
Evaluation of Factors-of-Interest in Bone Mimicking Models Based
on DFT Analysis of Ultrasonic Signals
Aleksandrs Sisojevs
a
, Alexey Tatarinov
b
and Anastasija Chaplinska
Institute of Electronics and Computer Science, 14 Dzerbenes Str., Riga, Latvia
Keywords: Pattern Recognition, DFT, Bone Models, Axial Quantitative Ultrasound.
Abstract: Bone fragility in osteoporosis is associated with a decrease in the thickness of the cortical layer CTh in long
bones and the development of internal porosity P in it. In the present work, an attempt was made to predict
the factors-of-interest CTh and P based on the pattern recognition approach, where DFT analysis was applied
to ultrasonic signals in surface transmission through a soft tissue layer. Compact bone was modeled with
PMMA plates with gradual changes in CTh from 2 to 6 mm, and internal porosity P was created by drilling
where the thickness of the porous layer P varied from 0 to 100% of CTh. The estimation method was based
on a statistical analysis of the magnitude of the DFT spectrum of the ultrasonic signals. Decision rules were
mathematical criteria calculated as ratios between the envelope functions of the magnitudes. Each of the
objects was chosen in turn as a test object, while other specimens composed the training set. The results of
the experiments showed the potential effectiveness of the CTh and P prediction, while additional physical
parameters may be used as decision rules to improve the reliability of the diagnosis.
1 INTRODUCTION
Osteoporosis is a systemic skeletal disease
characterized by low bone density and
microarchitectural deterioration of bone tissue with a
consequent increase in bone fragility (WHO, 2003).
It is a severe symptom of aging and a complication in
many metabolic diseases. Cortical bone or compact
bone tissue, the main load-carrying component of the
skeleton, suffers from osteoporosis by reducing the
thickness of the compact layer and increasing the
internal porosity in it, progressing from the side of the
channel (Osterhoff et al., 2016). An adequate
assessment of these manifestations of osteoporosis
can help in timely prevention and treatment.
Conventionally, the diagnosis of osteoporosis is made
using dual x-ray absorption techniques by measuring
the bone mineral density (Guglielmi, 2010).
However, planar radiography is not able to
distinguish reliably between changes associated with
bone thinning and porosity and thus distinguish
between thin normality and osteoporosis.
Ultrasonic techniques based on measuring the
parameters of elastic waves are a perspective
a
https://orcid.org//0000-0002-2267-4220
b
https://orcid.org//0000-0002-5787-2040
modality to assess bone conditions in respect of
osteoporosis (Laugier, 2008). Axial bone
ultrasonometers use to measure ultrasound velocity in
the compact bone of long bones, such as the tibia and
forearm bones. Although it demonstrated sensitivity
to osteoporosis and mineralization disorders, its
clinical use is compromised by the inability to discern
multiple factors influencing the bone condition by
this single input. New approaches are focused on
analysing guided wave propagation at several
frequencies that provide extensive information about
bone structure and properties (Tatarinov et al., 2014).
However, discrimination of the factors of interest
such as cortical porosity and thickness of the cortical
layer against the background of the influence of the
surrounding soft tissues requires advanced data
processing. Traditional approaches based on the
measurement of single parameters such as ultrasound
velocity do not allow separating the complex
influences of these acting factors. Artificial
intelligence methods, particularly, pattern recognition
applied to a complex of propagated ultrasonic signals
at different frequencies are expected to help solve the
problem.
914
Sisojevs, A., Tatarinov, A. and Chaplinska, A.
Evaluation of Factors-of-Interest in Bone Mimicking Models Based on DFT Analysis of Ultrasonic Signals.
DOI: 10.5220/0011742800003411
In Proceedings of the 12th International Conference on Pattern Recognition Applications and Methods (ICPRAM 2023), pages 914-919
ISBN: 978-989-758-626-2; ISSN: 2184-4313
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
The purpose of this study was to investigate the
possibility to detect differentially two independent
factors of interest, cortical thickness and intracortical
porosity as diagnostically valuable determinants of
bone fragility in osteoporosis. Synthetic solid
phantoms modeling the cortical layer with the gradual
variation of both factors were used. The very
formulation of the problem suggested the need to
apply pattern recognition methods, but unlike the
classical classification problem, in this case, there
was no need to determine the belonging of the object
under study to any known class, but just to find the
values of the factors, which were a-priori unknown.
