormal cells on the cervical tissue) prior to the
development of cervical cancer. The golden standard
for cervical screening is Visual Inspection with
Acetic Acid (VIA) during the colposcopic exam, also
known as a colposcopy (Sankaranarayanan et al.,
2003).
A colposcopy requires a speculum to hold the
vaginal walls open and a light source to illuminate the
surface of the cervix during the examination. The
colposcope is then used to visually examine the
cervix and surrounding tissues. The cervix is first
rinsed with saline solution and then stained with 3-
5% acetic acid. Acetic acid causes abnormal proteins
in the epithelium to coagulate and appear white and
opaque when inspected under light. These abnormal
cells on the surface of the cervix are termed as
cervical intraepithelial neoplasia (CIN) and are
usually caused by certain types of HPV. While CIN
is not cancer, it may become cancer and spread to
adjacent normal tissue. The density of acetowhitening
(AW) indicates the grade of the precancerous cells (Li
& Poirson, 2006). CIN can be easily treated, and full
recovery is possible if it is detected early. Depending
on the severity, a diagnosis of CIN1, CIN2, or CIN3
is given, where a mild case is likely to heal on its own,
and moderate to severe cases require immediate
treatment (Castle et al., 2007). However, even for an
expert in the field, it is difficult to diagnose and
investigate the presence of such lesions unless a
biopsy is performed (Kudva & Prasad, 2018). Other
contributing factors to the high mortality rate in low-
and middle-income countries include lack of
adequate medical facilities, high power requirements
for existing colposcopes, social stigma around
women’s healthcare, and the need for multiple visits
to medical clinics, which may be inaccessible,
unpleasant, and time-consuming for some women
((WHO), 2022). Therefore, there is a need for a tool
with screening capabilities that is easy to use and
reliable in communities that lack the necessary
resources.
Automated detection of CIN has been made
possible by several studies conducted to obtain
images of the cervix. Archived digitized cervical
72
Andrade, P. and Commuri, S.
A Portable System for Screening of Cervical Cancer.
DOI: 10.5220/0011777700003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 1: BIODEVICES, pages 72-79
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

images from screening, taken with a fixed-focus
camera, were used to develop a method based on deep
learning algorithm for automated visual evaluation of
cervical images (Hu et al., 2019). This dataset was
obtained during a study of HPV and cervical cancer
in Costa Rica (Bratti et al., 2004). Li and
coresearchers (Li et al., 2021) collected a large
dataset consisting of colposcopic images collected
from 8,604 patients, which include pathological
information and annotations from expert physicians.
Finally, the Atlas of Colposcopy (Basu &
Sankaranarayanan, 2017) was developed by the
International Agency for Research on Cancer (IARC)
with the intention to provide a freely accessible
learning tool for researchers and medical personnel.
Challenges to the automated detection of CIN
include low quality of the devices used at times,
which impair the image resolution; lighting
conditions, which can make shadows appear,
hindering the ability to find the cervical region of
interest (ROI); distortion of the images due to the
presence of glare or specular reflections (SR) from
the light source; and the appearance of artifacts such
as the speculum and surrounding tissue.
Efforts to overcome these challenges include
classification of cervical dysplasia using machine
learning methods based on image pre-processing and
feature extraction (Asiedu et al., 2019; Bai et al.,
2019; Fernandes et al., 2018). Other researchers have
focused on image pre-processing techniques to detect
and remove SR from the cervical epithelium (Das &
Choudhury, 2017; Lange, 2005; Meslouhi et al.,
2011). While these research teams have achieved
promising results in the field, there is still a need for
complete systems that can rely on effective
techniques for cervical image analysis while in low-
resource settings.
In this paper we describe a portable colposcope
and its use in automated detection of CIN. We present
details about our ongoing pilot study for data
collection and validation of our image analysis
algorithms. Furthermore, we use a technique that can
help remove specular reflections prior to identifying
the presence of lesions and determining their size in
relation to the cervical ROI. Images from the Atlas of
Colposcopy are used to demonstrate the removal of
SR. The steps in detecting and classifying lesions on
the surface of the cervix are presented in the next
section, and the results are discussed in section 3.
