
Lessons Learned from mHealth Monitoring in the Wild
Pedro Almir M. Oliveira
1,2 a
, Rossana M. C. Andrade
1 b
and Pedro A. Santos Neto
3 c
1
Group of Computer Networks, Software Engineering and Systems (GREat), Federal University of Cear
´
a, Cear
´
a, Brazil
2
Federal Institute of Maranh
˜
ao (IFMA), Pedreiras, Maranh
˜
ao, Brazil
3
Laboratory of Software Optimization and Testing (LOST), Federal University of Piau
´
ı, Piau
´
ı, Brazil
Keywords:
Health Monitoring, Self-reported Quality of Life, Practical Report, Lessons Learned.
Abstract:
In the modern world, it is no overstatement to say that “our devices know us better than we know ourselves”.
In this sense, the vast amount of data generated by wearables, mobile devices, and environmental sensors has
enabled the development of increasingly personalized and intelligent services. Among them, there is a growing
interest in the delivery of medical practice using mobile devices (i.e., mobile health or mHealth). mHealth
makes it possible to optimize healthcare systems based on continuous and transparent health monitoring,
aiming to detect the emergence of diseases. However, mHealth monitoring in the real world (i.e., uncontrolled
environment or, as labeled in this paper, “in the wild”) has many challenges. Therefore, this practical report
discusses ten lessons learned from the Quality of Life (QoL) monitoring of twenty-one volunteers over three
months. The main objective of this QoL monitoring was to collect data capable of training Machine Learning
algorithms to infer users’ Quality of Life using the WHOQOL-BREF as a reference. During this period, our
research team systematically recorded the problems faced and the strategies to overcome them. Such lessons
can support researchers and practitioners in planning future studies to avoid or mitigate similar issues. In
addition, we present strategies for dealing with each challenge using the 5W1H model.
1 INTRODUCTION
Our world is becoming mobile (Palos-Sanchez et al.,
2021). As a benchmark, 67.1% of the world’s pop-
ulation uses smartphones, which means 5.31 billion
of unique users by the start of 2022
1
. In addition, a
similar percentage – 62.5% of the world’s population
– has Internet access. This outstanding diffusion as-
sociated with advances in hardware (such as, cost re-
duction, sensor miniaturization, and expansion of pro-
cessing power) has enabled a massive transformation
in access to a variety of healthcare services, especially
in the area called mobile health (mHealth, for short)
(Bravo et al., 2018).
mHealth can be defined as the delivery of medical
practice by mobile devices, including smartphones,
tablets, or wearable monitoring devices (Bostrom
et al., 2020). mHealth apps facilitate the collection
and sharing of health data and the delivery of health
services (Qudah and Luetsch, 2019).
a
https://orcid.org/0000-0002-3067-3076
b
https://orcid.org/0000-0002-0186-2994
c
https://orcid.org/0000-0002-1554-8445
1
Digital 2022 Global Report: wearesocial.com.
Unique features such as accessibility, context
awareness, and personalized solutions have made the
use of mHealth attractive for the healthcare industry
(Akter and Ray, 2010). The mobile health market was
valued at USD 63 billion in 2021 and is projected to
reach more than 230 billion by 2027
2
. Furthermore,
mHealth has emerged as an opportunity to optimize
health systems resources, promoting high-quality at a
low-cost (Islam et al., 2015).
As stated by Varshney (2014), mobile health is
more than just some healthcare applications on a mo-
bile phone. Mobile health makes possible many kinds
of applications such as non-intrusive Quality of Life
(QoL) monitoring (Oliveira et al., 2022c), older adults
fall detection (Ara
´
ujo et al., 2022), gait and posture
analysis (Junior et al., 2021).
The analytical model of mobile health generally
includes applications that assist patients (i.e., users)
during their treatment. For example, the patient can
be a child, an adult, or an older person. They can also
have chronic or acute illnesses, and these health issues
make them dependent or independent.
In addition, healthcare professionals follow up
2
mordorintelligence.com/industry-reports.
Oliveira, P., Andrade, R. and Santos Neto, P.
Lessons Learned from mHealth Monitoring in the Wild.
DOI: 10.5220/0011689600003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 5: HEALTHINF, pages 155-166
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
155
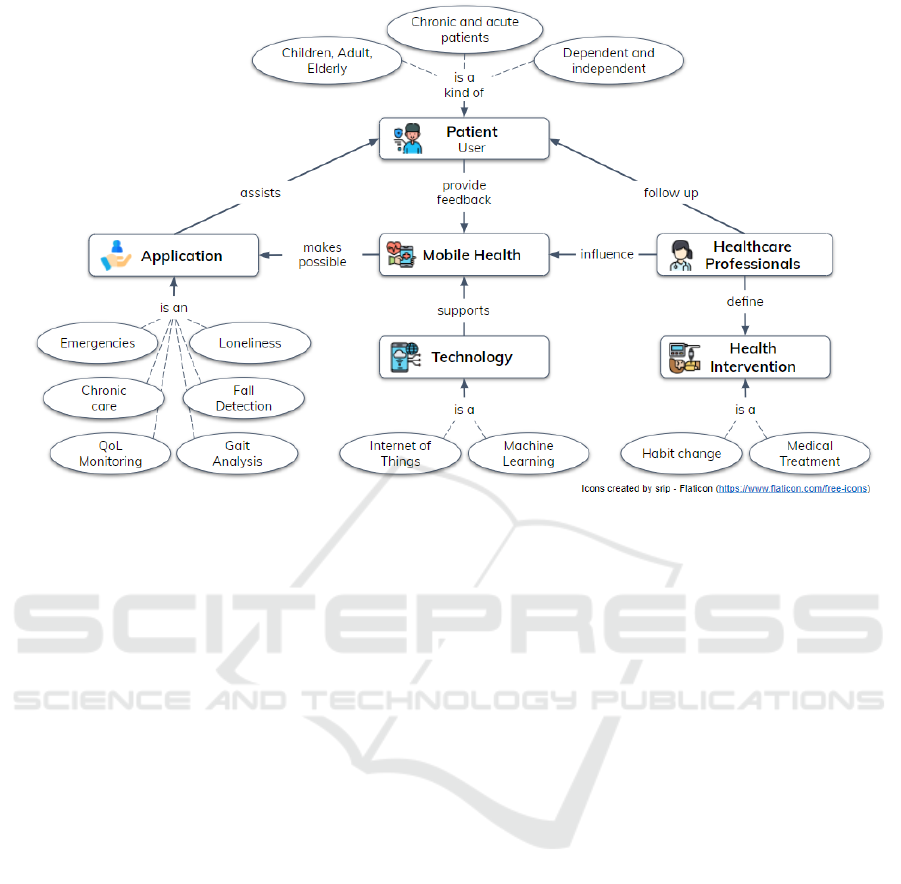
Figure 1: Mobile Health analytical model. Adapted from (Varshney, 2014).
with the patient and define health interventions (e.g.,
habit change). Figure 1 reinforces this analytical
model, highlighting that the Internet of Things (Ro-
drigues et al., 2018) and Machine Learning (Ian
and Eibe, 2005) are technologies applied to support
mHealth.
In this scenario, monitoring personal QoL using
mobile health applications has attracted the interest
of many researchers (Oliveira et al., 2022b) due to the
ability of these technologies to get data capable of un-
derstanding human behavior. Furthermore, this kind
of monitoring is relevant due to the health benefits that
can be achieved from an accurate QoL analysis, such
as disease detection and early healthcare interventions
(Oliveira et al., 2022c). Dohr et al. (2010) also rein-
forces that these benefits have individual impacts by
increasing well-being, economic impacts by improv-
ing the cost-effectiveness of healthcare resources, and
social impacts by promoting better living conditions.
