
Design and Manufacturing of Microtextured Patient-Specific
Coronary Stent
Francisco Franco-Martínez
1a
, William Solórzano-Requejo
1, 2 b
, Alejandro de Blas-de Miguel
1c
,
Matthias Vostatek
3
, Christian Grasl
3,4
, Marta Bonora
3d
, Francesco Moscato
3,4,5 e
and Andrés Díaz Lantada
1f
1
ETSI Industriales, Universidad Politécnica de Madrid, Madrid, Spain
2
Department of Mechanical and Electrical Engineering, Universidad de Piura, Piura, Peru
3
Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria
4
Ludwig Boltzmann Institute for Cardiovascular Research, Vienna, Austria
5
Austrian Cluster for Tissue Regeneration, Vienna, Austria
Keywords: Metasurfaces, Metamaterials, Additive Manufacturing, Coronary Artery Disease, Personalized Medicine.
Abstract: Currently, the most usual treatment for coronary artery disease is the use of stents, which are produced with
standard dimensions and shapes and the surgeon selects the one that best fits the patient’s anatomy. Due to
this treatment, likelihood of restenosis might reach 40%. Additionally, thrombi formation is an important risk
for these patients that is treated with anticoagulant medicines. Therefore, a design and manufacturing method
to produce microtextured patient-specific coronary stent is developed with the aim to minimize the likelihood
of restenosis and thrombosis. Stents consisting of unit cells structures that are regularly repeated to form a
ring and, sometimes connectors to join the rings. To improve the fitting between artery and stent, parametric
design of unit cell as a function of the length and mean radius of coronary artery is required. Then, the unit
cell is microtextured to improve hemocompatibility using a bioinspired design in shark skin, which provide
superhydrophobicity, drag reduction and oleophobicity under water conditions. Once the unit cell is
micropatterned, a reverse engineering reconstruction is done to obtain the stent model. Finally, the design is
manufactured with a 3D printer using two-photon polymerisation technology. SEM is used to evaluate the
design and manufacturing method.
1 INTRODUCTION
Coronary artery disease is a major cause of mortality
and morbidity (Ho et al., 2016), each year causes 3.9
million deaths in Europe and is estimated to cost the
European Union €210 billion per year (CVD
Statistics, n.d.). It occurs when fat, calcium and
cellular debris accumulate in the artery wall blocking
blood flow and causing an inadequate supply of
oxygen and nutrients to the myocardium; resulting in
infarction, cerebral hemorrhage, or ischemic stroke
a
https://orcid.org/0000-0002-7894-7478
b
https://orcid.org/0000-0002-2989-9166
c
https://orcid.org/0000-0003-2375-8327
d
https://orcid.org/0000-0003-3667-0965
e
https://orcid.org/0000-0003-0279-6615
f
https://orcid.org/0000-0002-0358-9186
(Pan et al., 2021). The most popular treatment is
coronary angioplasty (CA), (Ho et al., 2016; Canfield
& Totary-Jain, 2018). CA is a minimal invasive
procedure used to expand the blood vessels and
restore the function of the cardiovascular system by
inserting a stent inside it. Constructively, coronary
stents are small, complex, hollow and cylindrical-
shaped tubes consisting of unit cells structures that
are regularly repeated to form a ring; the ring, a group
of unit cells that are held together; and the connectors,
which join rings to build the stent.
142
Franco-Martínez, F., Solórzano-Requejo, W., de Blas-de Miguel, A., Vostatek, M., Grasl, C., Bonora, M., Moscato, F. and Díaz Lantada, A.
Design and Manufacturing of Microtextured Patient-Specific Coronary Stent.
DOI: 10.5220/0011691600003414
In Proceedings of the 16th Inter national Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 1: BIODEVICES, pages 142-149
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

The design of the components defines the
biomechanical performance of the stent, the rings
determine radial support and expandability; the
number of connectors sets longitudinal stability,
flexibility, and longitudinal integrity; and the reduced
number of connectors provides greater flexibility and
reduces arterial injuries (Polanec et al., 2020;
Tomberli et al., 2018).
According to follow-up studies, 10% of patients
require a replacement prosthesis one year after
implantation and the likelihood of restenosis might
reach 40% (Pan et al., 2021; Schillinger et al., 2007).