2 EQUIPMENT AND METHODS
2.1 System Overview
In this paper, we propose a low-cost and portable tool
for screening and detecting abnormal changes in the
cervix. The Cervitude Imaging System (CIS) (Patent
Appl. 63/121,432, 2020) consists of a digital
microscope used in conjunction with a speculum
during the colposcopic exam and a desktop
application to analyze the captured images. The
digital microscope is capable of 50 to 1000X
magnification and provides illumination to the cervix
via an embedded LED ring. The microscope is placed
at the opening of the speculum, outside the patient's
body, using the setup shown in Figure 1. It is then
powered on and used to capture digital images of the
cervix. The images are then processed using the
proprietary CIS algorithm to detect the presence, size,
and extent of lesions on the cervix.
Figure 1: CIS Digital Microscope with Speculum.
The CIS desktop application, developed in
MATLAB, is built around an image analysis
algorithm that can quickly detect and locate lesions
on the surface of the cervix as images are collected.
Figure 2 shows our framework for image acquisition
and analysis. After capturing images of the cervix
with our probe, the images are transferred via
Bluetooth to a laptop where the desktop application is
running. The CIS application pre-processes the image
in three stages: removal of specular reflections;
segmentation of the cervical region of interest; and
segmentation of acetowhite (AW) lesions.
Upon completing the image pre-processing steps,
classification to determine different severities of CIN
will be performed. In this paper, we cover the steps
involved in the removal of SR. Identification of ROI
can be found in (Cepeda-Andrade & Commuri,
2022).
A Portable System for Screening of Cervical Cancer
73

Figure 2: Steps for Lesion Detection and Classification.
2.2 Pilot Study
We obtained approval from the Institutional Review
Board (IRB number 1629150-4) from the University
of Nevada, Reno to conduct a pilot study to acquire
cervical images with CIS. This small-scale study,
consisting of 30 participants, is ongoing, and we
expect to finalize our image acquisition step in the
upcoming weeks.
The study is directed towards adult female
participants aged between 21 and 65 years old that
have been identified with having abnormal cervical
screening, and where follow-up evaluation is
recommended. Informed consent is required from all
patients participating in this study. The process of
consent is as follows: upon checking in for their
appointment, a member of the research team hands
the patient an information form explaining the details
of the research and any preliminary questions the
patient might have about the study will be answered
by the principal investigator. If the patient is
interested in participating in the study, she will go
through the consent form with the physician who will
be performing her colposcopy.
Following our protocol, standard procedure for
colposcopy is followed, that is, the speculum is
placed, the cervix visualized, the CIS probe is then
placed in a clean protective sleeve and inserted into
the speculum and an image is captured using the CIS