Soft tissues covering bones were considered as an
aside factor, so the datasets were obtained for three
thicknesses of the soft layer to assure the feasibility
of the method for persons of different constitutions.
The raw data were presented by sets of ultrasonic
signals acquired stepwise by surface profiling of the
object in the pitch-catch mode. The discrete Fourier
transform (DFT), one of the recognized methods of
signal analysis, transforming the signals from time to
frequency domains was used (Irrigaray et al., 2016).
A set of statistical parameters was extracted from the
set of magnitude signals, thus forming a set of
features describing the object. Extracting statistical
parameters from each object in the set, decision rules
are created to be the instrument for the evaluation of
parameters of interest in the examined objects.
2 PROPOSED APPROACH
The proposed approach for evaluating two factors-of-
interest using ultrasonic signals datasets was based on
pattern recognition principles. The evaluation method
consisted of two parts: creating a set of decision rules
from the data for a training set of phantoms and
validating the set of decision rules by substitution the
data for an examination specimen to make verify the
correctness of the approach.
2.1 Bone Phantoms and Ultrasonic
Data Acquisition
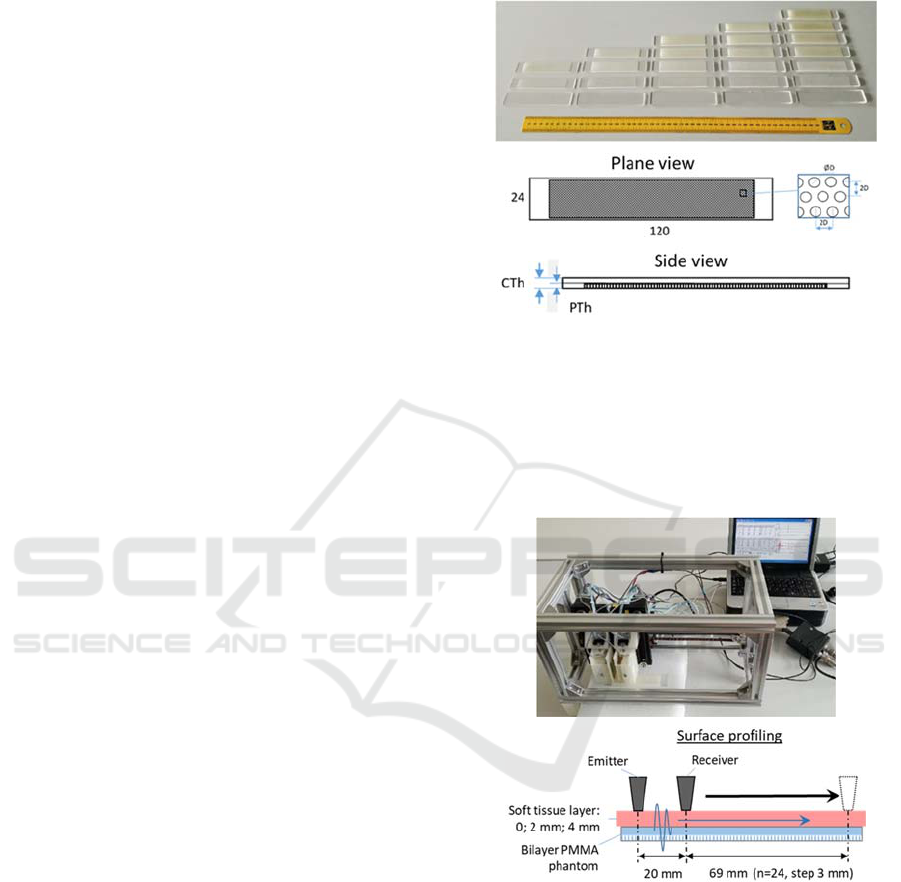
Bone models (phantoms) presented a set of bi-layer
PMMA (polymethyl methacrylate) plates (Figure1)
with gradually varied overall thicknesses 2, 3, 4, 5
and 6 mm that corresponded to the bone cortical
thickness CTh. The effect of intracortical porosity
progressing from the in-bone channel was imitated by
the regularly bottom-drilled holes. The volumetric
porosity of the porous layer was constant at the level
of 20%, but the gradual progress of porosity P from
zero to 100% of CTh was set by increasing the
thickness of the porous layer PTh with a step of 1 mm.