The history of the Quality of Life term began a
long time ago (Elkinton, 1966). Even so, despite be-
ing discussed a lot, this term can be observed from
many perspectives (Karimi and Brazier, 2016). For
example, the QoL can be related to the absence of
chronic diseases, perception of loneliness, physical
well-being, and understanding of the aging process.
The World Health Organization’s Quality of Life
definition is the primary reference in this work. Thus,
QoL can be defined as the individual perception of
life in a sociocultural context (Orley and Kuyken,
1994). Based on this definition, many instruments
to assess QoL have been proposed, such as the
WHOQOL-BREF questionnaire, SF-36 health sur-
vey, and KIDSCREEN-52 for children.
Unfortunately, the continuous application of this
kind of questionnaire is tedious, bothersome (Sanchez
et al., 2015), and can also include a bias as the pa-
tient needs to actively provide the information, which
makes it challenging to engage the participants (Hao
et al., 2017). Therefore, QoL continuous monitoring
is still an open problem due to the complexity of the
measuring instruments and the invasive approaches
that do not preserve privacy (Oliveira et al., 2022b).
Motivated by this context, we decided to start –
in a previous work (Oliveira et al., 2022c) – an in-
vestigation about the use of the Internet of Health
Things (IoHT) to collect data from Smart Environ-
ments and apply Machine Learning to infer QoL mea-
sures. Then, to evaluate this proposal, we conducted
a longitudinal study in which twenty-one (21) partic-
ipants were monitored “in the wild” for three months.
This expression “in the wild” reinforces the inherent
complexity of monitoring health data outside a con-
trolled environment such as a laboratory or hospital.
Thus, the main goal of this paper is to present
and discuss the lessons learned during this longitudi-
nal health monitoring. The systematization of these
lessons contributes to researchers and practitioners
anticipating possible issues and highlighting some
strategies to overcome them.
This paper is outlined as follows: Section 2 dis-
cusses similar studies focused on lessons learned from
HEALTHINF 2023 - 16th International Conference on Health Informatics
156

mHealth data monitoring; Section 3 details the lon-
gitudinal study design; Section 4 briefly exposes the
results obtained by the Machine Learning regressors;
then, Section 5 discusses the volunteers’ perceptions
using the Technology Acceptance Model; Section 6
presents all challenges and limitations faced in the
study; then, Section 7 summarizes lessons learned
and strategies to address possible challenges related
to health monitoring “in the wild”; and, finally, the
Section 8 brings final remarks and future work.
2 RELATED WORK
To compose our related work, we employed Google
Scholar to search for relevant publications using the
terms “lessons learned” and “mobile health monitor-
ing”. Though this search strategy does not cover a
wide range of terms, it found papers appropriate to
situate the reader about what has been developed in
this area. The similarity to our proposal was applied
as the primary filter, and the papers were sorted by
Google Scholar relevance metric.
Aranki et al. (2016) present a physical activity
monitoring system for patients with chronic heart fail-
ure. Similar to our work, they conducted a pilot study
with 15 participants in the real world. Among the
main lessons learned, the authors highlight that the
behavior of patients is neither static nor uniform and
that patients tend to suffer fatigue in using technol-
ogy. In addition, they discuss aspects related to bat-
tery consumption and the privacy of sensitive data.
The main difference between this study and ours is
that the data were collected only from smartphones
that should be located on the right hip at the waistline
level, which is not typical for users.
Bravo et al. (2018) describe mobile health as an
emerging field capable of transforming how people
manage their health. In this work, the authors discuss
lessons from the experiences obtained from mHealth
development by the MAmI Research Lab. However,
unlike our work, the lessons focus on developing and
representing data in mHealth systems. Also, the ex-
periences are diluted throughout the sections.
L’Hommedieu et al. (2019) provide recommen-
dations for conducting longitudinal sensor-based re-
search using both environmental sensors and wear-
ables in healthcare settings. Among the recommenda-
tions, it is possible to highlight the need to build trust
with the key stakeholders and volunteers and moni-
tor the data collected to identify possible issues in the
sensors. Although this work is similar to ours, the
recommendations presented in this paper are comple-
mentary and could compose a more comprehensive
set of recommendations.
Finally, Gjoreski et al. (2021) systematically com-
pare machine learning approaches when applied to
cognitive load monitoring with wearables and sum-
marize the learnings related to a machine learning
challenge. The recommendations presented by the au-
thors are relevant since there is a trend in using intel-
ligent algorithms to provide mHealth services.
3 STUDY DESIGN
In order to understand the lessons presented as the re-
sult of this paper, it is essential to figure out how our
longitudinal study was conducted.
This evaluation aimed to analyze the QoL infer-
ence process in physical and psychological domains,
using data collected from smartphones and commer-
cial wearables. These two QoL domains were chosen
from the observation that a large amount of data col-
lected by mobile devices can provide insights into the
users’ QoL (Ghosh et al., 2022). The physical do-
main assesses motor facets such as daily activities,
medicines dependence, mobility, sleep quality, and
work capacity. The psychological domain is related
to body image, negative and positive feelings, self-
esteem, and other mental health aspects (Orley and
Kuyken, 1994).
The evaluation was conducted to assess the feasi-
bility of Quality of Life inference concerning errors
(Mean Absolute Error and Root Mean Squared Error)
obtained by the machine learning regressors using as
a reference value the WHOQOL-BREF in the context
of independent adults.
3.1 Participants
Thirty adults were invited to participate as volunteers
given the following criteria:
a. age between 18 and 65 years;
b. prior knowledge in the use of smartphones;
c. availability for continuous use of wearables.
However, only 21 completed the study. Seven
accepted our terms but did not start due to lack of
availability or devices’ incompatibility (e.g., iOS de-
vices). In addition, one participant dropped out after
the initial setup reporting that he/she could not use
the wearable continuously, and another dropped out
in the middle of the study because he/she had a wrist
allergy.
The participants’ invitation prioritized members
of our research laboratory (due to COVID-19 restric-
Lessons Learned from mHealth Monitoring in the Wild
157
tions) and those who had a smart band or smart-
watch. This last criterion was essential to reduce
costs. Therefore, after accepting the invitation, the
procedure for starting the study had six steps:
1. agreeing to the informed consent form;
2. answering the WHOQOL-BREF supported by the
responsible researcher to clarify possible issues;
3. configuring the wearable to sync data;
4. installing the QoL Monitor app;
5. granting permissions to monitor health data;
6. effectively initiates monitoring.
After this initial procedure, participants were in-
structed to follow their activities normally.
The final profile of these participants comprises 15
men and six women aged between 19 and 47. Almost
half of the participants are single, and the other half
are married. Most of them have postgraduate degrees
and are full-time workers. Regarding income, ten (10)
participants reported receiving between 2 and 4 mini-
mum wages (Brazilian minimum wage R$ 1,100 was
used as the reference), and all claimed to live in an
urban area. Regarding the family arrangement, most
participants live with 1 or 2 more people at home,
and there are two large groups in terms of the num-
ber of children: those who do not have children (12)
and those who have 1 or 2 children (9).