These issues are the result from geometrical
alterations in a coronary artery once surgery is done
due to the size disparity between the blood vessel and
the expanded stent which is manufactured with
standard dimensions and shapes. A significant
interaction force will be produced if the stent does not
suit the diseased artery, injuring the artery and finally
causing restenosis (Wang et al., 2018). This
pathology is a major challenge and nowadays patients
usually need anticoagulant and antiplatelet therapies
(Movafaghi et al., 2019). Therefore, the combination
of medical and mechanical design can improve
notoriously the function of vascular prostheses and
minimize the use of anticoagulant drugs to meet the
needs of patients worldwide. Currently, many efforts
are being made to minimize the risk of restenosis and
thrombi formation, caused by the adsorption of
plasma proteins and platelets adhesion, modifying the
properties of the surface in contact with the blood
applying controlled micro- and nanopatterns (Koh et
al., 2010; Movafaghi et al., 2019).
Organisms as plants, animals and bacteria among
others, has carried out different solutions to survive
and adapt to the environment. The surface is where
organisms interact with other organisms and the
environment. Therefore, some of them has developed
textured surfaces in micro and nanoscales with
remarkable properties, such as the gecko, lotus flower
or sharks. Regarding the need, it could be chosen one
or another bioinspired microtexture and replicate it
artificially.
Researchers have studied the use of
superhydrophobic surfaces to improve the
hemocompatibility. These surfaces are super-
repellent to water with a static contact angle > 150º
and minimum contact angle hysteresis < 10º.
Moreover, superhydrophobic surfaces present low
blood protein adsorption and low blood cell
interaction which leads to improve
hemocompatibility (Movafaghi et al., 2019). Celik et
al. demonstrated the use of biocompatible materials
with superhydrophobic surfaces by manufacturing
samples rubbing the surface after coated the
polydimethylsiloxane (PDMS) with carnauba wax.
The static (169º) and sliding (3º and 5º respectively)
contact angle measurements with water and blood
showed super-repellency. Additionally, they studied
the contact angle with platelets suspension,
erythrocite concentrate and fresh plasma, all of them
showed superhydrophobic behaviour (Celik et al.,
2021).
Hoshian et al, also used PDMS with
microtextured inner surface coated with titania (TiO
2
)
to produce flexible tubes which repel water and
blood, where the blood contact angle for the
microtextured surface was 161 ± 3º compared with
the control surface (flat PDMS/titania surface) of 90
± 2º. The improvement in repellency is notorious.
Furthermore, they investigated the drag reduction
when tubes were microtextured compared with no-
textured tubes obtaining only 39% and 99%
respectively, when both measurements were
compared to a free falling droplet in air. Finally, some
test were performed comparing the superhydrophobic
surface with smooth titania and PDMS substrate
revealing that only the superhydrophobic one does
not present adhered platelets (Hoshian et al., 2017).
Considering the main causes of restenosis and
thrombi formation, in this study the potential of
designing and manufacturing a patient-specific
microtextured stent based on the disease will be
demonstrated by designing two patient-specific
stents, micropatterning and manufacturing one of
them to test the feasibility of the design methodology
presented above. The shark skin micropattern is
selected because his drag reduction under water
condition, superhydrophobicity in air with contact
angle 155º and oleophobicity in water environment
(Jung & Bhushan, 2009). It will be show a novelty
method to microtextured stents with the aim of
reducing restenosis and thrombi formation.
2 MATERIALS AND METHODS
2.1 Open-Source Coronary Stent
Design
Parametric design is one of the methods to produce
stents for each patient since it can effectively improve
their mechanical properties. For this purpose, the
parameterization of two coronary stents as a function
of the length (𝐿) and mean radius (𝑅) of the artery is
detailed. The first design is distinguished because the
unit cells are joined together, whereas in the second
they are linked by connectors.
