
In silico Tissue Engineering and Cancer Treatment Using Cellular
Automata and Hybrid Cellular Automata-Finite Element Models
Andrés Díaz Lantada
*a
, Miguel Urosa Sánchez and David Fernández Fernández
Department of Mechanical Engineering, ETSI Industriales, Universidad Politécnica de Madrid,
c/ José Gutiérrez Abascal 2, 28006 Madrid, Spain
Keywords: Software as Medical Device, in silico Tissue Engineering, Cell Simulation, Cellular Automata, Finite Element
Modelling, Multi-Scale Modelling.
Abstract: An innovative approach for in silico tissue engineering and cancer treatment is presented in this study. It is
based on the employment of cellular automata (CA) and cellular automata hybridised with finite-element
models (FEM) for simulating cells within tissue engineering scaffolds. Thanks to the presented strategy, it
has been possible to model cells colonising scaffolds, the interactions among different populations of cells
and between the cells and the scaffolds as extracellular matrices, and the effects of external stimuli, like
temperature, for treating disease. Among the advances incorporated to conventional models based on cellular
automata it is important to mention: the establishment of a direct connection between CAD models and the
simulation workspace, the incorporation of a wall factor for considering the affinity of cells for the
extracellular matrix, the coupling of FEM simulations to the cellular automata for rendering them more
versatile, and the modelling of interactions among different types of cells. Results, limitations, and potentials
of these simulation approaches are presented and discussed, in connection with current trends in software as
a medical device (SaMD).
1 INTRODUCTION
Software as a medical device (SaMD) is gaining
momentum and transforming healthcare. For decades
active medical devices have been smartly driven by
embedded software, but nowadays medical apps and
different kinds of standalone software are emerging
for a wide set of prevention, diagnosis and monitoring
purposes and must be considered medical devices in
themselves (Ludvigsen, 2022). These SaMDs not
only support medical practice but may render it much
more efficient and sustainable, from the different
economic, environmental, and social perspectives. In
large part they can also contribute to the 3R principles
(Replacement, Reduction, Refinement) for ethical
biomedical research (Aske, 2017), as the use of
simulations can be an excellent alternative to other in
vivo studies with animals or in vitro studies with cells
and tissues, along the development lifecycle of
innovative medical devices and drugs or in parallel to
medical practice.
a
https://orcid.org/0000-0002-0358-9186
*
Contact: andres.diaz@upm.es
To cite some examples, minimising the number of
animals required for validating innovative therapies
or reducing the use of cells and tissues employed for
studying disease, by means of in silico strategies -
based on software and simulations-, can have highly
positive ethical, economical, and procedural impacts
in remarkable fields such as tissue engineering,
biofabrication and cancer therapies. Indeed, in silico
tissue engineering (Geris, 2018, Keshavarzian, 2019)
and in silico cancer research (Edelman, 2010, Jean-
Quartier, 2018) constitute important trends aimed at
speeding up the (R & D & I) Research – Development
– Innovation cycle, while reducing associated costs
and minimising negative social impacts without
compromising safety. Regarding the simulations of
cells, several computational approaches, both
continuum and discrete, enable the modelling of their
collective behaviours, their interactions with
extracellular matrices, the progress of disease and the
eventual success or failure of a healing or
regenerative strategy (Spencer, 2013, Geris, 2013,
2016).
56
Díaz Lantada, A., Urosa Sánchez, M. and Fernández Fernández, D.
In silico Tissue Engineering and Cancer Treatment Using Cellular Automata and Hybrid Cellular Automata-Finite Element Models.
DOI: 10.5220/0011742300003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 1: BIODEVICES, pages 56-63
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

In vitro, the invention of tissue engineering
scaffolds, 3D or 4D porous structures that mimic the
extracellular environment providing cells in culture
with biomimetic cell niches, has been fundamental
for setting the foundations of tissue engineering and
biofabrication and for providing more physiological
environments for studying disease and therapies
(Khademhosseini, 2016).
In many ways, scaffolds can be employed to study
the healing and regeneration of tissue and to test the
development of innovative therapies for cancer, but
the cell sources, growth factors, reagents and cell
culture processes involved are often challenging,
expensive and require highly trained professionals. If
such processes could be simulated, fields like tissue
engineering and cancer research could advance even
more efficiently and sustainably. Therefore, it is
necessary to further progress in the simulation of cells
within scaffolds, in the modelling of cell-cell and cell-
material interactions, and in the coupling of these
simulations with the effects from environmental cues
and stimuli. Linking computer-aided designs of
scaffolds and biomaterials with the workspaces
employed for agent-based models, for considering
both the individual and collective behaviours of cells,
and with the input from FEM simulations, for
considering the effects of different physical/chemical
fields on cells’ behaviour and fate, is hence required.