image capturing system and colposcope. Next, 5%
acetic acid (AA) solution is applied to the cervix and
the cervix will be evaluated by the standard
colposcope. The findings are documented by the
standard colposcope and the CIS system. Directed
biopsy and ECC (endocervical curettage) is obtained
as indicated utilizing the standard colposcope.
To protect the privacy of research participants,
once the initial organization of data is completed, the
name of the participant will be replaced by her initials
and a five-digit identification number. In addition to
collecting images of the cervix, we also use the CIS
desktop application to collect demographic
information and relevant health information as
directed by the participating physicians. A
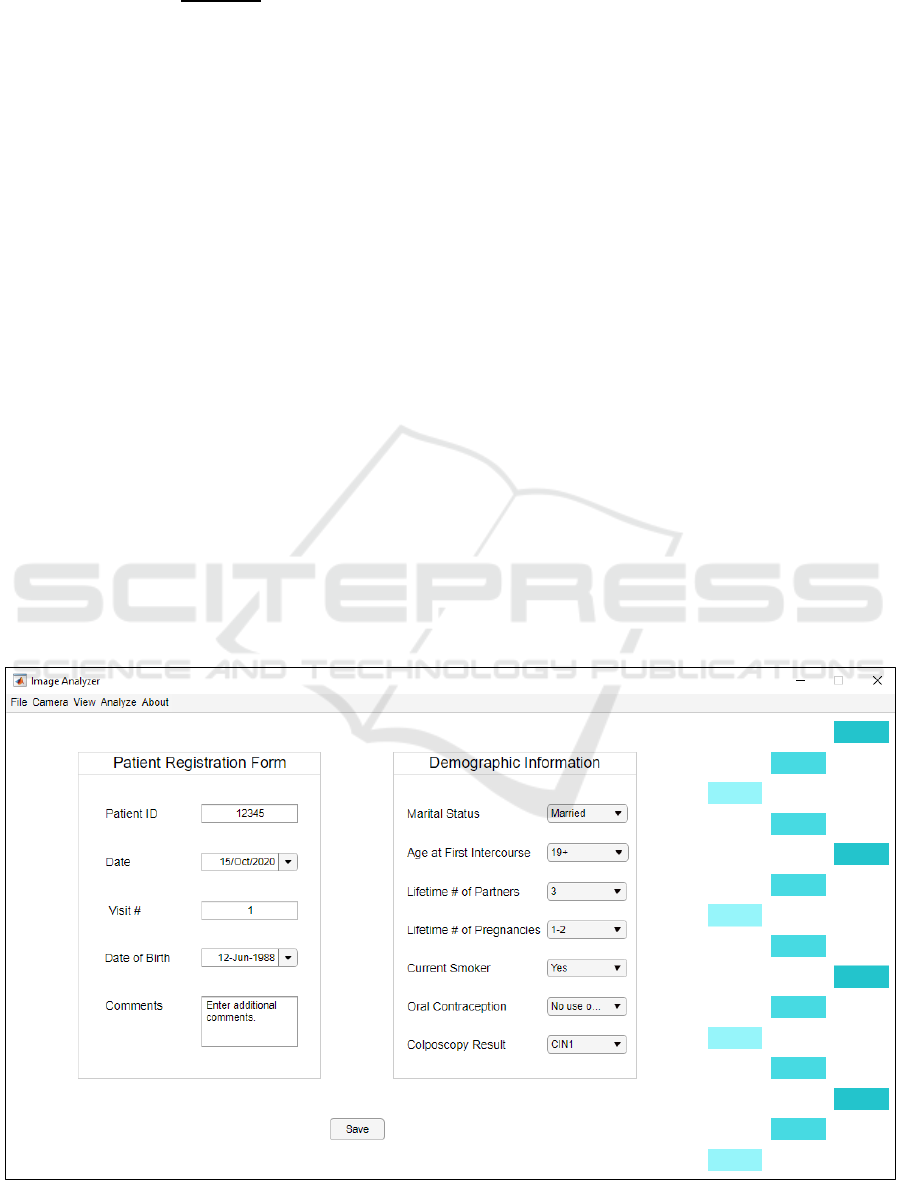
registration form (shown in Figure 3) is filled out for
every new patient. There are also options to attach the
camera and capture images or video, as well as an
option to quickly analyze and save these images. All
data except for identifying information is collected
through this application and is then encrypted and
stored in a secure server.
2.3 Removal of Specular Reflections
(SR)
As mentioned earlier, abnormal cells present in the
epithelium of the cervix become opaque when treated
with acetic acid. The reflected light from the opaque
epithelium gives it a white color, indicating the
presence of a precancerous lesion.
Specular reflections are observed when light from
the colposcope is reflected from the cervical
epithelium or from the speculum. SR on the cervical
images will also appear white, but with high
brightness and low saturation values. This could
cause a problem in which reflections of light on the
tissue could be misconstrued as AW lesions,
producing incorrect diagnostic results. Therefore, it is
necessary to identify and remove SR areas without
affecting the acetowhite regions in the image.
We approach the removal of specular reflections
through exemplar-based image inpainting (Criminisi
et al., 2004; Le Meur et al., 2013). It is important to
maintain the structure and texture of the surrounding
epithelial tissue or AW lesion, and this method has
been shown to take into account these factors to
properly restore images (Shroff & Bombaywala,
2019).
The method to achieve this begins with
identifying the target regions, Ω, to be removed and
inpainted. Given a patch, Ψ
p
, centered on a boundary
pixel, p, in the target region, its priority can be
computed as:
𝑃
(
𝐩
)
=𝐶
(
𝐩
)
𝐷
(
𝐩
)
(1)
where 𝐶
(
𝐩
)
is a confidence term that measures
the amount of reliable information surrounding the
pixel p and 𝐷
(
𝐩
)
is a data term that reflects the
presence of contour information. These are defined
as:
𝐶
(
𝐩
)
=
∑
𝐶(𝐪)
𝐪∈
∩
Ψ
𝐩
(2)
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
74
𝐷
(
𝐩
)
=
∇𝐼
𝐩
⋅𝐧
𝐩
𝛼
(3)
where |Ψ
p
| is the area of Ψ
p
, 𝛼 is a normalization
factor (𝛼 = 255 for an image of type uint8), ∇I
p
┴
shows the direction of curves of constant light
intensity on a surface (isophotes), and n
p
is a unit
vector orthogonal to the point p. The priority term is
calculated for every border patch, with different
patches for each pixel on the boundary of the target
region.