Design and Manufacturing of Microtextured Patient-Specific Coronary Stent
143
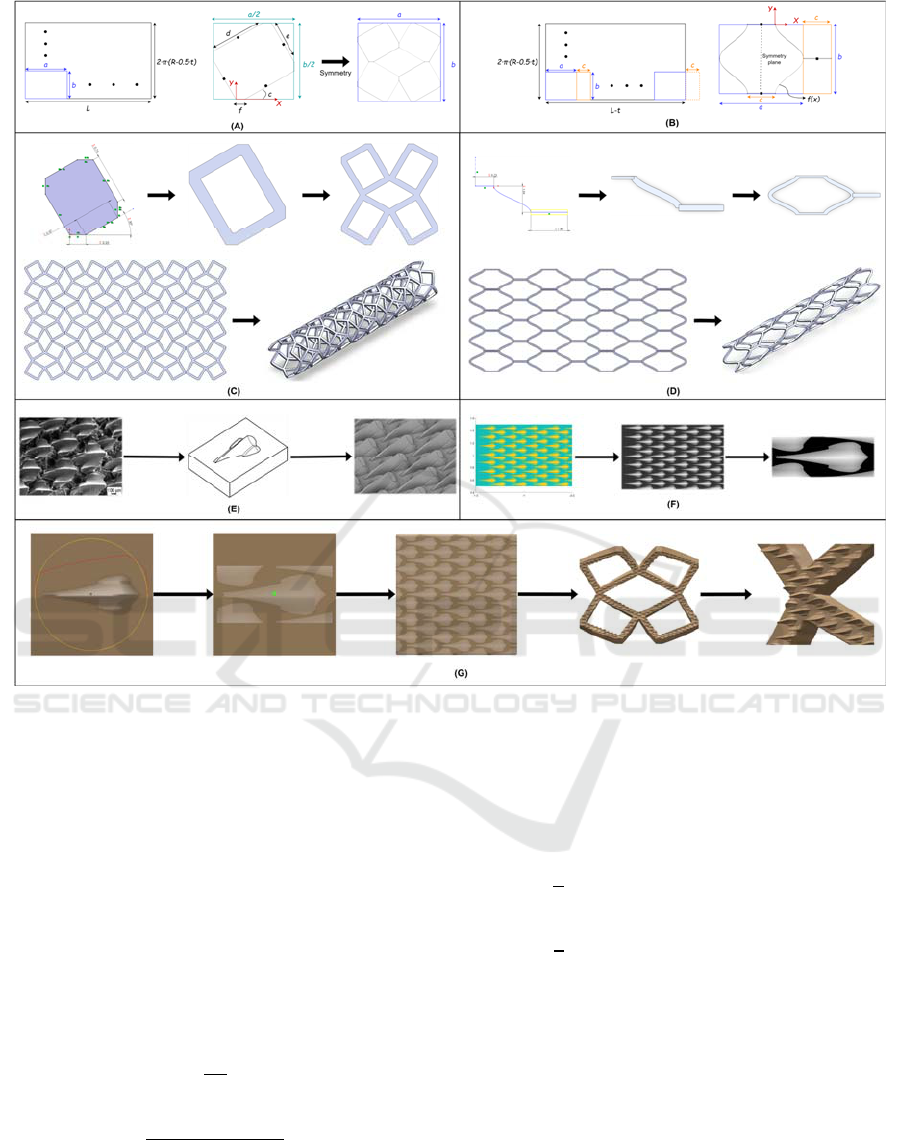
Figure 1: Macro and microstructure design. CAD design process of the (A, C) first and (B, D) second coronary stents. (E)
Bioinspired microstructures CAD design. (F) Mask acquisition. (G) Surface texturing.