Figure 1: PMMA phantoms modeling the progression of
osteoporosis in compact bone tissue: CTh – cortical
thickness; PTh – thickness of the porous layer.
Ultrasonic signals were acquired by means of a
custom-made scanning setup by stepwise profiling
the upper surface of the phantoms covered by soft
tissues with a profiling step of 3 mm (Figure 2).
Figure 2: Acquisition of ultrasonic signals in phantoms: A
– general view of ultrasonic setup, B – layout of
experiment.
Totally acquired 24 signals formed so called
ultrasonic spatiotemporal waveform profiles that
served as the source material for pattern recognition
(Figure 3). The profiles contained complex
information on temporal (velocities) and energetic
(attenuation) characteristics of different modes of
ultrasound propagation in the objects. Commonly, it
presents difficult to analyze those analytically using
signals decomposition (Bochud et al., 2017), but the
Evaluation of Factors-of-Interest in Bone Mimicking Models Based on DFT Analysis of Ultrasonic Signals
915

pattern recognition approach provided an integral
solution.
Figure 3: Examples of ultrasonic spatiotemporal waveform
profiles in bone phantoms: A – normalized within each
signal line; B – normalized upon the entire profile. The
abscissa is ultrasonic time; the ordinate is profiling
distance.
In one signal frame of a 1 ms duration, three
frequency excitation responses were collected: HF at
500 kHz, LF at 100 kHz, and a chirp signal with
frequency sweeping from 500 to 50 kHz. In this
frequency range, different modes of ultrasonic guided
waves manifested, including S0 and A0 Lamb waves.
2.2 Used Mathematical Method
The initial data set comprised raw ultrasonic signals
obtained at three different frequency regimes of
excitation (HF, LF, chirp) and forming the
spatiotemporal waveform profiles that contained 24
stepwise acquired signals. The general structure of
the initial data on objects (phantoms) is shown in
Figure 4.
Figure 4: Data structure of source objects in the space of
factors-of-interest CTh and P.
As the set of bi-layer phantoms consisted of
gradually varied solid and porous layers with an
increment of 1 mm, the objects’ grid in the space of
the factors-of-interest CTh and P was a non-
orthogonal one that made an additional challenge for
interpolation.
In this study, the method for evaluating of factors
of interest used a pattern recognition approach
applied to ultrasonic signals after DFT processing
(Sisojevs et al., 2022). The signal frame consisting of
three frequency regimes was divided into three-time
sub-frames. In each sub-frame, mathematical criteria
were calculated. For this, the magnitude functions of
the DFT signals received from the corresponding sub-
frames were used. Then, for each object, mathematical
criteria were calculated that presented various ratios
between the envelope functions of signal magnitudes
(Sisojevs et al., 2022). The total number of
mathematical criteria for one sub-frame was 13, and
considering three sub-frames in the time domain, the
number of mathematical criteria for one object was 39.
After calculating the mathematical criteria for all
objects in the training set, decision rules were built.
In this case, mathematical criteria were used as
attributes for pattern recognition rules. For decision
rule creation, the bilinear interpolation of a patch of
the surface was used (Sisojevs et al., 2022).
3 EXPERIMENTS
As part of the validation of the proposed approach,
experiments were carried out to assess the total
thickness of the bone phantom CTh and the thickness
of the porous part P with a-priori known values of the
soft tissue thickness. In the experiment, the soft tissue
thicknesses were 0, 2 and 4 mm.
