
Description of PD Phonation in Terms of EEG-Related Frequency
Bands
Pedro Gómez
1a
, Jiří Mekyska
2b
, Luboš Brabenec
3c
, Patrik Šimko
3d
Irena Rektorová
4e
,
Andrés Gómez
5f
and Victoria Rodellar
1g
1
NeuSpeLab, CTB, Universidad Politécnica de Madrid, 28220 Pozuelo de Alarcón, Madrid, Spain
2
Department of Telecommunications, Brno University of Technology, Brno, Czech Republic
3
Applied Neuroscience Research Group, Central European Institute of Technology - CEITEC,
Masaryk University, Brno, Czech Republic
4
First Department of Neurology, Faculty of Medicine and St. Anne’s University Hospital,
Masaryk University, Brno, Czech Republic
5
Usher Institute, Faculty of Medicine, University of Edinburgh, Edinburgh, U.K.
{lubos.brabenec, patrik.simko, irena.rektorova}@ceitec.muni.cz, a.gomezrodellar@ed.ac.uk
Keywords: Parkinson’s Disease, Functional Assessment of Phonation, Neuromotor EEG Activity Monitoring, Repetitive
Transcranial Magnetic Stimulation.
Abstract: Parkinson’s Disease (PD) is an increasing prevalence neurodegenerative condition affecting the life quality
of people suffering from its neuromotor and cognitive performance. PD symptoms include vocalization and
speech alterations, known as hypokinetic dysarthria (HD). One of the manifestations of HD is unstable
phonation. Repetitive Transcranial Magnetic Stimulation (rTMS) is a non-invasive method that may improve
some motor and non-motor symptoms of persons with PD (PwP). The present study concentrates on analyzing
and comparing the phonation behavior of two cases before (pre-stimulus) and after (post-stimulus) ten
sessions of rTMS treatment, to assess the extent of changes in their vocalization. Voice recordings of a
sustained vowel [a:] taken immediately before and after the treatment, and at follow-up sessions (at six, ten,
and fourteen weeks after the baseline assessment) were processed by inverse filtering to estimate a
biomechanical correlate of vocal fold stiffness, which band-pass filtered into EEG-related frequency bands.
Log-likelihood ratios between pre- and post-stimulus amplitude distributions of each frequency band, Mann-
Whitney U-tests, and normalized difference scores showed significant improvements in the actively
stimulated case, which were not observed in the sham case. Early preliminary insights into the capability of
phonation quality assessment on monitoring neuromechanical activity from acoustic signals are shown.
1 INTRODUCTION
Parkinson’s Disease (PD) is a neurodegenerative
disorder with a prevalence of around 200 cases per
100,000 persons, at a growing incidence rate of 15
cases per 100,000 (Dorsey et al., 2007). It has a severe
impact on the life quality of persons with PD (PwP)
a
https://orcid.org/0000-0003-3283-378X
b
https://orcid.org/0000-0002-6195-193X
c
https://orcid.org/0000-0002-8348-5757
d
https://orcid.org/0000-0002-0767-0918
e
https://orcid.org/0000-0002-5455-4573
f
https://orcid.org/0000-0003-3283-378X
g
https://orcid.org/0000-0001-9384-3290
affecting motor and non-motor symptoms (Duffy
2013). Perturbed respiration, phonation, articulation,
and prosody are among motor symptoms hampering
vocalization and speech, in what is known as
hypokinetic dysarthria (HD), characterized by mono-
pitch and mono loudness, imprecise articulation,
impaired speech rate, and rhythm, and irregular pitch
fluctuations. Repetitive transcranial magnetic
226
Gómez, P., Mekyska, J., Brabenec, L., Šimko, P., Rektorová, I., Gómez, A. and Rodellar, V.
Description of PD Phonation in Terms of EEG-Related Frequency Bands.
DOI: 10.5220/0011669100003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 4: BIOSIGNALS, pages 226-233
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
stimulation (rTMS) is a non-invasive method used to
modulate neuronal excitability which has been
proposed as a therapy to improve various symptoms
of PD (Brabenec et al. 2019). The purpose of the
present study is two-fold: on the one hand, to explore
the relationship between neuromotor activity and
phonation through the inversion of a cortico-muscular
coupling (CMC) model (Branbilla 2021); on the other
hand, to show how to characterize and monitor the
efficiency of rTMS in two study cases using CMC
model inversion.