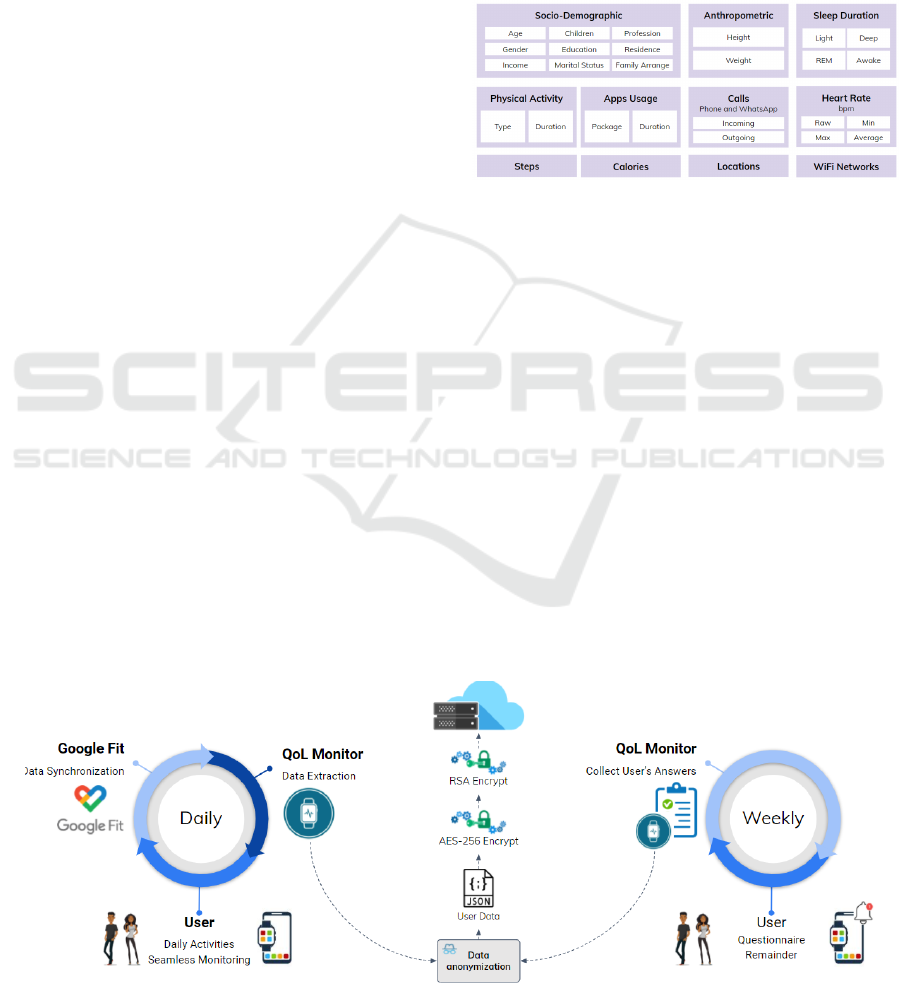
3.2 Data Collection
Data were collected daily and sent anonymously to
the cloud (Figure 2). Weekly, the QoL Monitor
app (developed for this work) warned the participant
to answer the WHOQOL-BREF only with questions
about the physical and psychological domains. This
data was also sent anonymized to the cloud.
Figure 3 highlights the data collected. Socio-
demographic and anthropometric data are needed to
understand the characteristics of the users. The other
raw data directly correlates with the physical and psy-
chological QoL domains. Also, all of them can be ob-
tained through common devices such as smart bands
and smartwatches. Additionally, it is worth mention-
ing that the location data only stores the number of
points visited throughout the day, i.e., the application
does not record the specific places. The same logic
was applied to the WiFi Networks field. This strategy
was adopted to preserve the users’ privacy.
Figure 3: Raw data collected from users.
Figure 4 puts light on how the training instances
are created. A sample has as predictors all data col-
lected from 18:00 of the previous day to 17:59 of the
current day. We decided to use this time slot because
the last night’s sleep directly impacts the current day’s
activities (Arora et al., 2020). The value to be pre-
dicted is obtained after answering the questionnaire
on Sundays. As the user must answer this question-
naire considering the past week, we can use this value
as a reference. Naturally, during the data collection,
some issues can arise (e.g., absence of network or bat-
tery issue). In this case, if the data is not recorded,
such intervals do not generate new training instances.
3.3 Operation
After obtaining the raw data, preprocessing activi-
ties are performed to prepare our dataset. Among
Figure 2: Data flow to collect health measures and self-reported QoL questionnaires.
HEALTHINF 2023 - 16th International Conference on Health Informatics
158

Figure 4: A representation of how the instances are created.
these activities are removing inconsistencies and out-
liers, data stratification, categorical variables encod-
ing, data sync, and computation of QoL scores based
on the questionnaire responses. Thus, at the end of the
data preparation, two datasets are obtained: a dataset
in which the last column is the QoL score for the
physical domain and a similar dataset for the psycho-
logical domain. The last column changes because it
is used as a reference for the learning process.
For the Machine Learning modeling, we decided
to use the Scikit-learn toolbox (Pedregosa et al., 2011)
due to its high acceptance in the scientific community
and the consistency of its results (Tanaka et al., 2019)
(G
´
eron, 2019). Then, four algorithms were selected
based on G
´
eron (2019) guidelines: Linear Regres-
sion, Decision Tree Regressor, Random Forest Re-
gressor, and GBoost Regressor.
The first algorithm search for linear relationships
within the dataset. It is considered a simple model
and an excellent choice to start investigating regres-
sion problems (Ian and Eibe, 2005). The second al-
gorithm is robust compared to linear regression and
can find nonlinear relationships in the data. The third
algorithm uses the concept of random forests to train
multiple decision trees. This algorithm performs well
for a wide variety of problems (Paul et al., 2018). Fi-
nally, the last algorithm uses gradient descent to mini-
mize the error function. In addition, this investigation
did not explore the hyperparameters strategy, and only
the default parameters were used.
We selected three metrics to assess our results:
Mean Absolute Error (MAE) and Root Mean Squared
Error (RMSE), to evaluate the error of the inference
algorithm and the time in seconds to estimate the
computational resource needed for training. MAE
measures how far the predictions are from the correct
output, and RMSE measures the square root of the
square of the differences between the predicted and
accurate values. The latter is one of the most used
metrics to evaluate regressors (Ian and Eibe, 2005).
After data collection and processing, we per-
formed a 10-fold cross-validation of four Machine
Learning techniques using the Scikit-learn toolbox.
4 RESULTS
Tables 1 and 2 summarize the results achieved for
each of the study metrics.
Table 1: Results for the physical QoL dataset.
ML Techniques
Physical Dataset
MAE RMSE
Linear Regression 6.5866 ± 1.7582 8.8457 ± 2.9102
Decision Tree 6.1465 ± 1.6188 9.3685 ± 2.7071
Random Forest 4.9477 ± 1.5283 7.2215 ± 3.0008
GBoost 4.9569 ± 1.4472 6.9191 ± 2.6899
In both datasets, the training time grows as the
classifier complexity increases. Naturally, the er-
rors tend to decrease with more robust classifiers.
However, there are exceptions. For example, the
model created by Random Forest for the Psychologi-
cal dataset had more minor errors than the metrics ob-
tained by GBoost. In this case, we can conclude that
the Random Forest and GBoost algorithms presented
similar results but with very different computational
costs. GBoost takes much longer to train. Thus, we
decided to use Random Forest as our reference.
Table 2: Results for the psychological QoL dataset.