Next, data is extracted from the source region, Φ,
which is computed by subtracting the target region
from the input image. Image texture is propagated by
directly sampling the source region until a patch,
centered around a point q, most similar to Ψ
p
is found:
Ψ
𝒒
=argmin𝑑(Ψ
𝐩
,Ψ
𝐪
)
(4)
where the distance between the two patches is
calculated through the sum of squared differences
(SSD). Finally, after finding the exemplar Ψ
q
, the
pixel values are copied into the target patch and the
confidence values are updated.
A summary of the described algorithm for
removal of specular reflections is given below:
1. Identify target regions, Ω, from the input
image, to be removed and inpainted.
2. Create a binary mask where the nonzero
values are the pixels that correspond to the
target regions.
3. Identify the source region, Φ, which
corresponds to the input image minus the
target region.
4. For every patch of size Ψ centered on a
boundary pixel in the target region, compute
the patch priority.
5. Find the patch with the maximum priority.
This patch constitutes the target patch to be
inpainted.
6. Given the target patch, search for the best-
matching patch in the source region by using
the sum of square difference (SSD).
7. Copy image data from the best-matching
patch to the target patch.
8. Update the input image, binary mask, and
patch priority.
Steps 4–8 are then repeated until all target regions
have been inpainted. We applied this algorithm to
regions identified as specular reflections in our
dataset, and the results are shown in section 3.
2.4 Segmentation of the Cervical
Region
We presented the steps to achieve segmentation of the
cervical region in detail in (Cepeda-Andrade &
Commuri, 2022). We follow a five-step approach to
the analysis of cervigrams and detection of
precancerous lesions on the cervix, summarized as
follows:
Figure 3: Example of the CIS desktop application.
A Portable System for Screening of Cervical Cancer
75
1. Convert the cervical image from sRGB to
LAB color space and combine the
information from the L* and a* channels.
2. Use the k-means algorithm (Luo et al., 2003;
Tariq & Burney, 2014) to obtain clusters,
segment the image, and identify the cervical
ROI.
3. Implement morphological filters (Burger &
Burge, 2016) to eliminate holes and connect
similar regions.
4. Automatically crop the segmented and
filtered image to maximize the ROI.
5. Identify AW lesions and calculate their area
in proportion to the cervix.
2.5 Annotation Tool
Through the pilot study, we seek to assess the
Cervitude Imaging System’s functionality and ease of
use at each step of colposcopy exam compared to a
standard colposcope while observing the patient’s
comfort level throughout the evaluation. The quality
of screening information and accuracy of detection
will also be evaluated.
For this purpose, we have developed our own
image annotation tool. The participating physicians
have agreed to help with annotating the images that
we collect. They can select between segmenting the
cervical ROI, lesions on the cervical epithelium, or
specular reflections. Their input is extremely valuable
to our ongoing research, as we will pursue
quantitative, rather than qualitative, assessments
regarding the performance of our segmentation and
lesion detection algorithms. Their input also helps us
as we increase the size of our dataset through further
clinical studies. A large data set is necessary to
increase the accuracy of automated detection through
machine learning techniques.
Figure shows an example of the information that
can be collected from cervigrams. The segmented
regions are saved as binary masks and masked images
to facilitate further analysis by the research team.