The structure of the paper is as follows. Section 2
is devoted to explaining the conducting narrative
capitalizing on the possibility of describing
phonation-related biomechanical correlates as vocal
fold stiffness in terms of EEG-related frequency
bands based on the well-known relationship between
EEG neuroelectrical activity in the premotor and
supplementary motor cortex and the neuromuscular
activity in the laryngeal nerves controlling voice
production explained by CMC (McKeown et al,
2006). Section 3 describes the signal inversion
methods to determine neuromotor activity in EEG-
related frequency bands, and their statistical
distributions in two cases of PwP submitted to active
and sham rTMS. Section 4 compares the results from
the two study cases, which are discussed in Section 5.
Contributions, findings, and conclusions are
disclosed in Section 6.
2 FUNDAMENTALS
The objective of the present study is to dive into the
relationship between neuromotor and acoustical
activity (neuroacoustical) involved in vocalization
(phonation and articulation) with application to the
characterization of PD hypokinetic dysarthria, as
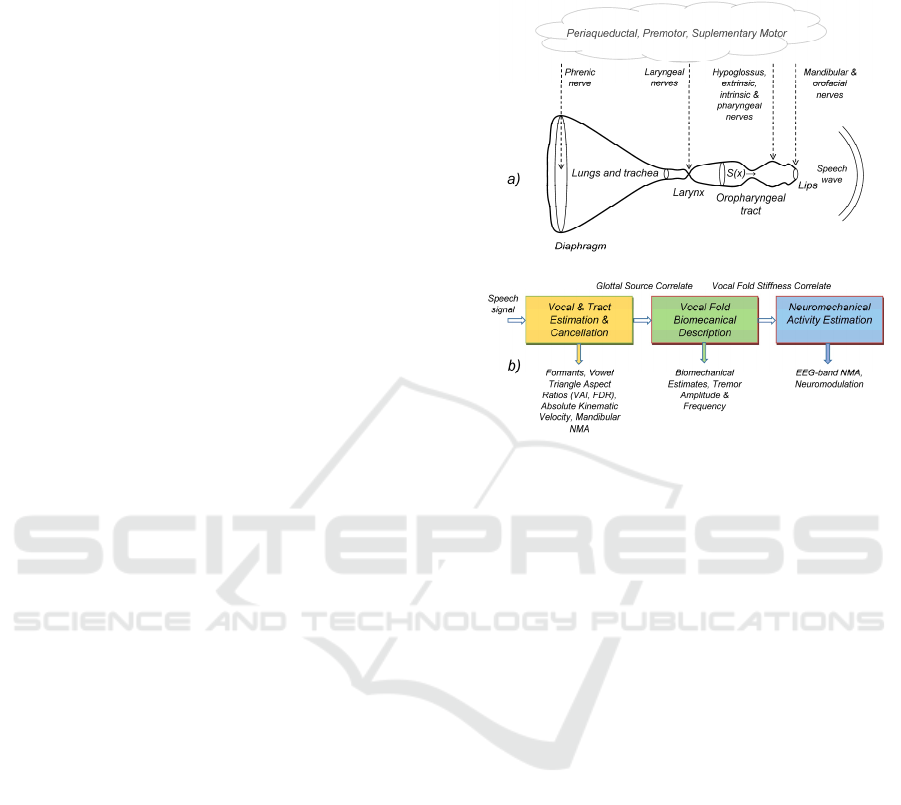
summarized in 0. The simplified neuroacoustical
model (acoustical and neuroneuromotor) of the
system controlling vocalization (top-down view) is
summarized in 0.a. The vocalization structure
involves the lungs, larynx, and the oro-naso-
pharyngeal cavities, considering the lips as the
radiation place where the speech wave is projected to
the surrounding media. Important muscular systems
control each substructure, such as the diaphragm and
intercostal (not shown) controlling lung pressure, the
laryngeal muscles (thyroarytenoid, cricothyroid, and
transverse and oblique arytenoid) of which the
thyroarytenoid (musculus vocalis) is responsible for
blocking and releasing airflow through the glottis and
producing the basic vibration in voiced speech
(phonation), the hypoglossus, extrinsic and intrinsic
glossal, controlling tongue movements, the
mandibular, regulating jaw raising and lowering, and
the orofacial, defining lip rounding gestures.