ML Techniques
Psychological Dataset
MAE RMSE
Linear Regression 8.1918 ± 1.9133 10.6146 ± 2.4728
Decision Tree 5.8000 ± 1.7678 9.5880 ± 2.3525
Random Forest 4.6830 ± 1.2204 6.8838 ± 2.2436
GBoost 4.9707 ± 1.3524 7.0034 ± 2.2327
Using MAE and RMSE, we can state that the error
obtained by the classifiers is reasonable on a scale that
Lessons Learned from mHealth Monitoring in the Wild
159
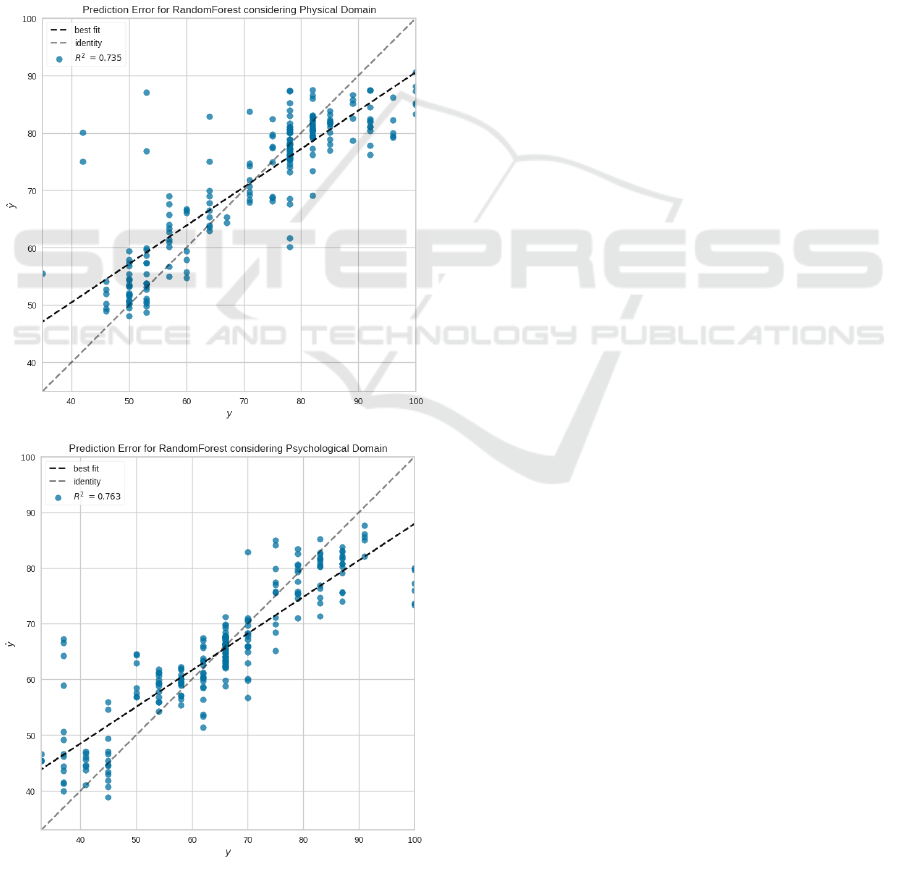
varies from 0 to 100. For example, considering the
RMSE metric for the physical dataset, Random Forest
has a mean error of 7.2215 with a standard deviation
of 3.0008.
Figure 5 brings the prediction error plot for the
Random Forest regressor considering both datasets.
This kind of plot has the actual (represented by the x-
axis) and predicted (represented by the y-axis) values
generated by the model. Thus, it is possible to analyze
the model variance. For example, the 45° degree gray
line (identity) represents a perfect scenario where the
predictor perfectly matches the actual values. In our
case, the prediction errors are distributed close to this
line, with few outliers. The graph also contains the
black line with the best fit obtained by the regressor.
Figure 5: Prediction error plots for RandomForest.
Furthermore, it is possible to observe (in Figure 5)
that the model obtained R
2
equal to 0.735. This met-
ric evaluates the performance of regressors consider-
ing the percentage of the sum of errors concerning the
mean error. In the worst case, R
2
is equal to zero, and
in the best scenario, it is equal to 1. This explanation
reinforces the claim that the results obtained in this in-
vestigation are satisfactory. We argue that the results
should improve once we get a more robust database
and algorithms with adjusted parameters.
5 TAM EVALUATION
At the end of the participant monitoring period, we
decided to apply a final survey developed based on
TAM3 (Technology Acceptance Model 3) (Venkatesh
and Bala, 2008) to collect feedback about the study
and concerning the tool used to monitor Quality of
Life. TAM helps in understanding aspects related to
the adoption of new technologies.
Thus, the applied questionnaire was subdivided
into four groups, each exploring an aspect. The as-
pects analyzed were: i) perceived usefulness; ii) per-
ceived ease of use; iii) self-efficacy when using the
tool; iv) intention to use the tool. Five possible al-
ternatives for each question were: a) I fully agree; b)
I partially agree. c) neutral; d) partially disagree; e)
I totally disagree. In the end, an open question was
included to include perceptions about the study.
Figure 6 presents the quantitative results of the
participants’ answers. It is worth mentioning that
the questionnaire was administered anonymously and
that only 13 of the 21 participants responded.
Regarding perceived usefulness, most participants
agreed that the QoL Monitor tool – previously de-
scribed by Oliveira et al. (2022c) – is helpful for
QoL monitoring. However, there was a partial dis-
agreement regarding the reduction in monitoring cost,
probably associated with the user’s need to use some
wearable to complement the data collected.
As for the perceived ease of use, most volunteers
considered the interaction clear and did not require
much mental effort. This result was expected because
the app was designed to simplify user interactions.
The third aspect observed was self-efficacy. In this
aspect, the aim was to assess the users’ ability to use
the tool in situations with little or no prior instruction.
In the results, it was possible to observe that some
users disagreed about the possibility of being able to
monitor their Quality of Life only with the support of
the tool or having used similar tools. Therefore, this
shows that initial training is necessary for people to
understand aspects related to QoL monitoring.
HEALTHINF 2023 - 16th International Conference on Health Informatics
160
1. Using the QoL Monitor would make easier to monitor your Quality of Life
69% (9) 23% (3)
8% (1)
I fully agree Partially agree Neutral Partially disagree I totally disagree
2. Using QoL Monitor tool would reduce QoL monitoring cost
92% (12) 8% (1)
3. QoL Monitor would make monitoring your QoL transparent in your routine
69% (9) 31% (4)
4. I consider the QoL Monitor useful for monitoring Quality of Life
85% (11) 15% (2)
5. My interaction with the QoL Monitor was clear and understandable
69% (9) 31% (4)
6. Interacting with the QoL Monitor doesn't require a lot of mental effort
77% (10) 23% (3)
7. QoL Monitor is easy to use
85% (11) 8% (1) 8% (1)
8. I find it easy to use the QoL Monitor for monitoring QoL
62% (8) 38% (5)
I could monitor my QoL using the QoL Monitor:
9. if there was no one close to me to provide me instructions
46% (6) 46% (6) 8% (1)
10. if someone showed me how to do it
77% (10) 16% (2) 8% (1)
11. if I used similar tools before
38% (5) 15% (2) 31% (4) 15% (2)
12. if I only had the help feature built into the application
38% (5) 38% (5) 8% (1) 8% (1) 8% (1)
13. Since I have access to the QoL Monitor, I will probably use it
38% (5) 62% (8)
14. I would rather use the QoL Monitor than other QoL monitoring tools
54% (7) 38% (5) 8% (1)
Perceived
Usefulness
Perceived
Ease of Use
Intention
to Use
Self-efficacy
Figure 6: Results of the TAM questionnaire.