3 RESULTS AND DISCUSSION
3.1 Removal of Specular Reflections
We implemented the algorithm described in section
2.3 on the Atlas of Colposcopy dataset. A sample of
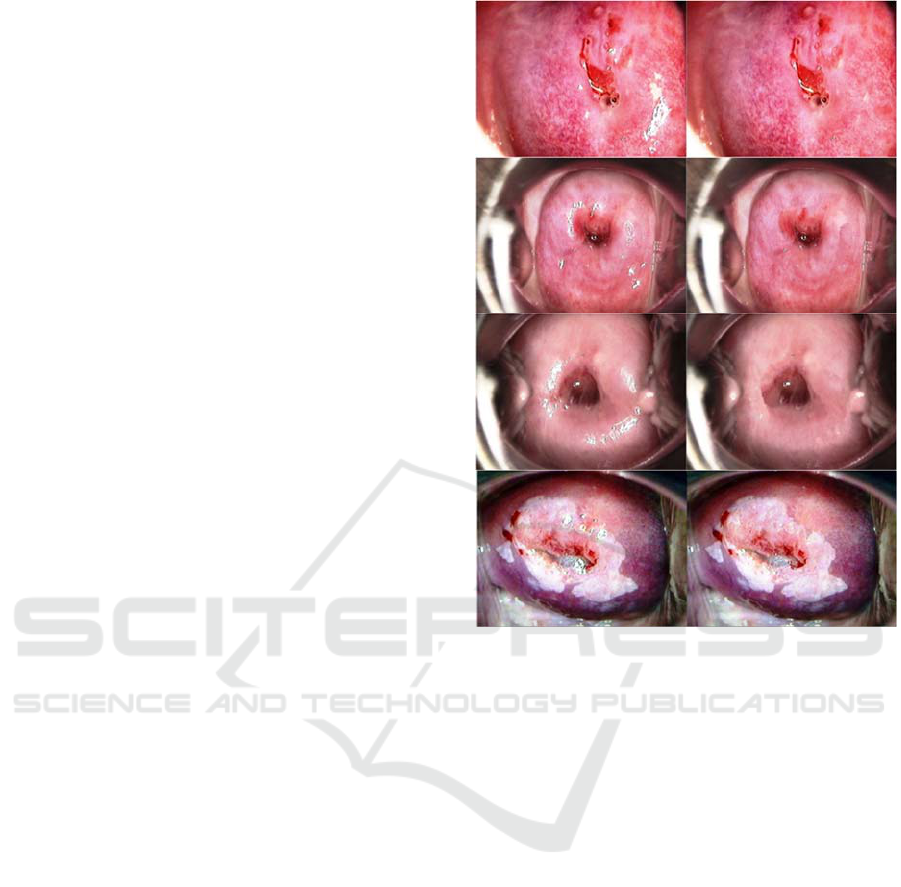
results is presented in Figure 4.
Figure 4: Left: Original image. Right: Results of SR
removal.
In the first three rows of the above figure, we
show images that do not present signs of
precancerous lesions. Without the removal of SR,
these images would likely be classified as containing
abnormalities, prompting the medical provider
conducting the examination to perform a biopsy in the
“white” areas for further analysis. The post-processed
images present a more accurate state of the epithelial
tissue, which reduces the possibility of a misdiagnosis
of misclassification by an automated system.
The algorithm also works when SR is present on
top of a lesion, as seen on the bottom row of Figure 4.
The reflection of light is no longer obstructing the
details on the lesion, while the texture and structure
matches the surrounding area. This enables the
medical provider to more easily understand where the
acetowhitening is occurring and decide what the best
treatment option is.
A limitation to this algorithm is that it will not
work properly if the target regions are relatively large.