Figure 1: Acoustical and neuromotor pathways in
vocalization: a) Top-down model from the neuromotor to
the acoustical (speech wave); b) Bottom-up model from
phonation to neuromechanical activity estimation.
The neuromotor activity (NMA) controlling each
group of muscles is driven by common areas of
activation for semantic processing, lexical selection,
syntactic construction as well as oral articulation,
involving mainly the periaqueductal, premotor, and
supplementary motor brain areas, among others
(Schulz et al., 2005). These areas project to the
specific muscles by different neural pathways, such
as the phrenic nerve (diaphragm), the superior and
recurrent laryngeal (laryngeal muscles), the
pharyngeal, hypoglossus, extrinsic, and intrinsic
tongue nerves (oropharyngeal), and the trigeminal
branch controlling mandibular and orofacial muscles.
The inverse bottom-up model shown in 0.b provides
a view of the model inversion methodology proposed
in the present study to estimate and visualize NMA
involved in phonation. The speech signal is processed
by a linear predictive algorithm (Deller, Proakis, and
Hansen, 1993) to produce a glottal source correlate.
The inverse filter used in the processing is used to
estimate acoustic-articulatory features, such as
formants, vowel triangle aspect ratios, tongue-jaw
kinematic movement, masseter NMA, etc. (Gómez et
al., 2021). Among the mentioned larynx muscles, the
thyroarytenoid is of special interest for the study
because it is a very small mass low-inertial muscle,
responding well to vibration frequencies beyond
Description of PD Phonation in Terms of EEG-Related Frequency Bands
227

1kHz, and is directly related to phonation. The study
capitalizes on the estimation of the thyroarytenoid
muscle stiffness on the glottal source correlate, which
is used to produce estimations of the vocal fold
biomechanical features. It is assumed the vocal fold
stiffness to be controlled by the inferior laryngeal
nerve NMA on the thyroarytenoid muscle, therefore,
it may be used to estimate EEG-related frequency
bands in the laryngeal nerve periaqueductal control.
This inverse bottom-up chain could help in better
monitoring phonation control in PwP (Rektorova et
al., 2012). Eventual instabilities of the laryngeal
NMA will appear as oscillations in the frequency
bands (tremors).
3 MATERIALS AND METHODS
The present research includes results from two PwP
participants (under active and sham rTMS) in a study
devised to HD in PwPs (Brabenec et al., 2021). The
NMA of both participants was monitored at several
evaluation periods since stimulation using sustained
emissions of the vowel [a:], and the results have been
compared.
Table 1: Participants’ demographic and clinical data; A:
active stimulation; S: sham stimulation; M: Male Gender;
Y: years. UPDRS-III: Unified Parkinson’s Disease Rating
Scale part III (motor).
PwP code
(pre)
Active/Sham Gender Age (Y) UPDRS
-III
1400 A M 64 10
1900 S M 77 8
The speech processing was based on fragments of
4s long vowel emissions between the time instants at
2s and 6s from the vowel onset, sampled at 16 kHz
and 16 bits. An inverse lattice-ladder filter (Deller,
Proakis, and Hansen, 1993, Alku et al., 2019)
evaluated the oropharyngeal tract model, estimating
the residual prediction error, which once integrated,
produced the glottal source correlate. The vocal fold
stiffness (VFS) was estimated from the glottal source
correlate adjusting its spectral power by a 2-mass
model of the vocal fold biomechanics (Gómez et al.
2009) The VFS was de-biased and de-trended by a
moving-average filter. See 0 for an example of
unbiased VFS (UVFS) estimation from case 1400.