Some recent inspiring studies can be cited, which
evolve from the foundational works with cellular
automata (CA) (Von Neumann, 1966). In short, CA
were developed as collections of elements or cells
defined upon grids that evolve through time steps or
iterations following certain rules. Along the time
steps, the state (i.e. colour or value, typically “0” or
“1”) of the cells within the grid changes according to
the rules and to the previous states of the neighbour
cells. Since the beginning, these models were
conceived as possible simulators for biological
systems with remarkable examples, such as
Conway’s game of life (Gardner, 1970), in which the
cells upon a 2D grid have two possible states, dead or
alive, and in which cells survive, reproduce, migrate,
or die, depending on the 8 neighbouring cells or the
previous state. Further studies led to verifying that
extremely complex systems could be modelled with
CA (Wolfram, 1984).
In connection with biodevices, these models have
also proven useful for studying the biodegradation of
tissue engineering scaffolds (Erkizia, 2010), for
studying scaffolds’ colonization processes (Garijo,
2012, Vivas, 2015), and, by our team, for simulating
and optimising biomimetic cell culture systems
(Ballesteros Hernando, 2019).
In this study, we intend to advance to the next step
by linking three-dimensional CAD models of tissue
engineering scaffolds with the grids of CA and by
hybridising CA and FEM simulations for obtaining
multi-scale and multi-physical/chemical simulators
with more versatile functionalities, as described in the
following sections. Applications in tissue engineering
and cancer research are foreseen and discussed.
2 MATERIALS AND METHODS
2.1 Software Resources
Siemens NX 12.0 and Autodesk Inventor 2020 are
employed as main CAD software resources. The
FEM capabilities of NX 12.0 are used for the thermal
simulations performed. Regarding programming,
Matlab r2020a is used as main resource for creating
the codes for cellular automata. Ultimaker Cura, a 3D
printing slicer is utilised for slicing the CAD models
and obtaining images used as input for generating the
workspaces for the CA models, as described below.
2.2 Fundamentals of Models Used
2.2.1 From CAD Models to Cellular
Automata
Once the usual lattice-like or porous structures of
tissue engineering scaffolds are designed, it is
possible to generate the workspaces or grids for CA
models by slicing their geometries, performing digital
tomographs, and processing the images obtained. The
process is based on previous studies by our team with
some minor modifications that allow us to work with
voxels, instead of pixels (Ballesteros Hernando,
2019), and is schematically illustrated in figure 1.
Figure 1: From CAD models of tissue engineering scaffolds
to the working grids for cellular automata. Left: CAD
models are sliced to generate grayscale images with
allowed and forbidden regions. Right: model space in
Matlab with allowable (red) and restricted (blue) voxels -or
volumetric pixels-.
In silico Tissue Engineering and Cancer Treatment Using Cellular Automata and Hybrid Cellular Automata-Finite Element Models
57
2.2.2 Modelling the Colonization of Tissue
Engineering Scaffolds
Once the working space is obtained, cell proliferation
can be modelled following different proliferation
rules and illustrated along the temporal iterations by
means of colour changes to the voxels, as shown in
figure 2. Depending on the resolution of the images
obtained through the slicing process and on the
distance between slices, voxel size can be adjusted to
represent single cells (i.e., voxels of c.a. 10 x 10 x 10
μm
3
) or cell populations or clusters. The size of the
scaffold employed as extracellular matrix and its
porosity, which defines the allowed space for cell
proliferation, together with the resolution or number
of voxels employed per volume unit, define the
computational cost of these simulations.
By means of example, figure 2a presents three
iterations of a cell or cell cluster proliferating
following a rule, by which all voxels normally
connected to a voxel filled with a seed cell or cluster
become populated by cells or clusters in the following
step. However, figure 2b presents four iterations of a
cell or cell cluster proliferating following an irregular
pattern, giving options for asymmetric growth
patterns and even for steps without any proliferation,
based on the incorporation of random functions.
Apart from the proliferation, the possibility of cell
death is taken into account by adding a probability of
death in each iteration. This is represented in figure
2c, in which green voxels represent living cells, while
dead cells are represented in red and, in general,
occupy that space until the end of the simulation.
In order to consider the affinity of cells for the
scaffold’s trusses and the effects of adhesion, we have
also decided to study the incorporation of a “wall
factor”, which modifies the proliferation rules or
probabilities, by employing different probability
proliferation values for cells surrounded by cells and
for cells in contact with trusses. To our knowledge,
this is reported for the first time and leads to results
that better mimic what happens with cells cultured
within real scaffolds, as additionally analysed in
section 3.1.