2.1.1 First Design Strategy
The parameterization of the first model starts with the
development of a hollow cylinder; a rectangle of
width equal to 𝐿 and length, to the average length of
the artery minus the half of the stent thickness (2𝜋∙
(𝑅− 0.5𝑡)). Macroscopically, the unit cell is another
rectangle of “𝑎” width and “𝑏” length embedded in
the development to be repeated “𝑁𝑥” and “𝑁𝑦” times
in the longitudinal ( 𝑋 ) and radial ( 𝑌 ) axes
respectively (Figure 1A). With these similarities, the
first equivalences are made:
𝑎=
𝐿
𝑁𝑥
(1)
𝑏=
2∙𝜋∙(𝑅−0.5∙𝑡)
𝑁𝑦
(2)
From the microscopic point of view, the unit cell
has a defined shape delimited by the rectangle “𝑎𝑏”
(Figure 1A). Hence, the geometric relationships
between the macro and microscopic parameters must
be expressed mathematically, considering that there
is a more basic unit that with even and odd symmetry
constitutes the unit cell of the stent:
𝑎
2
=𝑑∙𝑐𝑜𝑠
(
𝑐
)
+𝑒∙𝑠𝑖𝑛
(
𝑐
)
+
𝑓
(3)
𝑏
2
=𝑑∙𝑠𝑖𝑛
(
𝑐
)
+𝑒∙𝑐𝑜𝑠
(
𝑐
)
+
𝑓
(4)
The parameters “𝑎” and “𝑏” are constrained by
the artery dimensions (𝐿 and 𝑅) and the designer
controls “ 𝑁𝑥”, “𝑁𝑦” and “𝑡” to obtain the best
performance. So, the other variables are unknowns,
but there are only two equations ((3) and (4)) resulting
in an indeterminate system; therefore, it is considered
that the unit cell opening, quantified through the angle
“𝑐”, is a parameter regulated by the designer and “𝑓”
is equal to “𝑡” to make its cross-section a square. By
partially solving the system of equations:
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
144

𝑚1=𝑑+ 𝑒=
𝑎+𝑏−4∙
𝑓
2∙(𝑠𝑖𝑛
(
𝑐
)
+𝑐𝑜𝑠 (𝑐))
(5)
𝑚2=𝑒− 𝑑=
𝑎−𝑏
2∙
(
𝑠𝑖𝑛
(
𝑐
)
−𝑐𝑜𝑠
(
𝑐
))
(6)
The microscopic variables “𝑑” and “𝑒” are a
combination of “𝑚1” and “𝑚2”
𝑑=
𝑚1 − 𝑚2
2
(7)
𝑒=
𝑚1 + 𝑚2
2
(8)
All these parameters are imported in the
"Equations" command, as global variables, in
SolidWorks 2021
®
(Dassault Systèmes, Waltham,
MA, USA). This software is used for its simplicity
when modelling complex geometries because the
parameterization of parts is highly optimized, and its
interface is user-friendly.
For the modelling, a sketch is made in the “Right
plane” correctly defining the constraints to obtain the
geometrical changes under the modification of the
global variables. Then, it is extruded with a depth
equal to “𝑡” and the “Shell” tool allows get an internal
wall with same thickness. To compose the unit cell
from this basic structure, the “Symmetry” tool is used
and the “Linear Pattern” command replicates “𝑁𝑥”
and “𝑁𝑦” times the unit cell in the 𝑋 and 𝑌 axes to
elaborate a mesh, development of the coronary stent.
Finally, the “Flex” and “Combine” tool folds the
mesh and joins each unit cell to form the coronary
stent (Figure 1C).
2.1.2 Second Design Strategy
The design of the second model starts from the same
development but, in addition to the unit cell, the
connectors that link them must be parameterized.
Therefore, both elements are integrated forming a
larger unit cell that is repeated bidirectionally. From
Figure 1B, the large-scale equivalence is performed:
𝐿−𝑡=𝑁𝑥∙(𝑎+𝑐)−𝑐 (9)
𝑏=
2∙𝜋∙(𝑅−0.5∙𝑡)
𝑁𝑦
(10)
This system has three unknowns (“𝑎”, “𝑏” and
“ 𝑐”) and only two equations. Therefore, it is
indeterminate and one of the unknown variables must
be defined. Due to the symmetry of the design, the
width of the unit cell was related to the length of the
connector:
𝑐=
𝑎
3
(11)
As a result, introducing equation (11) in (9) we
obtain:
𝑎=
𝐿−𝑡
4
3
∙𝑁𝑥−
1
3
(12)
Unlike the previous case, the thickness also
affects the longitudinal axis with the term (𝐿−𝑡)
appearing in equation (12), a consequence of the unit
cell geometry, which is modelled in SolidWorks
®
through sine function:
𝑓
(
𝑥
)
=
𝑐
2
∙𝑠𝑖𝑛
2𝜋
b
∙𝑥−
𝑏
4
+
𝑐
2
;
𝑥∈[0,0.5𝑏]
(13)
The above equations are imported into the
Solidworks
®
file as global variables. The sinusoidal
structure and its connector are sketched at the origin
of the “Top plane” and the “Swept Boss/Base”
command is used to obtain them three-dimensionally
with square cross-section of side “𝑡”. Furthermore, a
double symmetry is performed to produce the
complete unit cell. Then, the “Linear Pattern” tool
replicates the cell “ 𝑁𝑥” and “𝑁𝑦” times, but the
connector only “𝑁𝑥− 1” times because the stent
must start and end with the unit cell. Once the mesh
is prepared, the elements are folded and combined to
form the vascular prosthesis (Figure 1D).