A separate experiment was carried out for each of
the soft tissue thickness values. The experiment
looked like this. Of all the scanned objects, one was
selected for the test sample. The rest made up the
training set. In this work, the method (Sisojevs et al.,
2022) was used. For each object, the magnitudes of
DFT signals were calculated for sub-frames in the
signal time domain.
𝑀
𝜔
𝑅𝑒
𝜔
𝐼𝑚
𝜔
where:
𝑅𝑒
𝜔
𝑠
𝑡
∙𝑐𝑜𝑠
2𝜋∙𝑡∙𝜔
𝑡
𝑡_𝑚𝑖𝑛
_
_
and
𝐼𝑚
𝜔
𝑠
𝑡
∙𝑠𝑖𝑛
2𝜋∙𝑡∙𝜔
𝑡
𝑡_𝑚𝑖𝑛
_
_
In the selected interval ω, the values of three functions
were calculated:
𝐹_𝑚𝑎𝑥
𝜔
𝑚𝑎𝑥
𝑀
𝜔
;
𝐹
𝑎𝑣𝑒𝑟𝑎𝑔𝑒
𝑀
𝜔
and
𝐹_𝑚𝑖𝑛
𝜔
𝑚𝑖𝑛
𝑀
𝜔
ICPRAM 2023 - 12th International Conference on Pattern Recognition Applications and Methods
916

Then, according to the values of these functions,
mathematical criteria were calculated:
First criteria cr#1 is the number of 𝜔 values that
fulfill the condition
𝐹
𝑎𝑣𝑒𝑟𝑎𝑔𝑒𝐹_𝑚𝑎𝑥
𝜔
,𝑐𝑟#1;
𝑐𝑟#2
_
_
; 𝑐𝑟#3
|
_
|
_
;
𝑐𝑟#4
|
_
|
_
; 𝑐𝑟#5
|
_
|
_
;
where:
𝑑𝐹_𝑚𝑎𝑥
𝜔
𝐹_𝑚𝑎𝑥
𝜔
𝐹_𝑚𝑎𝑥
𝜔1
.
𝑑𝐹_𝑎𝑣𝑟
𝜔
𝐹_𝑎𝑣𝑟
𝜔
𝐹_𝑎𝑣𝑟
𝜔1
.
𝑑𝐹_𝑚𝑖𝑛
𝜔
𝐹_𝑚𝑖𝑛
𝜔
𝐹_𝑚𝑖𝑛
𝜔1
.
𝑐𝑟#6
𝑐𝑟#7
𝑐𝑟#8
𝑊
∙
⎝
⎜
⎜
⎜
⎛
𝜔
∙𝐹_𝑚𝑎𝑥
𝜔
𝜔
∙𝐹_𝑚𝑎𝑥
𝜔
𝐹_𝑚𝑎𝑥
𝜔
⎠
⎟
⎟
⎟
⎞
where:
𝑊
⎝
⎜
⎜
⎜
⎛
𝜔
𝜔
𝜔
𝜔
𝜔
𝜔
𝜔
𝜔
𝜔
𝜔
⎠
⎟
⎟
⎟
⎞
𝑐𝑟#9
𝑚𝑎𝑥𝐹_𝑎𝑣𝑟
𝜔
𝑚𝑎𝑥𝐹_𝑚𝑎𝑥
𝜔
𝑐𝑟#10
; 𝑐𝑟#11
;
𝑐𝑟#12
; 𝑐𝑟#13
where:
𝑆
1
𝑚𝑎𝑥
𝐹_𝑚𝑎𝑥
𝜔
∙𝐹_𝑚𝑖𝑛
𝜔
_
_
𝑆
1
𝑚𝑎𝑥
𝐹_𝑚𝑎𝑥
𝜔
∙ 𝐹_𝑎𝑣𝑟
𝜔
_
_
𝑆
1
𝑚𝑎𝑥
𝐹_𝑚𝑎𝑥
𝜔
∙𝐹_𝑚𝑎𝑥
𝜔
_
_
The interest parameter estimation method was
based on the creation and use of decision rules. To
create decision rules, piecewise linear interpolation
was used for each of the parametric directions. As a
result, the decision rule was represented as a
piecewise bilinear function of two variables.