The UVFS was band-pass filtered at the EEG-related
frequency bands (δ: f≤4 Hz; ϑ: 4 Hz<f≤8 Hz; α: 8
Hz≤f≤16 Hz; β: 16 Hz<f≤32 Hz; γ: f>32 Hz; μ: 8
Hz<f≤12 Hz), producing a set of UVFS estimates
given by 𝜉
(𝑛), where i=(0,..., I) is the session index
(I=5), j=a (active case) or j=s (sham case) is the
participant index, and k=(1 for δ,…, 6 for μ) is the
frequency band index of the six frequency bands
defined above, and n is the time index. 0 shows the
results of the band-pass separation in the time domain
(left column) and their power spectrograms (right
column). The distributions p{ξ} of EEG-related
frequency bands were estimated from normalized
amplitude histograms as
𝑝
,
(
𝜉
)
=ℎ𝜉
,
(
𝑛
)
(1)
The distributions from post-stimulus recordings were
compared on their overlap interval ξ ϵ Ω with pre-
stimulus ones using log-likelihood ratios
𝜆
,
(
𝑝
|𝑝
)
= 𝑙𝑜𝑔𝑝
,
(
𝜉
)
𝑝
,
(
𝜉
)
𝑑𝜉
(2)
as well as Mann-Whitney U-tests (T
MW
)
𝑇
,
=𝑇
𝜉
,
(
𝑛
)
,𝜉
,
(
𝑛
)
(3)
and time-weighted scores (𝑠
and 𝑠
)
𝑠
,
=
〈
𝜉
,
(
𝑛
)〉
−
〈
𝜉
,
(
𝑛
)〉
〈
𝜉
,
(
𝑛
)〉
𝑤
;
𝑤
=(𝑑
−𝑑
)(𝑑
−𝑑
)
⁄
(4)
where the weight w
i
is a normalizing factor to take
into account the time interval between each post-
stimulus date d
i
and the corresponding pre-stimulus
date d
0
normalized to the longest interval (d
i
- d
0
) in
days, therefore, long-lasting beneficial effects were
given larger importance than short-duration effects.
The time intervals in days for the case 1400 were
T1=15, T2=47, T3=74, and T4=99. The
corresponding ones for case 1900 were T1=9, T2=42,
T3=65, and T4=96. Finally, the VFS unbalance (the
difference between two neighbor estimates of the
total UVFS relative to their average) is added as a
reference feature indirectly related to the jitter
𝑢
,
=2
𝜉
,
(
𝑛
)
−𝜉
,
(
𝑛−1
)
𝜉
,
(
𝑛
)
+𝜉
,
(
𝑛−1
)
(5)
4 RESULTS
Case 1400 corresponded with a participant who was
submitted to active stimulation. The pre-stimulation
recording of a sustained vowel [a:] produced an
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
228
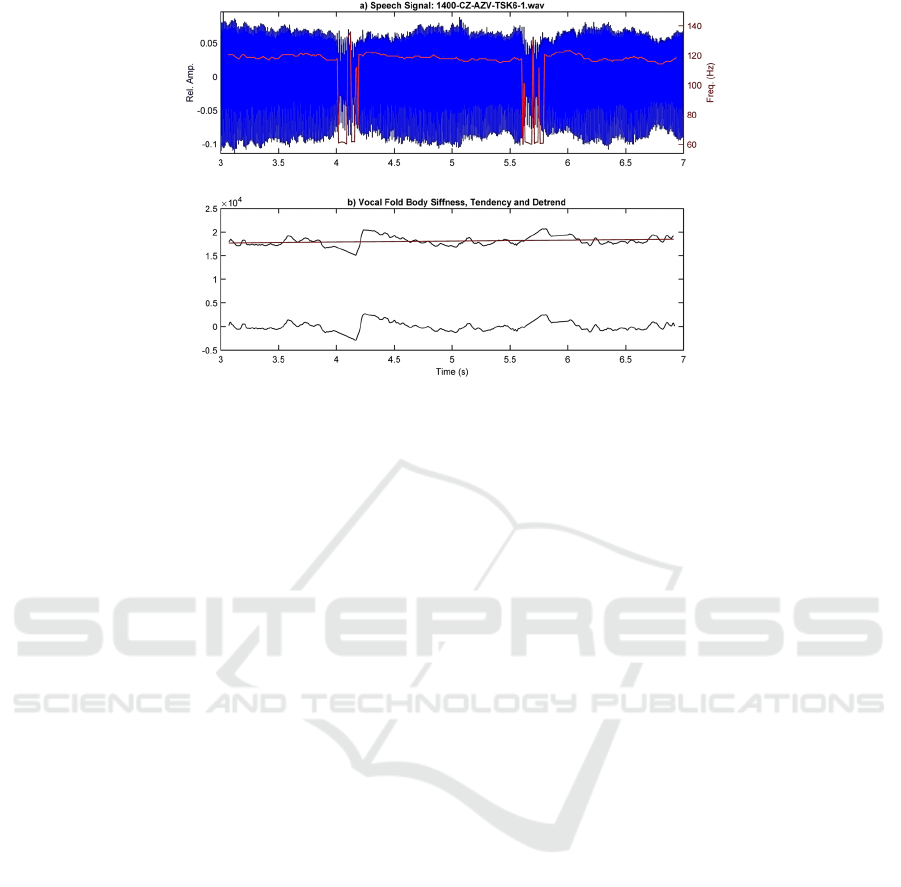
Figure 2: Example of the vocal fold body stiffness estimation from a segment of 4 s of phonation from case 1400, during the
utterance of a sustained vowel [a:]: a) speech segment showing two events of phonation blocking at intervals 4.0 s - 4.2 s and
5.6 s - 5.8 s, where the f0 drops down from 120 Hz to 60 Hz; b) vocal fold stiffness (black), its detrend tendency (red) and its
unbias (blue).
utterance with a duration of 11.163 s, during which