To conclude the quantitative results, most partici-
pants stated that they would use QoL Monitor again
instead of other similar tools.
In addition to the quantitative results, we summa-
rized some qualitative perceptions of the volunteers
regarding the difficulties they faced throughout the
study. Such perceptions were organized into three
groups: discomfort, privacy, and access to data. Fig-
ures 7 and 8 present the original comment in Por-
tuguese and its translation into English.
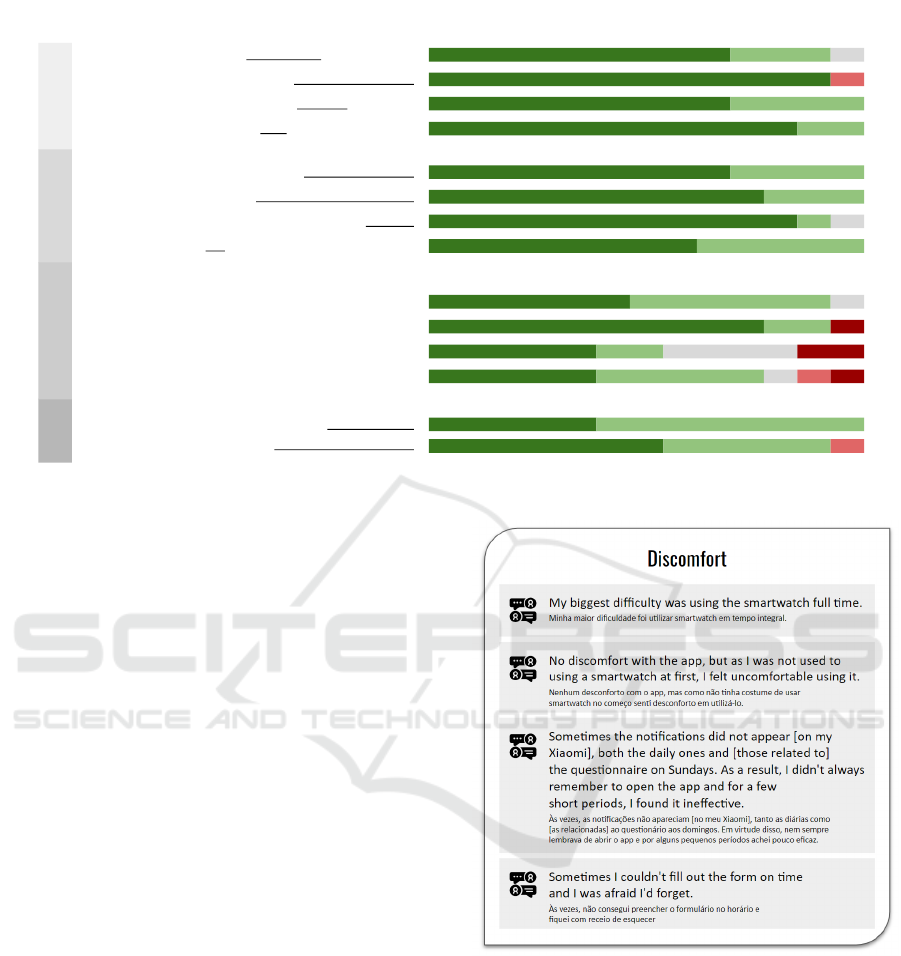
Regarding discomfort, the participants reported
difficulties in using the device uninterruptedly and
keeping the routine of filling in the surveys. In ad-
dition, on some devices, users reported issues with
receiving notifications due to restrictive policies con-
cerning background apps. For sure, this discomfort
can bias the collected data. Therefore, it is essential
to have strategies to reduce it.
Some participants reported that data collection
was a bit invasive. This perception is probably related
to the large amount of requested data and the need to
grant many permissions. However, machine learning
models would only perform satisfactorily with this
massive data. Therefore, we have comforted partici-
pants about data privacy using anonymization and en-
crypted request.
Finally, some participants reported issues in ex-
tracting the data from wearables. Usually, commer-
cial wearables do not deliver methods to access their
data. Thus, we decided to extract data through the
Google Fit platform. Nevertheless, some wearables
apps did not allow native integration with Google Fit,
Figure 7: Participants’ comments about discomfort.
requiring a third-party app to extract this information.
The complexity of this process frustrated volunteers
who used Samsung devices.
6 CHALLENGES
Facing challenges and limitations are common in the
empirical studies (Wohlin et al., 2012), and their dis-
cussion reinforces the work’s reliability since it is pre-
sented the main issues and strategies to mitigate them.
Also, this discussion represents a valuable contribu-
Lessons Learned from mHealth Monitoring in the Wild
161

Figure 8: Participants’ comments regarding privacy and
data access.
tion to researchers and practitioners who work or wish
to work in this investigation area. The scenario of
this paper has particular value due to the high inter-
est in developing mobile health monitoring solutions
(Oliveira et al., 2022a).
Based on the challenges discussed in this section,
it is possible to anticipate or even avoid issues when
conducting this kind of study. Therefore, this section
has organized the challenges discussion based on the
evaluation phases.
The conducting phase had many challenges. The
first was related to the participants’ recruitment. We
decided to recruit thirty (30) adults between the ages
of 18 and 65 since they usually have prior knowl-
edge of using smartphones and smartwatches. Due
to the restrictions imposed by the COVID-19 pan-
demic, we initially sought out members of our re-
search group (GREat/UFC) who already own a smart
band or smartwatch. Thus, the recruitment process
could be completely remote. However, only six par-
ticipants met such restrictions. Then, it was necessary
to expand the recruitment to close people (consider-
ing our social network). Even so, that number only
increased to nine participants.
Thus, purchasing some devices (Xiaomi Mi Band)
and sending them to interested participants was neces-
sary. Furthermore, the shipping and delivery logistics
delayed the start of data collection and increased the
study cost. Finally, some recruited participants with-
drew after the initial presentation, citing lack of time
and others having a smartphone incompatible with
our app (e.g., phones with the iOS system). There-
fore, despite our efforts, this evaluation’s relatively
low number of participants (21) is a limitation that
should be addressed in future studies.
After recruiting participants, we started collect-
ing data. During this step, we faced many issues re-
lated to noise in data collection. For example, the ab-
sence of an Internet connection when sending data,
devices without battery charge, sensors turned off,
and sensors or devices with different levels of accu-
racy. These situations caused noise in our registry,
making it difficult to clean the data.
Regarding failures in sending data to the cloud, it
was necessary to implement a mechanism in order to
perform retries on the connection and, after five failed
attempts, internally store the data for sending in the
next day. As for the disconnecting sensors, we warned
the participants about the continuous use of the de-
vices and about the need to keep at least GPS active.
However, we received feedback from participants that
the seamless GPS use increased battery consumption.
Therefore, some participants turned it off in moments
of low battery. Consequently, it was necessary to filter
inconsistencies during data processing.
Regarding charge frequency, we guided the par-
ticipants to charge their smartphones daily and their
wearables weekly. However, when charging the de-
vices, a data gap is created. Then, such gaps were
removed from the study.
Another area for improvement in our evaluation is
the non-standardization of using the same device for
monitoring. In an ideal scenario, all volunteers should
use the same device model to reduce inconsistencies
regarding the quality of the collected data. For exam-
ple, two different smart bands may differ in the num-
ber of steps recorded due to the detection algorithm.
However, budget limitations prevented all participants
from using the same devices. Even so, we decided to
proceed with the investigation because we understand
that it is impossible to guarantee that all users will use
similar devices in the real world.