2.2.3 Modelling Interactions Among Cells
Current tissue engineering strategies face the great
challenge of reconstructing large defects involving
different types of cells and tissues. In cancer research,
the progression of tumours affecting the various kinds
of cells and tissues within organs is also pivotal. In
consequence, simulating interactions among different
cell populations is essential.
The presented CA models can be also applied to
simulate the interactions among different types of
cells. From a visual point of view the voxels (e.g.,
green and blue in figure 2d). From a modelling
perspective, different kinds of cells are employed as
proliferation seeds, by initially selecting one or more
voxels in distinct regions of the allowable space of the
grid. The invasiveness of one cell type can be
modelled by establishing a simple colour change rule
whenever one invasive cell reaches the boundaries of
a normal cell, respectively illustrated in blue and
green in figure 2d. In this way, tumoral processes can
be simulated as further discussed in section 3.2.
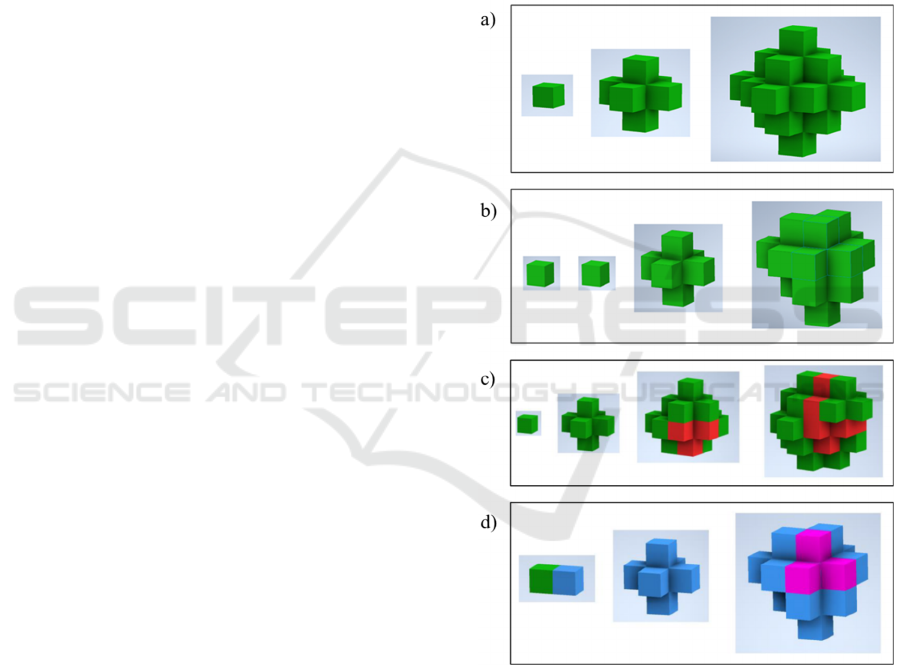
Figure 2: Examples of cell proliferation and cell-cell
interactions along different iterations using CA models. a)
Three iterations of a symmetric growth pattern. b) Four
iterations of an asymmetric growth pattern with a
proliferation probability lower than 1, due to which dead
cells (red) appear after some time steps. c) Four iterations
showing proliferation after including the probability of
death. d) Cell-cell iterations showing an invasive cell (blue)
attacking, invading, or cannibalising (Fais, 2018) a healthy
cell (green), subsequently proliferating, or dying (pink).