five events of phonation blocking were observed. The
segment selected for the analysis was extended
between 3 s and 7 s, to include the first two blocking
events, which are shown in 0.a, with an estimation of
its fundamental frequency (f0) profile superimposed
in red. 0.b shows the estimation of the vocal fold
stiffness (black), its detrend (red), and the unbiased
detrended result (blue). In its turn, 0 depicts the
results of splitting the unbiased vocal fold stiffness
(UVFS) into the EEG-related frequency bands
defined in Section 3 in the time domain (left column)
and as spectrograms (right column), the first one (0.b)
being given in a logarithmic representation to
compensate different amplitude levels, and the
remnant ones are shown in linear representation. The
distributions of the pre-stimulus and post-stimulus
EEG-related frequency bands are presented in 0.In
Figure 2 an example of VFS estimation from the
acoustic signal is shown, where two blocking events
are appreciated as 4 s and 5.2 s. These events are
marked by a descent in f0 (red line in Figure 2.a) from
120 Hz to 60 Hz during approximately 200 ms, during
which, the estimation of f0 becomes erratic and
unstable. This behavior is aligned with the UVFS
profile shown in Figure 2.b, where f0 instability is
explained by a decay in the VFS at 4 s, followed by a
correction action at 4.3 s to initiate a new slow decay
to be corrected again at 5.6 s.
The graphical example showing the frequency band
estimation protocol illustrated in Figure 3 adds new
information to the evolution of these events. The
activity on the δ-band in Figure 3.c shows the decay
starting really at 3.7 s, coming to a minimum at 4.1 s,
and being strongly incremented immediately after,
followed by a slow progressive decay and correction
bursts between 5.0 s and 5.6 s, where a new pull-up is
observed. The corrective actions are evident in all the
bands, but the most interesting ones are β and γ, given
in Figure 3.j and l. The β-band shows a strong activity
burst between 22-30 Hz at 4.1-4.3 s, followed by a
narrower one at 26 Hz and 4.4-4.7 s. The γ-band, on
the contrary, shows a narrow burst at 38 Hz between
4.4-4.5 s, and a wider and stronger one at 42-46 Hz
lasting from 5.4-5.7 s. According to recent research,
the activity in these bands could be related to the
neuromotor correction (β), and interaction of
different brain areas (γ), including the striato-cortical,
the cerebellum, and the temporal auditory areas
(Ibarra-Lecue, Haegens, and Harris, 2022).