We have also faced difficulties in user engage-
ment. Achieving and maintaining engagement in
healthcare technologies is such a complex challenge.
Because of that, many studies have been conducted
to find proper strategies to keep users active in an or-
ganic way (Wang et al., 2022) (Ganesh et al., 2022).
Our study faced engagement challenges, as users
had to follow a series of recurring actions, such as
opening a wearable app daily to ensure data sync
and weekly answering the QoL questionnaire. Even
with the application’s support to remind these actions,
we observed that at least one-third of the participants
failed to perform the questionnaire more than once.
HEALTHINF 2023 - 16th International Conference on Health Informatics
162

When investigating what could be happening, some
participants reported that day-to-day activities made
them forget. It was also clear that despite recognizing
the benefits of daily health monitoring, many partici-
pants ignored them and forgot to access the app’s no-
tifications. This challenge needs further investigation
to understand the real reasons for this lack of engage-
ment and what strategies can be used to overcome it.
We also received several reports of problems clas-
sified as “real-world issues”. For example, one par-
ticipant reported that he lost the smart band during
a bath in the sea; two participants reported that the
smart band was causing a wrist allergy and, there-
fore, they had to stop using it for a few days. An-
other participant caught chikungunya, which affected
his joints, preventing him from using the wearable for
a few days. These problems are inherent to “in the
wild” studies, and there are few strategies to avoid
such situations. We adopted a specific approach for
each of them, but, in general, they all generated data
gaps that were eliminated during preprocessing.
In the analysis of the results, we faced a data vari-
ability issue. This issue happens because the profile
of study participants has little variability. So, many
records have intermediate QoL scores and few high
or low scores. Consequently, this impairs the regres-
sors’ ability to generalize.
We expect to conduct a new assessment with more
participants (up to 100 members) to address this lim-
itation, varying the subjects’ profiles. Also, we ex-
plored a few algorithms (only four), as this was just
a Proof-of-Concept study. However, we understand
that it is necessary to expand the number of evaluated
Machine Learning techniques and include many repe-
titions (not only k-fold validation) in the experiments
to perform statistical tests.
7 LESSONS LEARNED
This section presents and discusses ten lessons
learned from conducting a longitudinal investigation
to monitor the Quality of Life using the Internet of
Health Things and Machine Learning. The lessons
were organized with a title, a short description, and
alternatives to overcome it. Finally, it is presented a
5W1H table to summarize this discussion.
- Study Design needs to be Carefully Validated
The planning phase is crucial for adequately conduct-
ing health monitoring studies “in the wild”. It is also
decisive in the approval by the ethics committee. On
the other hand, the absence of a rigorous planning
process can invalidate the data collected and increase
research costs. Thus, a possible alternative to vali-
date the planning is to conduct pilot studies. Accord-
ing to Van Teijlingen et al. (2010), pilot studies re-
fer to mini versions of a full-scale investigation, and
they can identify potential practical problems in the
research procedure.
- Data Privacy Must be a Priority
Currently, laws and regulations protect digital health
users from mishandling data (Purtova et al., 2015). In
this sense, privacy must be prioritized to create trust
with the volunteers. Moreover, from the feedback col-
lected in our qualitative assessment, it became evident
that participants will be hesitant to use an invasive
technology without a robust process for keeping their
data secure. In this regard, a good alternative is to use
data anonymization (Sneha and Asha, 2017). Another
option is to avoid using data that makes it possible to
re-identify the user, such as location or Internet access
data.
- Volunteer Engagement Requires Attention
from Beginning to End of the Study
Recruiting participants is not easy; keeping their en-
gagement is even more problematic. Thus, research
involving health data monitoring has the significant
challenge of finding volunteers. Regarding this chal-
lenge, a helpful strategy is establishing partnerships
with universities or health centers and making key
people in these organizations aware of the work’s rel-
evance. These people should become ambassadors to
attract volunteers. In addition, it is crucial to consider
strategies to keep volunteers committed until the end
of the work, for example, rewarding students who re-
main active or even gifting a wearable to those with
high engagement.
- The Technology Discomfort can be a Bias
Monitoring health data requires sensors (Rodrigues
et al., 2018). Such sensors can be wearable like
smart bands and smart rings, personal devices such
as smartphones, or even instruments fixed in the envi-
ronment such as cameras and infrared sensors. Dur-
ing planning, the researcher should decide which sen-
sors will be used and how to collect the data (us-
ing native apps, for example). For this decision, it
must be taken into account possible discomforts for
the users. For example, even commercial devices al-
ready established on the market, such as smart bands,
can provoke wrist-related allergies. Thus, it is rec-
ommended to investigate whether the participants are
already used to the selected technology to avoid dis-
comfort and, consequently, bias in the data.
- The Project Budget needs to be Considered
when Selecting Devices for Monitoring
Lessons Learned from mHealth Monitoring in the Wild
163

As stated before, the researcher must select sensors
for data collection during planning. Among the crite-
ria for this selection are the number, variety, and ac-
curacy of measurements, battery consumption, ease
of use, market availability, data access, and durabil-
ity. However, while conducting our case study, we
realized that the project budget is a vital criterion in
this selection. In general, most volunteers do not have
these devices, and even for those that have, there is the
problem of non-standardization since different brands
and models can cause inaccurate data. In addition,
as this type of study requires many participants, our
strategy was to opt for a low-cost device that would al-
low us to obtain the necessary measurements. Thus, it
would be possible to include a more significant num-
ber of participants. Therefore, we opted for the Xi-
aomi Mi Smart Band, which costs approximately 39
dollars in Brazil.
- Extracting Data from Wearables is Complex
A significant challenge for those who work with wear-
able devices is data extraction (Oliveira et al., 2022b).
If the researcher chooses to build their own device,
this new technology can face many additional issues
due to the lack of maturity. On the other hand, few
commercial wearables have methods for extracting
data. Furthermore, those commercial wearables that
share Web APIs to retrieve data tend to have a higher
cost, such as smartwatches with Android Wear or
Fitbit devices. An alternative is to look for devices
that allow data synchronization with platforms such
as Google Fit (for Android) and HealthKit (for iOS)
(Oliveira et al., 2022a). Such platforms were designed
to centralize users’ health information and have well-
documented APIs for data extraction.
- Data Collected “in the wild” Always has Noise
Monitoring patients in a controlled environment al-
lows the researcher or professional to establish the re-
quired minimum parameters for the system. It is pos-
sible, for example, to guarantee that the devices will
always have access to the Internet or even that there
will be no lack of battery supply. On the other hand,
collecting health data in real life (uncontrolled envi-
ronment) implies noise in the data. For example, data
gaps will be generated when removing devices for
charging. Also, synchronization problems can make
it impossible to record specific measures. Again, an-
other situation that can occur in studies that include
self-reported surveys is the user forgetting to answer
the survey. In addition to these examples, a wide va-
riety of other situations can occur; unfortunately, it
is impossible to avoid them all. Therefore, a suitable
strategy to deal with these issues is to intensify the ef-
fort dedicated to data cleaning and processing. This
step is crucial to remove noise.
- Constant Internet Access Cannot be a Premise
As stated by Rodrigues et al. (2018), IoHT architec-
ture for healthcare monitoring systems involves col-
lecting data by sensors and sending it to robust nodes
for processing and analysis. It is common for these
nodes to be at the edge or in the cloud. However, the
periodic sending of data cannot presuppose continu-
ous access to the Internet. In uncontrolled environ-
ments, it is common to have unavailable access for
a while, resulting in failures in sending data. In this
way, it is essential to implement strategies for resend-
ing in case of failure or even temporary storage for
later sending. This strategy should prevent informa-
tion loss.