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
58
2.2.4 Coupling Finite Element Models and
Cellular Automata
More complex behaviours of cells within scaffolds
should take account of existing physical/chemical
fields, microenvironmental cues and external stimuli
that may affect processes like gene expression, cell
differentiation and final cell fate. FEM prove
excellent for numerically solving partial differential
equations upon complex geometries and domains,
a)
b)
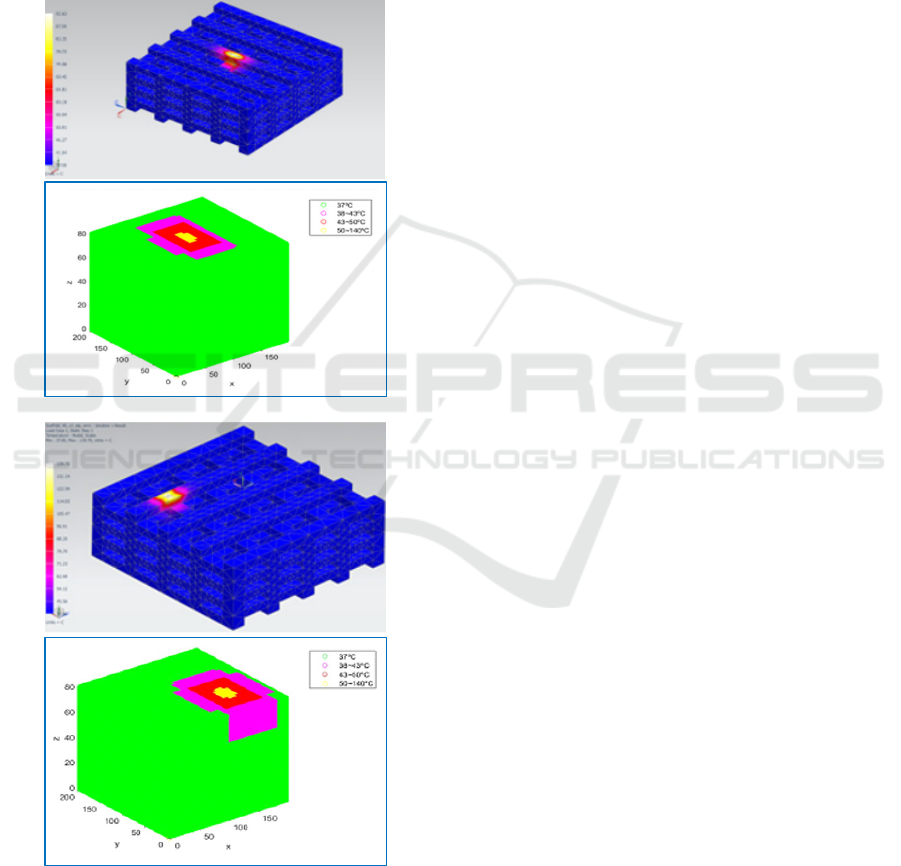
Figure 3: Temperature fields obtained by heating the central
upper (a) and upper corner (b) regions of tissue engineering
scaffolds and their mapping upon CA grids as ranges
associated to death probabilities.
hence being fundamental in modern engineering for
mechanical, electromagnetic, fluidic, and thermal
problems. All these physical domains can be used for
modulating cellular behaviour. Thus, the connection
of FEM simulations to agent-based models can prove
extremely useful, as we aim to demonstrate.
Accordingly, results from FEM simulations
stored in matrices have been mapped upon the three-
dimensional grids of CA models. So as to modulate
cellular responses, the values mapped can be
employed to modify the proliferation and survival or
death probabilities, depending on the actual fields
calculated with FEM simulations. To illustrate this
possibility, thermal simulations have been performed,
in connection with the possible cancer treatment
employing high temperatures (hyperthermia), and the
survival probabilities modified. Figure 3 presents the
temperature fields obtained by heating two tissue
engineering scaffolds and their mapping upon CA
grids, as ranges associated to death probabilities. In
these examples we consider temperatures around
37ºC as adequate, temperatures in the 38-43ºC as
risky, temperatures in the 43-50ºC as critical and
temperatures above 50ºC as necessarily deadly.
3 RESULTS
3.1 Cells Colonising Scaffolds
Figures 4 and 5 provide examples of cells colonizing
scaffolds measuring 10 mm in height and 10 in
diameter, which correspond to 100 x 100 x 100 voxels
with the slicing and resolution employed. The voxels
corresponding to scaffolds’ trusses are represented in
blue, while those voxels corresponding to living/dead
cells are respectively drawn in green/red. Figure 4
shows different colonization patterns emulating
colonization of scaffolds starting from distinct
regions. Proliferation and death probabilities of 0.9
and 0.05% are used. Four selected iterations (100,
150, 200, 250) of a simulation performed along 400
steps. Iterations represent time steps, which should be
adjusted by means of in vitro experiments monitoring
cell growth within real tissue engineering scaffolds,
as we reported previously for lab-on-a-chip devices
(Ballesteros Hernando, 2019). The challenges linked
to these experimental validations are discussed in
section 4. Figure 5 presents the influence of using a
wall factor for adjusting the simulations to the fact
that cells tend to colonize scaffolds by growing
preferentially along the trusses and finally filling the
voids.