The information provided in Figure 4.a and b,
corresponding to the activity on the β-band is also
quite meaningful, as it shows the profile of the
distributions for the five recordings corresponding to
the active case 1400. Whereas the first evaluation
(1400, pre-stimulus) is amplitude-widespread, all
post-stimulus evaluations (T1-T4) concentrate in
small amplitudes, all of them showing a χ2 profile.
The averages and standard deviations of the post-
stimulus evaluations are sensibly smaller than the pre-
stimulus one, pointing to a strong instability reduction
after stimulation. By contrast, the five recordings
corresponding to the sham case 1900 given in Figure
4.c and d, do not show any meaningful changes,
except possibly in the second evaluation (T1, 1900).
Description of PD Phonation in Terms of EEG-Related Frequency Bands
229
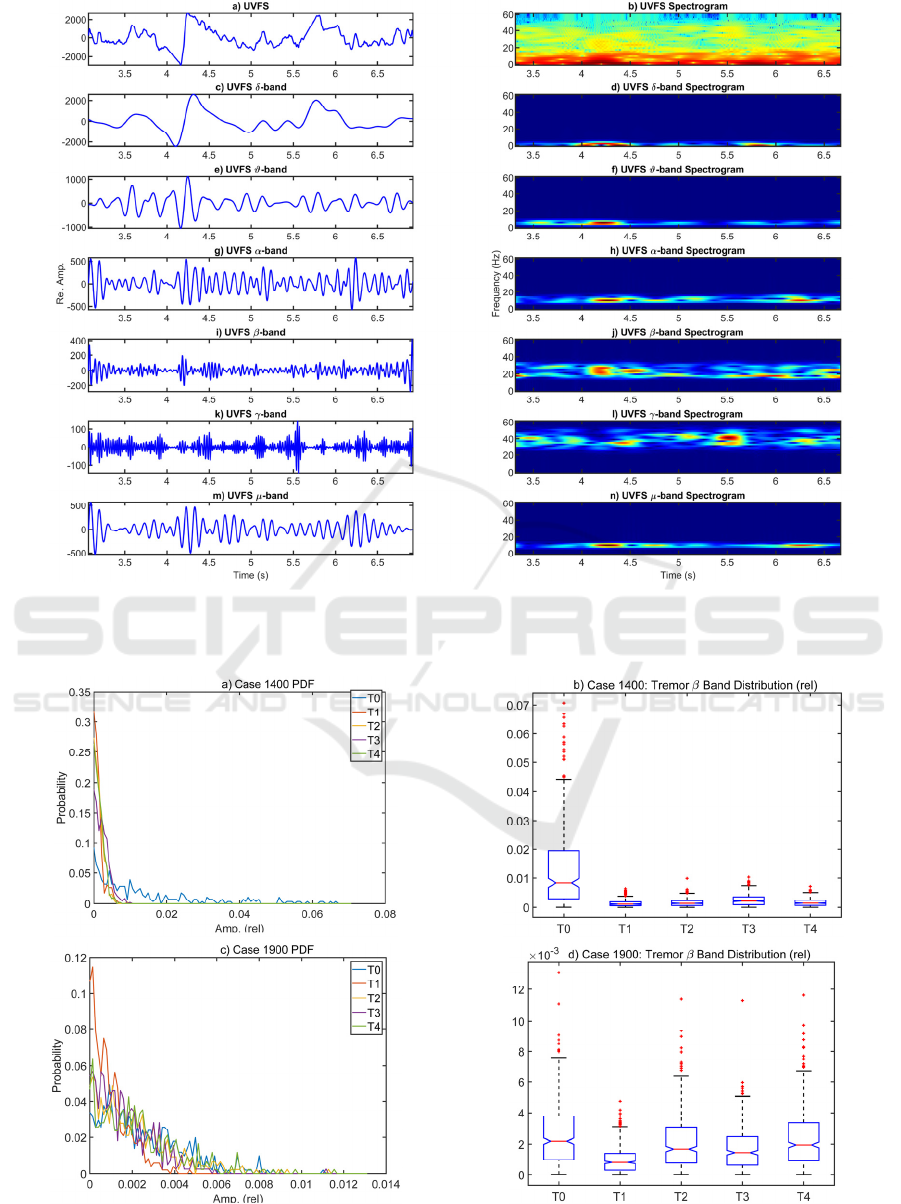
Figure 3: Spectral contents of the EEG-band description of 4 s of phonation from case 1400: a) unbiased vocal fold stiffness;
b) spectrogram of the vocal fold stiffness; c) δ-band component; d) spectrogram of the δ-band; e-f) id. of the ϑ-band; g-h); id.
of the α-band; i-j) id. of the β-band; k-l) id. of the γ-band; m-n) id. of the μ-band.