- Getting Feedback Should be Uninterrupted
After recruiting the participants, we held a session
to explain the study operation, configure the devices,
clarify doubts and sign the informed consent form.
On this occasion, we made it clear that the partici-
pants were free to withdraw at any time and that we
would be available to obtain feedback throughout the
monitoring period. Unfortunately, only some volun-
teers kept the practice of continuous feedback. In our
case, only using the final evaluation questionnaire was
possible to extract qualitative data, and probably some
details may have been lost. Thus, it is crucial to en-
courage volunteers to provide periodic feedback. For
future studies, we will leave an anonymous form open
from the beginning to the end of the research and ask
them to keep feeding whenever they face a positive or
adverse situation.
- Unexpected Problems Should Arise
Finally, the researcher must be prepared for unex-
pected issues. For example, a device being stolen
from the user or even a volunteer getting sick and hav-
ing to withdraw. Unfortunately, there is no specific
approach to dealing with these problems. However,
it is essential to keep the research team watchful to
reverse them as soon as possible.
Table 3 summarizes the lessons learned in this
study using the 5W1H model (in this case, who and
where were suppressed because the research team al-
ways conducts activities and location is not applied).
8 FINAL REMARKS
This paper presents ten (10) practical lessons from
mHealth monitoring of twenty-one (21) volunteers
over three months. The main contribution of these
lessons is that future studies can use this knowledge
HEALTHINF 2023 - 16th International Conference on Health Informatics
164
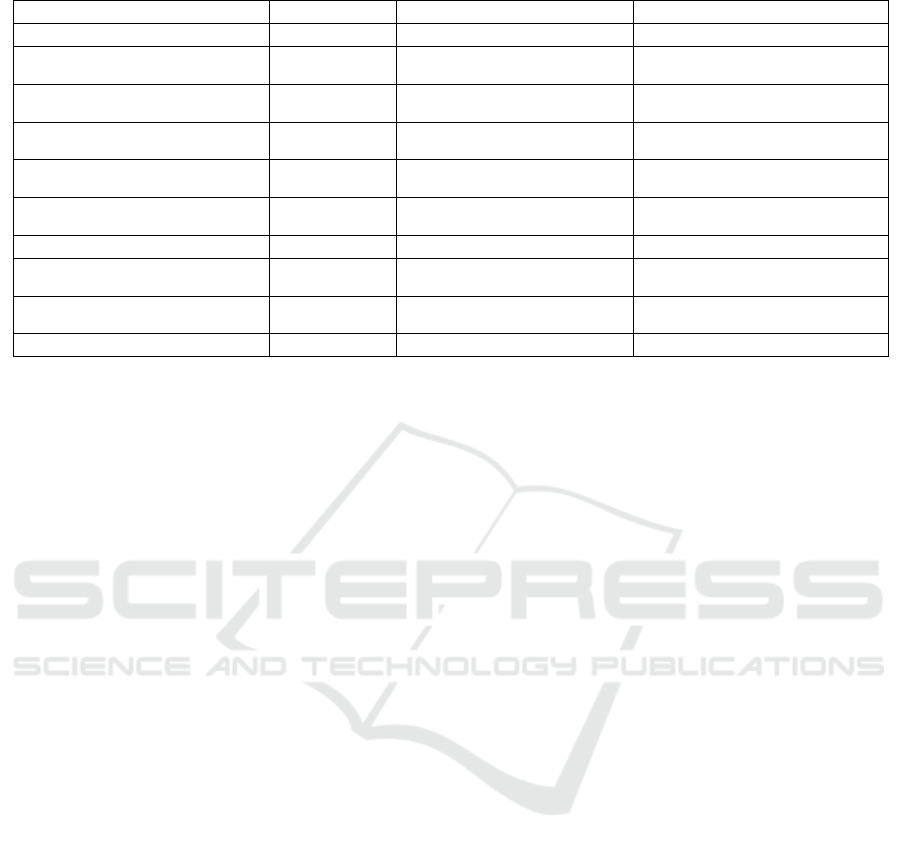
Table 3: Summarized lessons learned.
What When Why How
Study design needs to be validated Planning It can increase project costs Conducting pilot studies
Data privacy must be a priority Planning and
Recruiting
It can hamper recruitment, in addition to
legal issues
Anonymizing data and making privacy
policies clear
Volunteer engagement requires attention From the beginning
to the end
It can lead volunteers to withdraw Encouraging volunteer participation
The technology discomfort can be a bias Planning, and
Conduction
It can insert bias in data Selecting usual technologies
Project budget needs to be considered when
selecting devices
Planning It can increase project costs Weighing the cost against the device resources
required by the study
Extracting data from wearables is complex Conduction Without data, there is no health monitoring Using health data hub platforms like Google
Fit and Health Kit
Data collected “in the wild” has noise Conduction Noise can lead to inaccuracies Investing in cleaning and processing activities
Constant Internet access cannot be a premise Planning, and
Conduction
It can cause data loss Implementing data sending retries and data
staging
Getting feedback should be uninterrupted From the beginning
to the end
To avoid missing relevant feedback Allowing continuous sending of anonymous
feedback
Unexpected problems should arise Conduction To ensure proper study conduction Keeping the research team on its toes
to plan how to mitigate common issues related to
mHealth monitoring “in the wild” (i.e., uncontrolled
environments). As future work, we expect to refine
this learning by replicating this study.
ETHICAL APPROVAL
Our project (nº 56153322.0.0000.5054) was approved
by the coordination of the ethics committee located at
the Federal University of Cear
´
a (UFC). Furthermore,
it complied with CONEP laws and followed all the
international ethical standards. Finally, all volunteers
signed the informed consent document.
DATA AVAILABILITY
The QoL dataset is proprietary of GREat lab and is
not available for public use yet. However, the project
submitted to the ethics committee, the notebooks used
to process data, and the higher-resolution images are
available in https://bit.ly/3SSlkqs.
ACKNOWLEDGMENTS
The authors would like to thank National Council for
Scientific and Technological Development (CNPQ)
for the Productivity Scholarship of professor Rossana
Andrade DT-1D (N
o
306362 / 2021-0) and professor
Pedro Santos Neto DT-2 (N
o
315198 / 2018-4).
REFERENCES
Akter, S. and Ray, P. (2010). mhealth-an ultimate platform
to serve the unserved. Yearbook of medical informat-
ics, 19(01):94–100.
Aranki, D., Kurillo, G., Yan, P., Liebovitz, D. M., and Ba-
jcsy, R. (2016). Real-time tele-monitoring of patients
with chronic heart-failure using a smartphone: lessons
learned. IEEE Transactions on Affective Computing,
7(3):206–219.
Ara
´
ujo, I., Pereira, M. B., Silva, W., Linhares, I., Marx, V.,
Andrade, A. M., Andrade, R. M., and de Castro, M. F.
(2022). Machine learning and cloud enabled fall de-
tection system using data from wearable devices: De-
ployment and evaluation. In Anais do XXII Simp
´
osio
Brasileiro de Computac¸
˜
ao Aplicada
`
a Sa
´
ude, pages
413–424. SBC.
Arora, A., Chakraborty, P., and Bhatia, M. (2020). Analysis
of data from wearable sensors for sleep quality esti-
mation and prediction using deep learning. Arabian
Journal for Science and Engineering, 45(12):10793–
10812.
Bostrom, J., Sweeney, G., Whiteson, J., and Dodson, J. A.