2.2 3D Coronary Artery Model
A STL file of an artery (Model ID 3DPX-012589),
based on a CT scan and segmented by researchers at
the University of Toronto and Toronto General
Hospital, has been downloaded from the NIH 3D
Print Exchange repository (Phantom Coronary
Artery Models | NIH 3D Print Exchange, n.d.) to
quantify its length and average radius, being 22 and
1.71 mm respectively. Accordingly, the two coronary
stent models described above are adapted to extract
the unit cell and connector in the final position for
texturing.
2.3 Attainment of Microtextures
To create a microtexture to pattern the inner surface
of the stent, three different steps are required, from
the bioinspired microtexture to patterning the surface
with it (Figure 1E-G). The design process followed to
create the microtextured stent is described in detail in
(Franco-Martínez et al., 2022) where the authors
explained a design methodology to pattern the 3D
objects surfaces.
Design and Manufacturing of Microtextured Patient-Specific Coronary Stent
145

2.3.1 Microtexture Selection and Computer
Aided Design (CAD)
Previous studies showed that superhydrophobic
surfaces enhance hemocompatibility. Consequently,
shark skin is an adequate surface with the required
attribute. In this study, bioinspired shark skin replica
from (Jung & Bhushan, 2009) was chosen (first image
shown in Figure 1E) because of its high similarity to
nature, complex shape, high superhydrophobicity and
the authors provided the dimensions of replicated
shark scales. Then, one image and dimensions are
required to replicate the shape in CAD software,
obtaining a bioinspired surface model. Autodesk
Inventor 2021
®
(Autodesk, Inc.) was used to design
the pattern; to replicate the chosen shape, one
bioinspired shark scale is done using the “loft” tool
twice. Afterwards, this single model is replicated
along a flat surface with the “Pattern sketch driven”
command (Figure 1E).
2.3.2 Mask Acquisition
A grayscale mask is required to transfer the designed
pattern to the unit cell that works as the base for the
stent. Matlab R2022a
®
(Mathworks, Inc.) was used to
convert the STL file with shark skin pattern into a
grayscale mask. The STL is imported by “stlread”
tool, plotted with “patch” tool, transformed into an
image in grayscale with “rgbtogray” command and
“rescale” function is used to obtain the final mask
with better resolution. Finally, the image is trimmed
and saved to use in the next step (Figure 1F).
2.3.3 Surface Texturing
Once the unit cell is designed in CAD, the STL file is
imported to 3D Coat V4.9.65
®
(Pilgway). This
software offers a wide range of tools for digital
sculpting over the STL by “displacement mapping”,
a method that apply height maps to displace the points
or voxels of the mesh.
Regarding that, the shark skin mask is imported as
image in 3D Coat. Displacement’s height is directly
proportional to intensity in gray scale. The mask is
projected from the front view and it is used as
template over the surface micropatterned with the
chosen brush, determined by shape (in this case shark
scale), height and radius. To sculpt the surface, “live
clay” tool is used, which allows to add more density
of triangles where it is required on the surface to
achieve micrometer and well-defined range of the
micropatterning.
2.3.4 Manufacturing Process
Technology used to manufacture the stent prototype
is two-photon polymerisation (2PP), NanoOne
(UpNano GmbH, Vienna, Austria). It is a lithography
process capable of generating 3D structures with
nanometer feature sizes. The model was prepared in
Think3D (UpNano GmbH, Vienna, Austria) and
printed with UpPhoto (2-photon resin, UpNano)
which is biocompatible and non-cytotoxic (Materials
– UpNano – High-Resolution 3D Printing, n.d.), in
printing mode “vat” with a laser power of 250 mW at
a writing speed of 750 mm/s. To save time infill mode
“coarse” is selected and structuring was done in one
step with Fluar 5x/0.25 objective (Carl Zeiss
Microscopy, New York, United States).