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
76
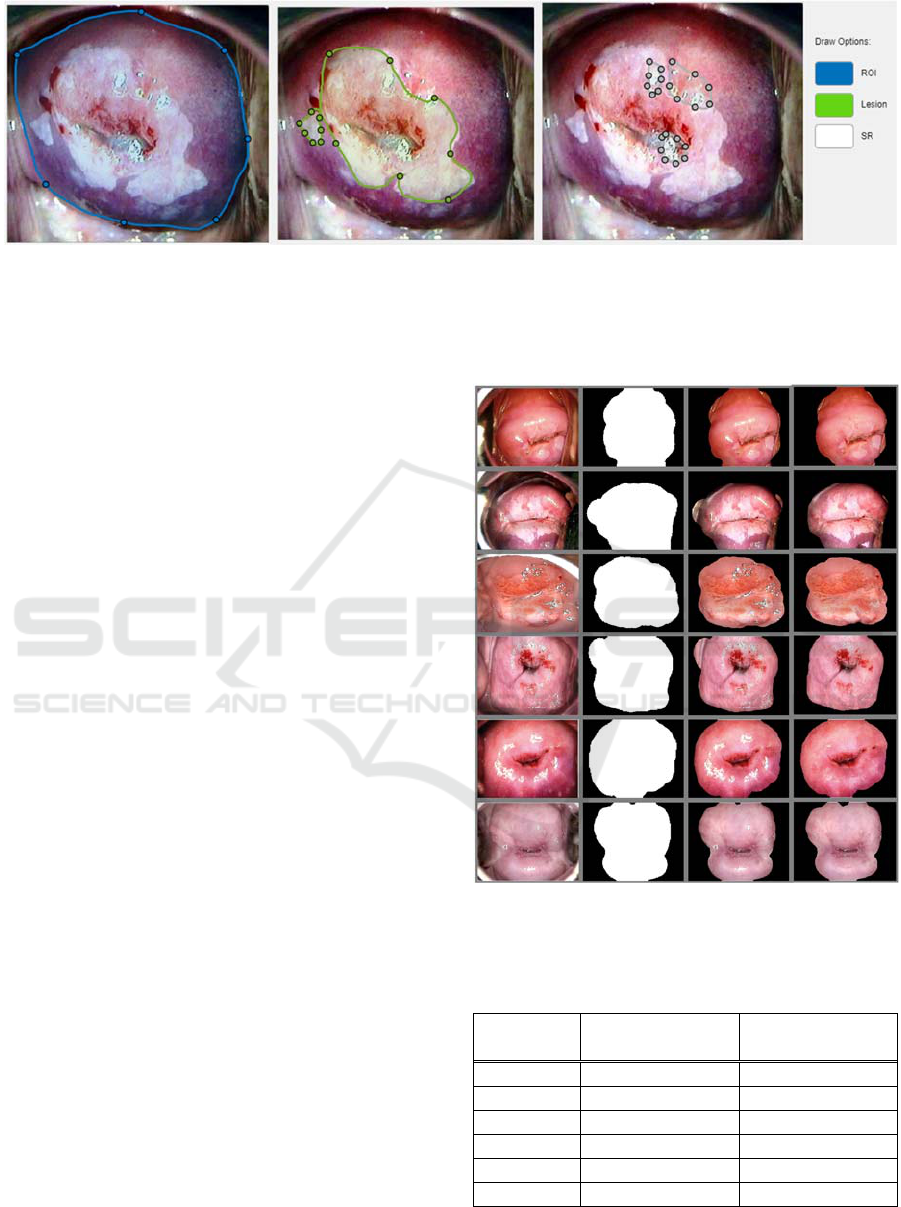
Figure 5: Segmentation options through our annotation tool. From left to right: cervical ROI, AW lesions, and SR.
As seen on the third row of Figure 5, the texture
remaining after removing the SR does not blend very
smoothly with the surrounding tissue. This may affect
analysis in situations where it is crucial to examine
morphological characteristics such as tissue shape,
mosaics, and punctuation vessels, which are also used
for determining a diagnosis. Further work is being
conducted to improve the observed results.
3.2 Segmentation of the Cervical
Region
Figure 6 shows the region of interest (ROI)
segmentation results on the Atlas of Colposcopy
dataset. The first column shows the original RGB
colposcopy image. The second column displays the
resulting binary mask outline after our segmentation
algorithm. The third column represents the segmented
colposcopic image without implementing the SR
removal algorithm. We add a fourth column
displaying segmentation results after removing SR.
To evaluate the performance of the SR removal
algorithm when automatically segmenting the
cervical region, we compare the area of the ROI when
removing SR and when SR are not removed. Table 1
shows the area of the cervical ROI of the images
presented in Figure 6 when performing our
segmentation algorithm for each of these cases.
By removing areas with specular reflections, the
area of the ROI becomes smaller. A visual assessment
also indicates that in most cases, the accuracy of ROI
segmentation increased after removing SR. Two clear
examples of this are in rows 2 and 4 of Figure. When
minimizing sources of error, such as reflection of
light on the speculum and cervix, as well as
surrounding tissue, it is possible to increase the
accuracy of detection of abnormalities that may lead
to cervical cancer.
This is a promising step towards automation of
AW segmentation and classification of precancerous
lesions.