The use of a decision rule to evaluate an unknown
object was reduced to the calculation of mathematical
criteria and their comparison with the corresponding
decision rules. In this case, the value of the criterion
expanded to the interval ±ε (eps). The result of
evaluation according to one rule is a set (segment) of
possible correct answers. Figure 5 illustrates such a
case.
Figure 5: An example of using a single decision rule to
evaluate an unknown object.
When using several decision rules, a sub-segment
of possible answers is found that is included in the
maximum number of initially found segments. It is
this sub-segment that is taken as the final evaluation
of the method used.
Figure 6: Result of evaluation of object with CTh = 3 mm
and P = 33.3% (PTh = 1 mm), soft tissue thickness = 0 mm
and eps = 25%.
After this estimate was obtained, this result was
compared with the a-priori known values of CTh and
P of this object. Within the framework of one
experiment, each of the objects was chosen in turn as
a test object.
Examples of how the method of the estimation
factors-of-interest works are shown in Figures 6-8. In
the figures, the red segment is the obtained estimate
Evaluation of Factors-of-Interest in Bone Mimicking Models Based on DFT Analysis of Ultrasonic Signals
917
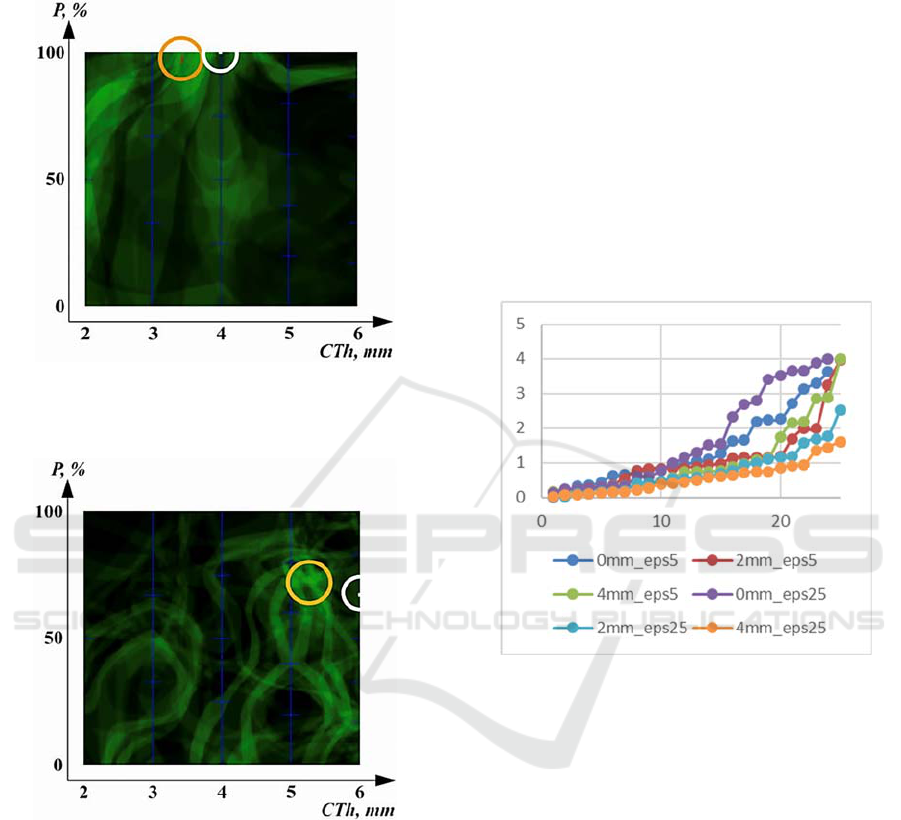
of the non-test object by the method (Sisojevs et al.,
2022), the white dot denotes the position of the test
object.
Figure 7: Result of evaluation of object with CTh = 4 mm,
P = 100% (PTh = 4 mm), soft tissue thickness = 2 mm and
eps = 25%.