In silico Tissue Engineering and Cancer Treatment Using Cellular Automata and Hybrid Cellular Automata-Finite Element Models
59
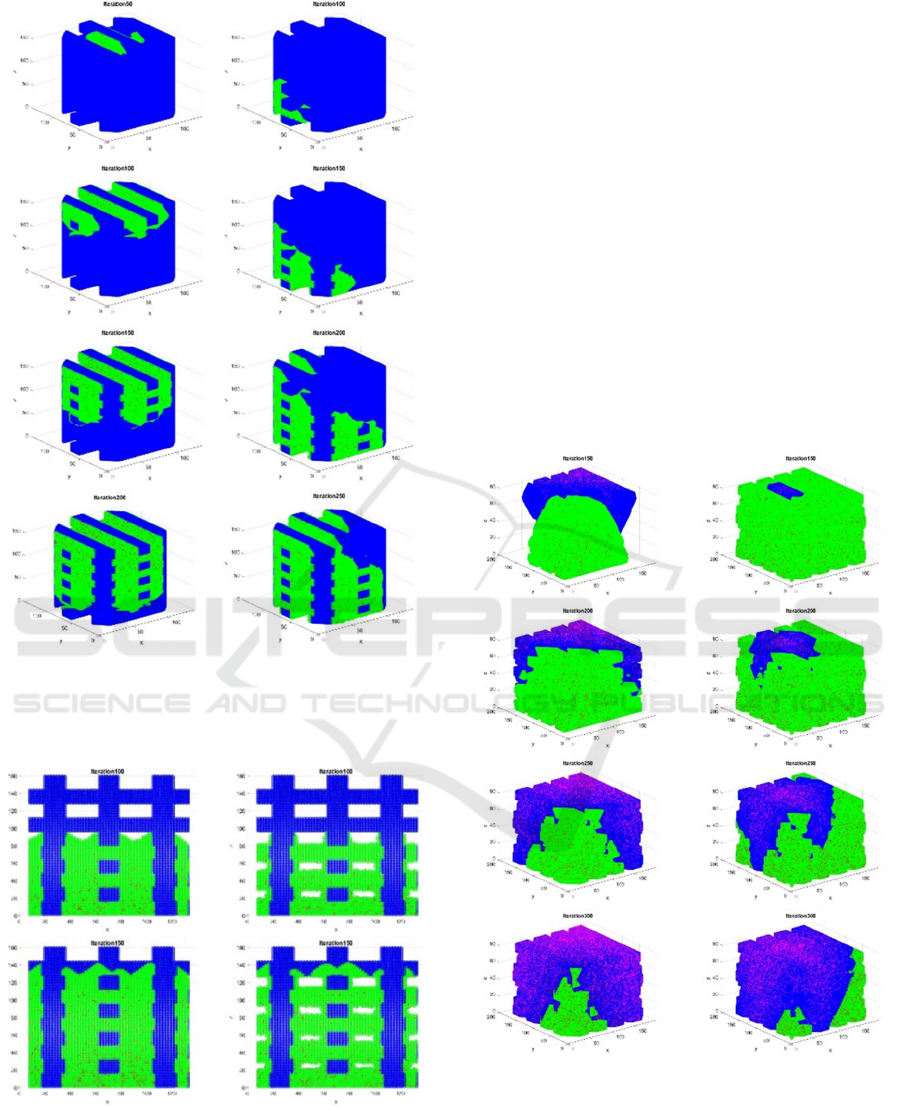
a) b)
Figure 4: Cells (green) colonizing scaffolds (blue). a) Four
iterations showing colonization from above. b) Four
iterations starting from a lower side.
a) b)
Figure 5: Influence of wall factor on colonization patterns.
a) Without wall factor. b) With increased proliferation
probability for cells touching scaffold’s trusses as
compared to cells far from the trusses (0.9 vs 0.5% as
proliferation probabilities for this model).
3.2 Cellular Interactions
To illustrate the possibility of modelling these
interactions, Figure 6 provides two examples of cells
interacting within a tissue engineering scaffold. For
visualization purposes the trusses of the scaffold are
not drawn, although they are considered as forbidden
regions for the cells, and the different cell types are
shown in green/red and in blue/pink, respectively for
living/dead healthy and invasive cells. Both examples
show features from patterns typically obtained when
diseases progress within in vitro, which have been
previously reported as extension along the scaffold,
cell clumps on the borders, and large cell clusters
(Zhang, 2013). In these examples no wall factor is
used and, for each iteration, proliferation probabilities
of 0.5 and 0.9 and death probabilities of 0.05% and
0.5% respectively for healthy and cancerous or
invasive cell types are employed.
a) b)
Figure 6: Examples of cell-cell interactions within a tissue
engineering scaffold. a) Scaffold being colonized by
healthy and invasive (cancerous) cells. b) Tumour growing
within an already colonized scaffold. Colour code: green =
healthy cells, blue = invasive cells, red = dead healthy cells,
pink = dead invasive cells. Four selected iterations for each
case.