Figure 6: ROI Segmentation Results. Left to right: Original
image; binary mask; segmented image; segmented image
after SR removal.
Table 1: ROI Segmentation Results.
Row
(Fi
g
ure)
ROI area with
SR (%)
ROI area
without SR (%)
1 51.89 50.38
2 58.82 49.88
3 64.02 57.37
4 53.32 42.28
5 71.17 64.42
6 50.60 49.28
A Portable System for Screening of Cervical Cancer
77

4 CONCLUSIONS
In this paper, we propose a portable, low-cost, reliable
system for automatic detection of precancerous
lesions on the cervix. We described our ongoing pilot
study, where we seek to assess the functionality and
reliability of the Cervitude Imaging System (CIS) and
validate our image analysis algorithms. By
developing an application where physicians can
collect relevant information about their patients, as
well as storing images from each visit within the same
location, we facilitate the process around screening
for cervical cancer.
We used a technique that helps remove specular
reflections as the first step in our image pre-
processing procedure. Through this algorithm, we can
remove specular reflections around and within areas
in the cervix that show precancerous lesions. It is an
important step, given that it is important to not only
detect signs of abnormal cells in the cervix, but also
to reduce misdiagnosis and unnecessary biopsies.
Removing specular reflections also improves the
results of segmentation of the cervical region of
interest. Therefore, our image pre-processing method
further decreases the chances of incorrect diagnosis
and treatment.
Future work includes implementing our methods
to images that we collect through our pilot study.
Extensive analysis to increase the accuracy of CIS
will be performed to our images as we increase the
size of our dataset. We believe that our low-cost
bioinformatics-based tool addresses the challenges to
cervical cancer screening in areas where there is
limited access to technology and trained specialists.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Charles Johnson,
MD, and Dr. Alison Westfall, MD, for their
participation and support in this pilot study.
REFERENCES
(WHO), W. H. O. (2022). Cervical Cancer. Retrieved May
12, 2022 from https://www.who.int/news-room/fact-
sheets/detail/cervical-cancer
Asiedu, M. N., Simhal, A., Chaudhary, U., Mueller, J. L.,
Lam, C. T., Schmitt, J. W., . . . Ramanujam, N. (2019).
Development of Algorithms for Automated Detection
of Cervical Pre-Cancers With a Low-Cost, Point-of-
Care, Pocket Colposcope. IEEE Trans Biomed Eng,
66(8), 2306-2318. https://doi.org/10.1109/TBME.2018
.2887208
Bai, B., Du, Y., Li, P., & Yuchun, L. (2019). Cervical
Lesion Detection Net. 2019 IEEE 13th International
Conference on Anti-counterfeiting, Security, and
Identification (ASID),
Basu, P., & Sankaranarayanan, R. (2017). Atlas of
Colposcopy – Principles and Practice. IARC
CancerBase. https://screening.iarc.fr/atlascolpo.php
Bratti, M. C., Rodríguez, A. C., Schiffman, M., Hildesheim,
A., Morales, J., Alfaro, M., . . . Herrero, R. (2004).
Description of a seven-year prospective study of human
papillomavirus infection and cervical neoplasia among
10000 women in Guanacaste, Costa Rica, . Rev Panam
Salud Publica, 15(2), 75-89. https://doi.org/10.1590/
s1020-49892004000200002
Burger, W., & Burge, M. J. (2016). Digital Image
Processing (2 ed.). Springer-Verlag London.
https://doi.org/10.1007/978-1-4471-6684-9
Castle, P. E., Stoler, M. H., Solomon, D., & Schiffman, M.
(2007). The relationship of community biopsy-
diagnosed cervical intraepithelial neoplasia grade 2 to
the quality control pathology-reviewed diagnoses: an
ALTS report. Am J Clin Pathol, 127(5), 805-815.
https://doi.org/10.1309/PT3PNC1QL2F4D2VL
Cepeda-Andrade, P., & Commuri, S. (2022). Automatic
Segmentation of the Cervical Region in Colposcopic
Images. 15th International Joint Conference on
Biomedical Engineering Systems and Technologies -
BIODEVICES,
Criminisi, A., Pérez, P., & Toyama, K. (2004). Region
filling and object removal by exemplar-based image
inpainting. IEEE Trans Image Process, 13(9), 1200-
1212. https://doi.org/10.1109/tip.2004.833105
Das, A., & Choudhury, A. (2017). A novel humanitarian
technology for early detection of cervical neoplasia:
ROI extraction and SR detection. 2017 IEEE Region 10
Humanitarian Technology Conference (R10-HTC),
Dhaka.