After printing, the sample was rinsed in isopropyl
alcohol and air dried. A scanning electron microscopy
(SEM) (Zeiss EVO MA10, Oberkochen, Germany)
was used for imaging, and previously, the sample was
gold coated with a Quorom Q150R ES sputter coater
(Quorum Technologies Ltd, Laughton, United
Kingdom). Manufacturing and imaging process
followed in this study is similar to (Franco-Martínez
et al., 2022).
3 RESULTS
3.1 Micropatterning Unit Cell
Figure 2, shows the micropatterned unit cell
demonstrating the possibility of patterning 3D object
with curved surfaces. Due to the performance of 3D
Coat, the dimensions of the textures in CAD (Figure
2A) and 3D Coat (Figure 2B) varied a few microns
because of the size and place of the mesh triangles, as
it is commented in section 2.3.3. It is assumed that
this deviation will not affect the superhydrophobicity
state, since the fabrication creates the same or more
due to its accuracy. Additionally, the imprecisions
could enhance hemocompatibility thanks to produce
a non-uniform area, as shown in (Koh et al., 2010)
when the high aspect ratio textures are deformed and
bent complicating the adhesion of platelets.
Figure 2: (A) CAD microtextures with dimensions. (B) 3D
Coat microtextures with dimensions.
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
146
3.2 Reverse-Engineered
Reconstruction of Microtextured
Stent
From the textured unit cell, the coronary stent is
reconstructed. First, the cell is replicated “𝑁𝑦” times
radially to form the ring, which is then cloned “𝑁𝑥”
times linearly to assemble the patient-specific
textured coronary stent, whose length and outer
radius are equal to the artery dimensions (Figure 3).
However, in the second model, the connector is
replicated only “ 𝑁𝑥-1” times due to its design
(section 2.1).
This simplicity in the reconstruction is a
consequence of the macroscopic and microscopic
view explored in the computational design of
coronary stents.
Figure 3: Reconstruction of textured coronary stents.
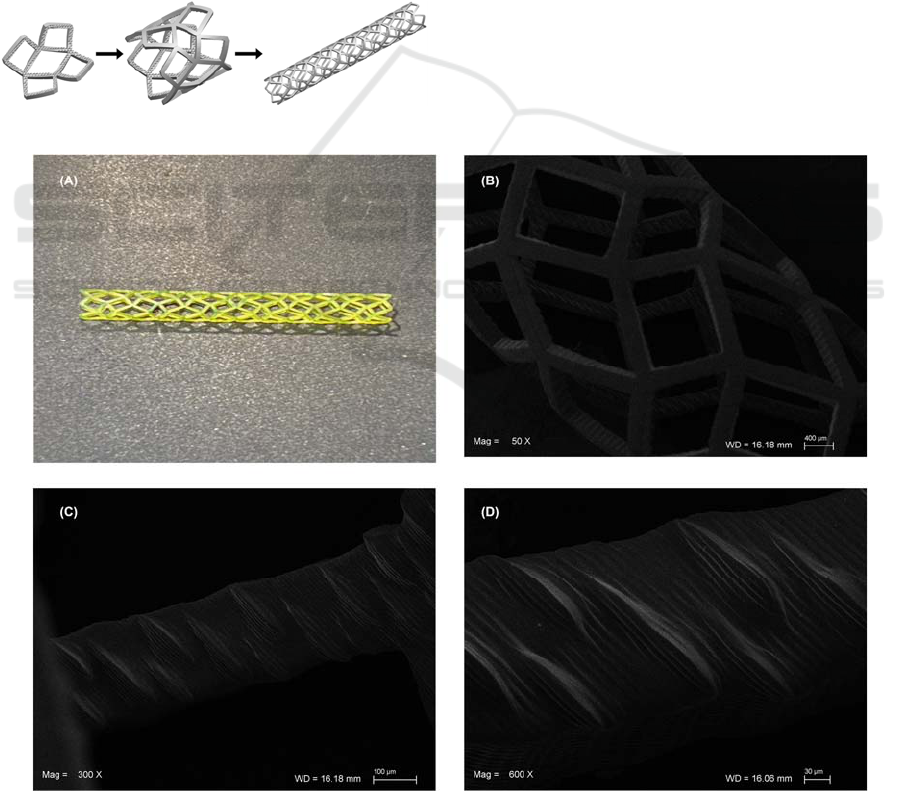
3.3 Microtextured Stent Manufacturing
3D manufacturing process is used to fabricate the
microtextured first design strategy stent prototype
(Figure ). The second design (Figure 1B) has not been
manufactured yet.