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
60
3.3 Hyperthermia Therapy
Hyperthermia, as a therapy, refers to the controlled
heating of a region of the human body for a medical
purpose, usually cancer treatment. It has shown high
potential for cancer therapy, either in conjunction
with immunotherapy, chemotherapy, radiotherapy,
and surgery (Yagawa, 2017), or as standalone
technique, although the heating affects surrounding
healthy tissues, which is still concerning. Different
approaches are being studied for minimizing its
invasiveness and reaching remote regions, based on
magnetic and optical systems (Casanova-Carvajal,
2021, Zeinoun, 2021). The use of simulations is
expected to support the 3Rs in this field.
a) b)
Figure 7: CA simulation coupled to thermal FEM
representing cancer treatment by hyperthermia. Misaligned
intervention with large HAZ.
a) Rapid intervention stopping the tumour.
b) Delayed and unsuccessful intervention.
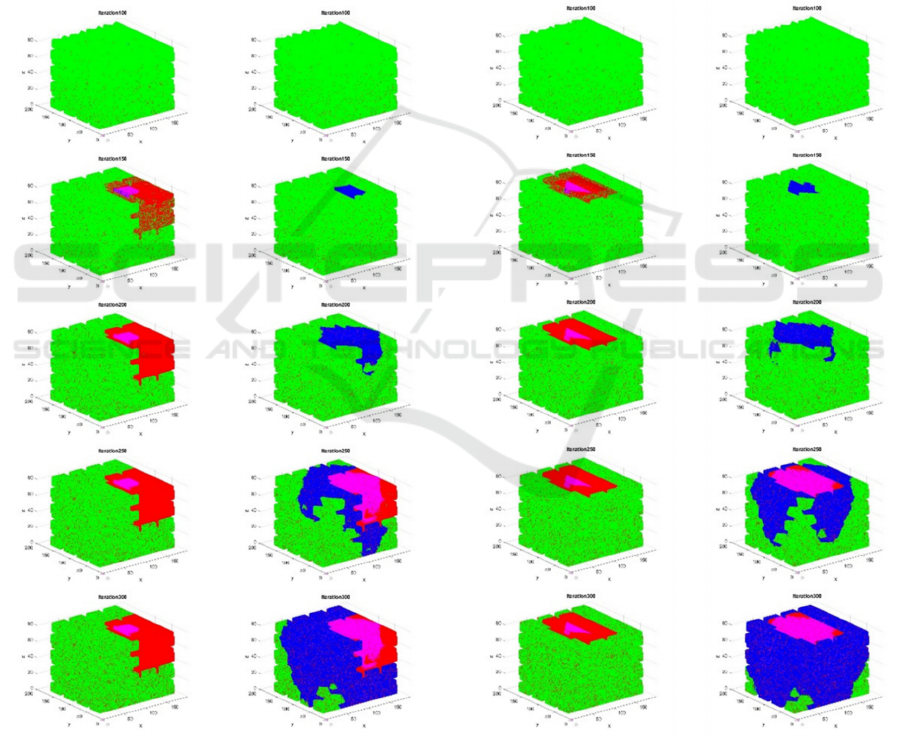
Figures 7 and 8 present two examples of CA
simulations coupled to thermal FEM representing
cancer treatments by hyperthermia. First the scaffolds
are colonized by healthy cells, as shown in the first
rows of images corresponding. Just before iteration
150 a tumoral seed is added by modifying some
voxels in the upper regions of the scaffolds. Rapid
interventions (figs. 7a and 8a) show the killing effect
of the thermal hyperthermia applied in iteration 150,
while delayed interventions (figs. 7b and 8b) apply
the thermal field in iteration 200, when the tumour
progress cannot be halted anymore. Figure 8a shows
the result of a thermal field more aligned with the
tumour, which helps to minimise the heat-affected
zone (HAZ) without compromising effectivity.
a) b)
Figure 8: CA simulation coupled to thermal FEM
representing cancer treatment by hyperthermia. Focused
intervention with reduced HAZ.
a) Rapid intervention stopping the tumour.
b) Delayed and unsuccessful intervention.
In silico Tissue Engineering and Cancer Treatment Using Cellular Automata and Hybrid Cellular Automata-Finite Element Models
61

4 LIMITATIONS AND FUTURE
RESEARCH PROPOSALS
Main limitation of the study is linked to the still
pending experimental validation with real cells. In the
previous study from our team, in which CA were
employed for modelling cells within lab-on-a-chip
devices (Ballesteros Hernando, 2019), we
demonstrated the possibility of adjusting cell growth
patterns using proliferation rates obtained directly
from cultures in Petri dishes. In those systems, simple
microscopy upon the lab-on-a-chip platforms is
adequate for monitoring growth. However, within
tissue engineering scaffolds the visualization is much
more challenging and monitoring the colonization
and the cell-cell and cell-material interactions in their
core requires alternatives to microscopy. Currently
we are exploring the applicability of magnetic
resonance microscopy, as a non-invasive technology
capable of exploring the inside of scaffolds with cells,
following the example of pioneering research
(Führer, 2017).