Figure 8: Result of evaluation of object with CTh = 6 mm,
P = 66.6% (PTh = 4 mm), soft tissue thickness = 4 mm and
eps = 5%.
3.1 Estimation Accuracy
The estimation accuracy in the experiment was
determined for each of the factors-of-interest (CTh
and P). The error was calculated as the modulus of the
difference between the computed estimate of the
factor’s value and the a-priori known value of this
factor in the object
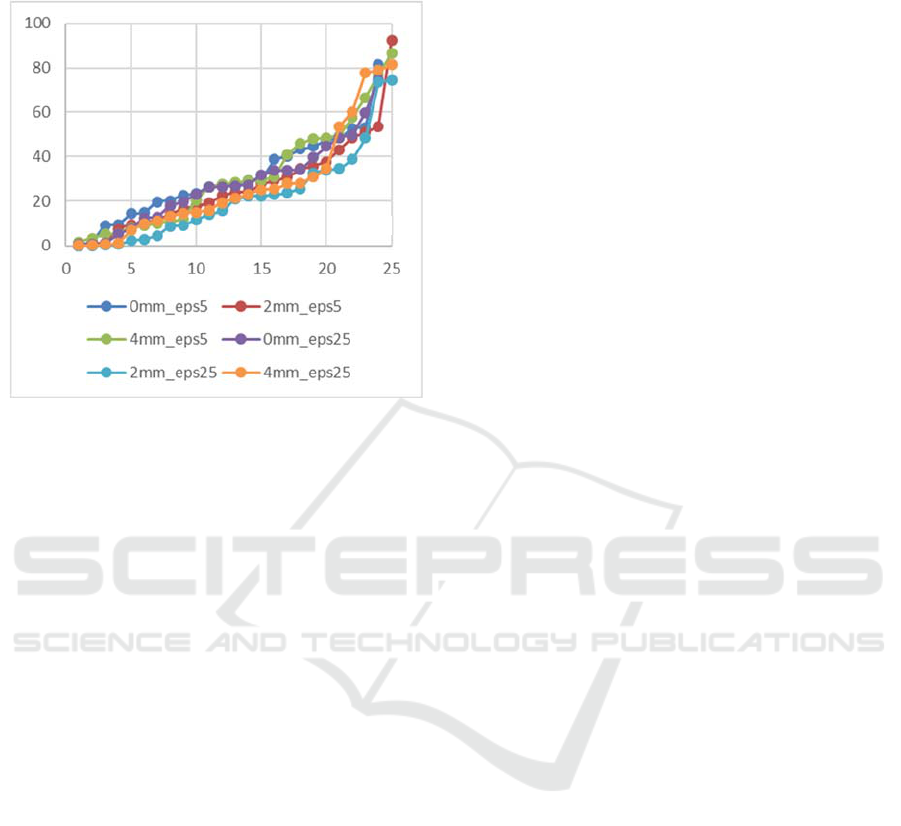
The estimate of the error in determining CTh
ranged from 0.0021 to 3.992 mm (i.e. between very
precise and completely incorrect). The average
estimation error was in the range of 0.561 - 1.675 mm
(depending on soft tissue thickness and eps value).
After sorting the errors in ascending order, the
distribution of errors is shown in Figure 9. The best
results or the results showing the smallest deviation
for CTh estimation are obtained with a value of the
soft tissue thickness layer of 4 mm and 2 mm at
eps=25% (average error 0.561 - 0.749 mm, maximum
1.606 - 2.532mm). Contrary, the worst results (the
highest deviation) are obtained for the value of the
soft tissue thickness layer 0 mm, regardless of the eps
value (average error 1.372 - 1.675 mm, maximum
3.623 - 3.983 mm). If the acceptable diagnostic
deviation threshold for CTh is 1.2 mm with a general
range of its changes from 2 to 6 mm, then the number
of correct estimations is 20 out of 25 or 80%.
Figure 9: Distribution of errors for CTh estimations
depending on the thickness of the soft tissue layer and eps.
The estimate of the error in determining the porosity
ranged from 0 to 92.478%, i.e. from the lowest to the
almost highest possible). The average estimation
error was in the range of 21.736 - 31.162%.