The microtextured stent prototype can be
observed in Figure . It is shown the inner surface
microtextured with remarkable shark’s scales, despite
of the sides riblets cannot be seen in most of them
(Figure D). Indeed, the stent was printed in “vat”
mode with infill “coarse” which is very fast and the
printing time was 20 minutes approximately. It is
feasible to reach higher accuracy with more precise
objective and longer printing time.
This prototype demonstrates that 3D objects with
complex and controlled surface topography can be
manufactured. In literature, most of microstructures
are made on a flat surface, as done in (Koh et al.,
2010), (Celik et al., 2021) or (Jung & Bhushan, 2009).
Hoshian et al. developed a manufacturing process
to design flexible tubes with microstructures on inner
surface. However, the structures where completely
random in shapes and position, in contrast to the 2PP
Figure 4: Microtextured Stent. (A) Real prototype. (B) SEM image outer surface with 50x magnification. SEM images inner
microtextured surface (C) 300x and (D) 600x magnification.
Design and Manufacturing of Microtextured Patient-Specific Coronary Stent
147

printing process that allows controlled and complex
structures to be made.
Regarding the material employed, despite its
biocompatibility, it may not be appropriate to use as
a stent due to its mechanical properties. In this case,
authors believe that it is feasible to use it as a
template, manufacturing the final stent in another
material, for example by applying the process
developed in (Hoshian et al., 2017). The material,
interaction with blood and implantation have to be
investigated in following studies.
4 CONCLUSIONS
In this study, a patient-specific microtextured stent
was designed and manufactured to minimize
restenosis and thrombi formation without the use of
anticoagulant medicines, whose design process is
characterized by its low computational and time cost,
due to the exposed three-dimensional object
modelling and texturing strategies.
Based on the design strategies described above,
considering their macroscopic (development) and
microscopic (unit cell and connector) view, it is
possible to computationally model any type of
coronary stent. Furthermore, the parameterization
favors the customization of these biodevices, since
the equation approach is a function of the length and
mean radius of the artery. Shark scales is well-known
because its drag reduction and superhydrophobicity
in air conditions. Additionally, it is oleophobic under
water conditions. Therefore, the authors chose it as
bioinspired microtexture for the inner surface and
believe that this topography is interesting because its
oleophobic behaviour. If the microstructures reach
oleophobicity under blood conditions, the adsorption
of proteins will not be possible and, consequently,
platelets adhesion will be reduced.
Finally, the authors demonstrated that it is
possible to design complex micropatterns on 3D
objects through this study of the stent. Even this novel
method could be applied to texture other implants
with different patterns, such as tracheal stents, grafts
or dental implants to achieve better biocompatibility
and avoid problems after surgery. However, it should
be noted that the study in its current form is mainly
the design process. Indeed, according to the European
Medical Devices Regulation 2017/745, customized
implants cannot reach patients without prescription
and involvement in the design procedure of
physicians and surgeons, therefore, this research
brings an engineering point of view. Consequently,
before the presented designs can be considered
successful solutions, collaboration with healthcare
personnel would be essential to improve the work by
taking into account the problems that may arise in the
pre-, intra- and postoperative phases; and by
performing systematic in vitro and in vivo
evaluations.
ACKNOWLEDGEMENTS
This research study has been funded by the European
Union’s Horizon 2020 Research and Innovation
Programme under grant agreement No. 953134
(INKplant project: Ink-based hybrid multi-material
fabrication of next generation implants).
"Optimized Hydrodynamic Flow Behaviour by
Selective Surface Structured of Ceramic 3D Printed
Rotodynamic Blood Pumps - OPTIFLOW-3D"
funded by the Austrian Research Promotion Agency
(FFG), Nr. 891239.