Considering future research, together with the
experimental validation employing in vitro cultures
for adjusting the CA models, it is important to
consider the following: First, the coupling of FEM
simulations with CA models has proven useful for
mapping temperature fields and simulating cancer
treatment using hyperthermia. Apart from that,
several phenomena can be modelled following this
hybrid CA-FEM approach, including: the effects of
fluid flow and shear stresses on scaffolds’
colonization, if computational fluid dynamics
simulations are used; the impact of nutrients and
drugs’ diffusion on cell viability or disease
progression; or, even more challenging, potential
mechanobiological effects (vibrations, cyclic
compressions / tractions, pulsatile stimulation) on cell
differentiation during tissue repair processes.
Second, the geometries of scaffolds employed
and the control volume and working space for the
performed simulations remain fixed during the
calculations, that is, a 3D grid with a fixed number of
elements with fixed sizes is always employed. The
size of the grid and elements depends only on the
actual scaffold’s size and slicing employed, which
can be adjusted to the dimensions of individual cells
or cells’ clusters. From a computational point of view
this is not yet optimal; it would be interesting to
explore models, in which the grid’s size and the
number of elements varies along the simulation, as
has proven useful in advanced FEM simulations, in
which elements can be switched off and on during a
simulation.
In addition, size changes are often involved in
gene expression and differentiation processes, so
counting with agents or cells within the automata
capable of modifying their size would be an
interesting incorporation.
Finally, towards societal impact, once validated,
these models should undergo a certification process
under the appropriate regulation, the Medical Device
Regulation 2017/745 in the case of the European
Union. Thus, they could become commercially
available solutions that healthcare professionals and
engineers devoted to medical technologies could
employ, in parallel to their research on tissue
engineering and cancer therapies, for supporting both
the design of medical devices like scaffolds and the
development of drugs for cancer treatment. Ideally,
for increased reach out, these SaMDs may be shared
as open-source solutions accessible to all.
5 CONCLUSIONS
This study has presented an innovative approach for
modelling cells colonising scaffolds, the interactions
among different populations of cells and between the
cells and the scaffolds as extracellular matrices, and
the effects of external stimuli, like temperature, for
treating disease. To this end, different advances have
been incorporated to conventional models based on
cellular automata. First, a direct connection between
CAD models and the simulation workspace has been
provided, by using digital tomographs, employing
additive manufacturing slicers upon the CAD files, to
create the grid. Furthermore, the option of
incorporating a sort of wall factor, capable of
considering the affinity of cells for the extracellular
matrix, has been discussed. In addition, FEM
simulation upon the scaffolds’ environment have
been coupled to the cellular automata for making
them more versatile. Finally, interactions among
different types of cells have been simulated.
Despite the pending in vitro experiments,
simulations presented show the possibility of
modelling complex interactions and phenomena
directly working with the CAD models of
biomaterials and scaffolds. Besides, a connection
between designed geometries and the grids used for
agent-based simulations has been established, and the
utility of hybridising CA with FEM for studying cells,
tissues, scaffolds, and cancer therapies has been
illustrated and discussed. The fact that wisely
implemented cellular automata can perform as
universal Turing machines (Wolfram, 1984) points
out the relevance and potentials of this approach.
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
62

ACKNOWLEDGEMENTS
This research was performed with the support of the
following programmes and projects: Programa estatal
de generación de conocimiento y fortalecimiento
científico y tecnológico del sistema de I+D+i,
subprograma estatal de generación de conocimiento
del Ministerio de Ciencia e Innovación y Agencia
Estatal de Investigación, ref. PGC2018-097531-B-
I00 (Talenano: Estudio de la eficacia de tecnologías
alternativas de liberación de energía térmica y
mecánica mediante nanoestructuras de óxido de
hierro y de oro con aplicación en terapias), as regards
the modelling of cancer hyperthermia; European
Union’s Horizon 2020 Research and Innovation
Programme under grant agreement n. 953134
(INKplant project: Ink-based hybrid multi-material
fabrication of next generation implants), as regards
the modelling of cell-scaffold interactions; and
Programa de estancias de movilidad de profesores e
investigadores en centros extranjeros de enseñanza
superior e investigación del Ministerio de
Universidades, ref. PRX21/00460 (Ingeniería de
tejidos in silico facilitada mediante microscopía de
resonancia magnética), as regards validation strategy.
REFERENCES
Aske, K.C., Waugh, C.A. (2017). Expanding the 3R
principles: More rigour and transparency in research
using animals. EMBO Reports, 18(9), 1490-1492.