Depending on soft tissue thickness and eps value.
After sorting the errors in ascending order, the
distribution of errors for P is shown in Figure 10. The
best results from the point of least deviation for
porosity P were obtained with a soft tissue thickness
layer 2 mm at eps=25% (average error 21.736%,
maximum error 74.558%). At the same time, the
parameters (thickness of soft tissue and eps) giving
the worst performance in terms of accuracy are not
indicated. If the acceptable diagnostic deviation
threshold for P is 30% with a general range of its
changes from 0 to 100% mm, then the number of
correct estimations is 15 out of 25 or 60%. This shows
that the accuracy of determining bone thickness CTh
is somewhat better than that of its porosity P, using
CTh
,
mm
n
ICPRAM 2023 - 12th International Conference on Pattern Recognition Applications and Methods
918
DFT-based criteria applied to the ultrasonic signals
related to the guided waves propagation.
.
Figure 10: Distribution of porosity P estimation errors
depending on the thickness of the soft tissue layer and eps.
4 CONCLUSIONS
The results of the experiments showed the potential
effectiveness of the earlier proposed pattern
recognition method (Sisojevs et al., 2022) in the tasks
of determining the factors of interest in osteoporosis
diagnostics (total thickness of the cortical bone and
the degree of inner porosity), using ultrasonic surface
profiling.
The use of only the DFT analysis does not give
full agreement between the obtained estimates and the
a priori predicted ones. The application of additional
evaluation criteria based on the physical parameters
of guided wave propagation may improve the
reliability of the diagnosis.
The small number of available objects (bone
phantoms) and the approximate nature of the
mathematical criteria did not allow us to estimate the
factors of interest with high accuracy. However, the
results obtained demonstrated the prospects for using
this method and increasing its accuracy with an
increase in the number of objects with a priori known
values of the factors.
ACKNOWLEDGEMENTS
The study was supported by the research project of
the Latvian Council of Science lzp-2021/1-0290
“Comprehensive assessment of the condition of bone
and muscle tissues using quantitative ultrasound”
(BoMUS).
REFERENCES
WHO Scientific Group on the Prevention and Management
of Osteoporosis (2000: Geneva, Switzerland). (2003)
Prevention and management of osteoporosis: report
of a WHO scientific group. WHO Technical Report
Series 921.
Osterhoff G, Morgan EF, Shefelbine SJ, Karim L,
McNamara LM, Augat P. (2016) Bone mechanical
properties and changes with osteoporosis. Injury.; 47,
Suppl 2: 11-20.
Guglielmi G, Scalzo G. (2010) Imaging tools transform
diagnosis of osteoporosis. Diagnostic Imaging Europe.
26: 7–11.
Laugier P. (2008) Instrumentation for in vivo ultrasonic
characterization of bone strength. IEEE Trans Ultrason
Ferroelectr Freq Control; 55(6):1179-96.
Tatarinov A, Egorov V, Sarvazyan N, Sarvazyan A. (2014)
Multi-frequency axial transmission bone
ultrasonometer. Ultrasonics. 54(5):1162-1169.
Irrigaray, M. A. P., Pinto R.C. and Padaratz I. J (2016) A
new approach to estimate compressive strength of
concrete by the UPV method. Revista IBRACON de
Estruturas e Materiais 9: 395-402.
Bochud N, Vallet Q, Minonzio JG, Laugier P. (2017)
Predicting bone strength with ultrasonic guided waves.
Sci Rep.; 7 (3):43628.
Sisojevs A., Tatarinov A., Kovalovs M., Krutikova O.,
Chaplinska A. (2022) An Approach for Parameters
Evaluation in Layered Structural Materials based on
DFT Analysis of Ultrasonic Signal. Proceedings of the
11th International Conference on Pattern Recognition
Applications and Methods, Volume 1: ICPRAM, 307-
314.
CTh
,
mm
n
Evaluation of Factors-of-Interest in Bone Mimicking Models Based on DFT Analysis of Ultrasonic Signals
919