REFERENCES
Canfield, J., & Totary-Jain, H. (2018). 40 Years of
Percutaneous Coronary Intervention: History and
Future Directions. Journal of Personalized Medicine,
8(4), Article 4. https://doi.org/10.3390/jpm8040033
Celik, N., Sahin, F., Ruzi, M., Yay, M., Unal, E., & Onses,
M. S. (2021). Blood repellent superhydrophobic
surfaces constructed from nanoparticle-free and
biocompatible materials. Colloids and Surfaces B:
Biointerfaces, 205, 111864. https://doi.org/10.1016/
j.colsurfb.2021.111864
CVD Statistics. (n.d.). Retrieved 29 December 2022, from
https://ehnheart.org/cvd-statistics.html
Franco-Martínez, F., Grasl, C., Kornfellner, E., Vostatek,
M., Cendrero, A. M., Moscato, F., & Lantada, A. D.
(2022). Hybrid design and prototyping of metamaterials
and metasurfaces. Virtual and Physical Prototyping,
17(4), 1031–1046. https://doi.org/10.1080/17452759.
2022.2101009
Ho, M.-Y., Chen, C.-C., Wang, C.-Y., Chang, S.-H., Hsieh,
M.-J., Lee, C.-H., Wu, V. C.-C., & Hsieh, I.-C. (2016).
The Development of Coronary Artery Stents: From
Bare-Metal to Bio-Resorbable Types. Metals, 6(7),
Article 7. https://doi.org/10.3390/met6070168
Hoshian, S., Kankuri, E., Ras, R. H. A., Franssila, S., &
Jokinen, V. (2017). Water and Blood Repellent Flexible
Tubes. Scientific Reports, 7(1), 16019. https://doi.org/
10.1038/s41598-017-16369-3
Jung, Y. C., & Bhushan, B. (2009). Wetting Behavior of
Water and Oil Droplets in Three-Phase Interfaces for
Hydrophobicity/philicity and Oleophobicity/philicity.
Langmuir, 25(24), 14165–14173. https://doi.org/10.
1021/la901906h
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
148

Koh, L. B., Rodriguez, I., & Venkatraman, S. S. (2010). The
effect of topography of polymer surfaces on platelet
adhesion. Biomaterials, 31(7), 1533–1545. https://doi.
org/10.1016/j.biomaterials.2009.11.022
Materials – UpNano – high-resolution 3D printing. (n.d.).
Retrieved 31 October 2022, from https://www.
upnano.at/materials/
Movafaghi, S., Wang, W., Bark, D. L., Dasi, L. P., Popat,
K. C., & Kota, A. K. (2019). Hemocompatibility of
super-repellent surfaces: Current and future. Materials
Horizons, 6(8), 1596–1610. https://doi.org/10.1039/
C9MH00051H
Pan, C., Han, Y., & Lu, J. (2021). Structural Design of
Vascular Stents: A Review. Micromachines, 12(7),
770. https://doi.org/10.3390/mi12070770
Phantom Coronary Artery Models | NIH 3D Print
Exchange. (n.d.). Retrieved 11 April 2022, from
https://3dprint.nih.gov/discover/3dpx-012589
Polanec, B., Kramberger, J., & Glodez, S. (2020). A review
of production technologies and materials for
manufacturing of cardiovascular stents. Advances in
Production Engineering & Management, 15(4), 390–
402. https://doi.org/10.14743/apem2020.4.373
Schillinger, M., Sabeti, S., Dick, P., Amighi, J., Mlekusch,
W., Schlager, O., Loewe, C., Cejna, M., Lammer, J., &
Minar, E. (2007). Sustained Benefit at 2 Years of
Primary Femoropopliteal Stenting Compared With
Balloon Angioplasty With Optional Stenting.
Circulation, 115(21), 2745–2749. https://doi.org/10.
1161/CIRCULATIONAHA.107.688341
Tomberli, B., Mattesini, A., Baldereschi, G. I., & Di Mario,
C. (2018). Breve historia de los stents coronarios.
Revista Española de Cardiología, 71(5), 312–319.
https://doi.org/10.1016/j.recesp.2017.11.016
Wang, Q., Fang, G., Zhao, Y.-H., & Zhou, J. (2018).
Improvement of Mechanical Performance of
Bioresorbable Magnesium Alloy Coronary Artery
Stents through Stent Pattern Redesign. Applied
Sciences, 8(12), 2461. https://doi.org/10.3390/
app8122461.
Design and Manufacturing of Microtextured Patient-Specific Coronary Stent
149