(2020). Mobile health and cardiac rehabilitation in
older adults. Clinical Cardiology, 43(2):118–126.
Bravo, J., Herv
´
as, R., Fontecha, J., and Gonz
´
alez, I. (2018).
M-health: lessons learned by m-experiences. Sensors,
18(5):1569.
Dohr, A., Modre-Opsrian, R., Drobics, M., Hayn, D., and
Schreier, G. (2010). The internet of things for am-
bient assisted living. In 7th int. conf. on information
technology: new generations, pages 804–809. IEEE.
Elkinton, J. R. (1966). Medicine and the quality of life.
Annals of Internal Medicine, 64:711–714.
Ganesh, D., Balaji, K. K., Sokkanarayanan, S., Rajan, S.,
and Sathiyanarayanan, M. (2022). Healthcare apps
for post-covid era: Trends, challenges and potential
opportunities. In 2022 IEEE Delhi Section Conference
(DELCON), pages 1–7. IEEE.
Lessons Learned from mHealth Monitoring in the Wild
165

G
´
eron, A. (2019). M
˜
aos
`
a Obra: Aprendizado de M
´
aquina
com Scikit-Learn & TensorFlow. Alta Books, Rio de
Janeiro.
Ghosh, S., L
¨
ochner, J., Mitra, B., and De, P. (2022). Your
smartphone knows you better than you may think:
Emotional assessment ‘on the go’via tapsense. Quan-
tifying Quality of Life: Incorporating Daily Life into
Medicine, page 209.
Gjoreski, M., Mahesh, B., Kolenik, T., Uwe-Garbas, J.,
Seuss, D., Gjoreski, H., Lu
ˇ
strek, M., Gams, M., and
Pejovi
´
c, V. (2021). Cognitive load monitoring with
wearables–lessons learned from a machine learning
challenge. IEEE Access, 9:103325–103336.
Hao, T., Walter, K. N., Ball, M. J., Chang, H.-Y., Sun, S.,
and Zhu, X. (2017). Stresshacker: towards practical
stress monitoring in the wild with smartwatches. In
AMIA Annual Symposium Proceedings, volume 2017,
page 830. American Medical Informatics Association.
Ian, H. W. and Eibe, F. (2005). Data mining: Practical ma-
chine learning tools and techniques.
Islam, S. R., Kwak, D., Kabir, M. H., Hossain, M., and
Kwak, K.-S. (2015). The internet of things for health
care: a comprehensive survey. IEEE access, 3:678–
708.
Junior, E. C., de Castro Andrade, R. M., and Rocha, L. S.
(2021). Development process for self-adaptive appli-
cations of the internet of health things based on move-
ment patterns. In 9th International Conference on
Healthcare Informatics (ICHI), pages 437–438. IEEE.
Karimi, M. and Brazier, J. (2016). Health, health-related
quality of life, and quality of life: what is the differ-
ence? Pharmacoeconomics, 34(7):645–649.
L’Hommedieu, M., L’Hommedieu, J., Begay, C., Schenone,
A., Dimitropoulou, L., Margolin, G., Falk, T., Ferrara,
E., Lerman, K., Narayanan, S., et al. (2019). Lessons
learned: Recommendations for implementing a longi-
tudinal study using wearable and environmental sen-
sors in a health care organization. JMIR mHealth and
uHealth, 7(12):e13305.
Oliveira, P., Costa Junior, E., Santos, I. D. S., Andrade, R.,
and Santos Neto, P. d. A. (2022a). Ten years of ehealth
discussions on stack overflow. International Confer-
ence on Health Informatics (HEALTHINF1’22).
Oliveira, P. A. M., Andrade, R. M. C., Neto, P. S. N., and
Oliveira, B. S. (2022b). Internet of health things for
quality of life: Open challenges based on a systematic
literature mapping. In 15th International Conference
on Health Informatics (HEALTHINF). INSTICC.
Oliveira, P. A. M., Andrade, R. M. C., Neto, P. S. N., and
Oliveira, B. S. (2022c). Towards an ioht platform to
monitor qol indicators. In 15th International Con-
ference on Health Informatics (HEALTHINF). IN-
STICC.
Orley, J. and Kuyken, W. (1994). The development of
the world health organization quality of life assess-
ment instrument (the whoqol). In Quality of life as-
sessment: International perspectives, pages 41–57.
Springer Berlin Heidelberg, Berlin, Heidelberg.
Palos-Sanchez, P. R., Saura, J. R., Martin, M.
´
A. R.,
and Aguayo-Camacho, M. (2021). Toward a bet-
ter understanding of the intention to use mhealth
apps: Exploratory study. JMIR mHealth and uHealth,
9(9):e27021.
Paul, A., Mukherjee, D. P., Das, P., Gangopadhyay, A.,
Chintha, A. R., and Kundu, S. (2018). Improved ran-
dom forest for classification. IEEE Transactions on
Image Processing, 27(8):4012–4024.
Pedregosa, F., Varoquaux, G., Gramfort, A., Michel, V.,
Thirion, B., Grisel, O., Blondel, M., Prettenhofer,
P., Weiss, R., Dubourg, V., Vanderplas, J., Passos,
A., Cournapeau, D., Brucher, M., Perrot, M., and
Duchesnay, E. (2011). Scikit-learn: Machine learning
in Python. Journal of Machine Learning Research,
12:2825–2830.
Purtova, N., Kosta, E., and Koops, B.-J. (2015). Laws and
regulations for digital health. In Requirements Engi-
neering for Digital Health, pages 47–74. Springer.
Qudah, B. and Luetsch, K. (2019). The influence of mo-
bile health applications on patient-healthcare provider
relationships: a systematic, narrative review. Patient
education and counseling, 102(6):1080–1089.
Rodrigues, J. J., Segundo, D. B. D. R., Junqueira, H. A.,
Sabino, M. H., Prince, R. M., Al-Muhtadi, J., and
De Albuquerque, V. H. C. (2018). Enabling tech-
nologies for the internet of health things. Ieee Access,
6:13129–13141.
Sanchez, W., Martinez, A., Campos, W., Estrada, H., and
Pelechano, V. (2015). Inferring loneliness levels in
older adults from smartphones. Journal of Ambient
Intelligence and Smart Environments, 7(1):85–98.
Sneha, S. and Asha, P. (2017). Privacy preserving on
e-health records based on anonymization technique.
Global Journal of Pure and Applied Mathematics,
13(7):3367–3380.
Tanaka, K., Monden, A., and Zeynep, Y. (2019). Effective-
ness of auto-sklearn in software bug prediction. Com-
puter Software, 36(4):46–52.
Van Teijlingen, E., Hundley, V., et al. (2010). The im-
portance of pilot studies. Social research update,
35(4):49–59.
Varshney, U. (2014). Mobile health: Four emerging themes
of research. Decision Support Systems, 66:20–35.
Venkatesh, V. and Bala, H. (2008). Technology acceptance
model 3 and a research agenda on interventions. De-
cision sciences, 39(2):273–315.
Wang, Z., Xiong, H., Zhang, J., Yang, S., Boukhechba, M.,
Zhang, D., Barnes, L. E., and Dou, D. (2022). From
personalized medicine to population health: A sur-
vey of mhealth sensing techniques. IEEE Internet of
Things.
Wohlin, C., Runeson, P., H
¨
ost, M., Ohlsson, M. C., Reg-
nell, B., and Wessl
´
en, A. (2012). Experimentation in
software engineering. Springer Science & Business
Media.
HEALTHINF 2023 - 16th International Conference on Health Informatics
166