Ballesteros Hernando, J., Ramos Gómez, M., Díaz Lantada,
A. (2019). Modeling Living Cells Within Microfluidic
Systems Using Cellular Automata Models. Scientific
Reports, 9, 14886.
Casanova-Carvajal, O., …, Serrano-Olmedo, J.J. (2021).
The use of silica microparticles to improve the
efficiency of optical hyperthermia (OH). Int. J. Mol.
Sci., 22, 5091.
Edelman, L.B., Eddy, J.A., Price, N.D. (2010). In silico
models of cancer. Wiley Interdiscip. Rev. Syst. Biol.
Med., 2(4), 438-459.
Erkizia, G., Rainer, A., De Juan-Pardo, E. M. & Aldazabal,
J. (2010). Computer simulation of scaffold degradation.
Journal of Physics: Conference Series, Surface
Modifications and Functionalisation of Materials for
Biomedical Applications, 252, 012004.
Fais, S., Overholtzer, M. (2018). Cell-in-cell phenomena,
cannibalism, and autophagy: is there a relationship?
Cell Death Dis., 9, 95.
Führer, E., et al. (2017). 3D carbon scaffolds for neural stem
cell culture and magnetic resonance imaging. Adv.
Health. Mat., 7(4), 1700915.
Gardner, M. (1970). Mathematical games: The fantastic
combinations of John Conway’s new solitaire game
“life”. Scientific American, 223, 120–123.
Garijo, N., Manzano, R., Osta, R., Perez, M. (2012).
Stochastic cellular automata model of cell migration,
proliferation and differentiation: Validation with in
vitro cultures of muscle satellite cells. Theoretical
Biology, 314, 1–9.
Geris, L. (2013). Computational modelling in tissue
engineering, Springer-Verlag Berlin Heidelberg.
Geris, L., Guyot, Y., Schrooten, J., Papantoniou, I. (2016).
In silico regenerative medicine: how computational
tools allow regulatory and financial challenges to be
addressed in a volatile market. Interface Focus, 6,
20150105.
Geris, L., Lambrechts, T., Carlier, A., Papantoniou, I.
(2018). The future is digital: In silico tissue
engineering. Current Opinion in Biomedical
Engineering, 6, 92-98.
Jean-Quartier, C., Jeanquartier, F., Jurisica, I., Holzinger,
A. (2018). In silico cancer research towards 3R. BMC
cancer, 18(1), 408.
Khademhosseini, A., Langer, R. (2016). A decade of
progress in tissue engineering. Nat. Protoc., 11, 1775–
1781.
Keshavarzian, M., Meyer, C.A., Hayenga, H.N. (2019). In
Silico Tissue Engineering: A Coupled Agent-Based
Finite Element Approach. Tissue Eng Part C Methods,
25(11), 641-654.
Ludvigsen, K., Nagaraja, S., Daly, A. (2022). When is
software a medical device? Understanding and
determining the “intention” and requirements for
software as a medical device in European Union law.
European Journal of Risk Regulation, 13(1), 78-93.
Spencer, T.J., Hidalgo-Bastida, L.A., Cartmell, S.H.,
Halliday, L., Care, C.M. (2013). In silico multi-scale
model of transport and dynamic seeding in a bone tissue
engineering perfusion bioreactor. Biotechnol. Bioeng.,
110, 1221-1230
Vivas, J., Garzón-Alvarado, D. & Cerrolaza, M. (2015).
Modelling cell adhesion and proliferation: A cellular
automata-based approach. Advanced Modelling and
Simulation in Engineering, Sciences 2, 32.
Von Neumann, J., Burks, A.W. (1966). Theory of self-
reproducing automata. Urbana, University of Illinois
Press.
Wolfram, S. (1984). Universality and complexity in cellular
automata. Physica, 10D, 1–35.
Yagawa, Y., Tanigawa, K., Kobayashi, Y., Yamamoto, M.
(2017). Cancer immunity and therapy using
hyperthermia with immunotherapy, radiotherapy,
chemotherapy, and surgery. J. Cancer Metastasis
Treat., 3, 218-230.
Zhang, M., Boughton, P., Rose, B., Soon Lee, C., Hong,
A.M. (2013). The use of porous scaffold as a tumor
model. International Journal of Biomaterials, 396056.
Zeinoun, M., …, Serrano Olmedo, J.J. (2021). Enhancing
magnetic hyperthermia nanoparticle heating efficiency
with non-sinusoidal alternating magnetic field
waveforms. Nanomaterials, 11(12), 3240.
In silico Tissue Engineering and Cancer Treatment Using Cellular Automata and Hybrid Cellular Automata-Finite Element Models
63