Fernandes, K., Cardoso, J. S., & Fernandes, J. (2018).
Automated Methods for the Decision Support of
Cervical Cancer Screening Using Digital Colposcopies.
IEEE Access, 6, 33910-33927. https://doi.org/
10.1109/ACCESS.2018.2839338
Franco, E. L., Duarte-Franco, E., & Ferenczy, A. (2001).
Cervical cancer: epidemiology, prevention and the role
of human papillomavirus infection. CMAJ, 164(7),
1017-1025.
Hu, L., Bell, D., Antani, S., Xue, Z., Yu, K., Horning, M.
P., . . . Schiffman, M. (2019). An Observational Study
of Deep Learning and Automated Evaluation of
Cervical Images for Cancer Screening. J Natl Cancer
Inst, 111(9), 923-932. https://doi.org/10.1093/jnci/
djy225
Kudva, V., & Prasad, K. (2018). Pattern Classification of
Images from Acetic Acid-Based Cervical Cancer
Screening: A Review. Crit Rev Biomed Eng, 46(2),
117-133. https://doi.org/10.1615/CritRevBiomedEng.
2018026017
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
78

Lange, H. (2005). Automatic glare removal in reflectance
imagery of the uterine cervix. Proceedings of SPIE -
The International Society for Optical Engineering,
Le Meur, O., Ebdelli, M., & Guillemot, C. (2013).
Hierarchical super-resolution-based inpainting. IEEE
Trans Image Process, 22(10), 3779-3790.
https://doi.org/10.1109/TIP.2013.2261308
Li, W., & Poirson, A. (2006). Detection and
Characterization of Abnormal Vascular Patterns in
Automated Cervical Image Analysis. In G. Bebis, R.
Boyle, B. Parvin, D. Koracin, P. Remagnino, A. Nefian,
G. Meenakshisundaram, V. Pascucci, J. Zara, J.
Molineros, H. Theisel, & T. Malzbender, Advances in
Visual Computing Berlin, Heidelberg.
Li, Y., Liu, Z. H., Xue, P., Chen, J., Ma, K., Qian, T., . . .
Qiao, Y. L. (2021). GRAND: A large-scale dataset and
benchmark for cervical intraepithelial Neoplasia
grading with fine-grained lesion description. Med
Image Anal, 70, 102006. https://doi.org/10.1016/
j.media.2021.102006
Luo, M., Ma, Y.-F., & Zhang, H.-J. (2003). A Spatial
Constrained K-means A pproach to Image
Segmentation Joint Conference of the
Fourth International Conference on Information,
Communications and Signal Processing, 2003
and Fourth Pacific Rim Conference
on Multimedia Singapore.
Meslouhi, O., Kardouchi, M., Allali, H., Gadi, T., &
Benkaddour, Y. (2011). Automatic detection and
inpainting of specular reflections for colposcopic
images. Open Computer Science, 1(3).
Sankaranarayanan, R., Wesley, R., Thara, S., Dhakad, N.,
Chandralekha, B., Sebastian, P., . . . Nair, M. K. (2003).
Test characteristics of visual inspection with 4% acetic
acid (VIA) and Lugol's iodine (VILI) in cervical cancer
screening in Kerala, India. Int J Cancer, 106(3), 404-
408. https://doi.org/10.1002/ijc.11245
Shroff, M., & Bombaywala, M. (2019). A qualitative study
of Exemplar based Image Inpainting. SN Applied
Sciences, 1(12). https://doi.org/https://doi.org/
10.1007/s42452-019-1775-7
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M.,
Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global
Cancer Statistics 2020: GLOBOCAN Estimates of
Incidence and Mortality Worldwide for 36 Cancers in
185 Countries. CA Cancer J Clin, 71(3), 209-249.
https://doi.org/10.3322/caac.21660
Tariq, H., & Burney, S. M. A. (2014). K-Means
Cluster Analysis for Image Segmentation.
International Journal of Computer Applications, 96.
A Portable System for Screening of Cervical Cancer
79