Figure 4: Probability density distributions of the EEG β-band activity from cases 1400 and 1900: a) pdfs from each evaluation
of the active case (1400: T0-T4); b) their corresponding boxplots; c-d) id. from the sham case (1900: T0-T4).
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
230
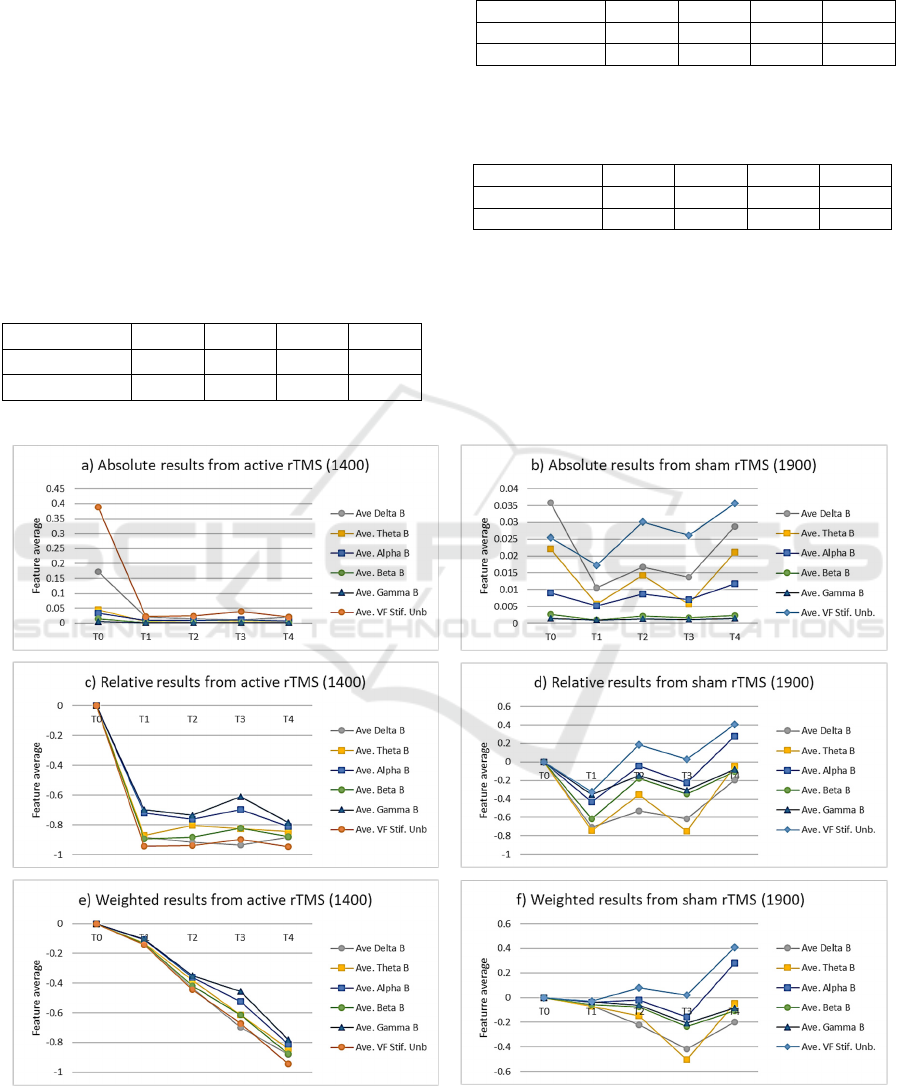
The results of the comparisons between pre-
stimulus and post-stimulus frequency distributions
estimated following expressions (2)-(3) are given
inTable 2 and Table 3. The results of evaluating the
improvement scores following expression (4) are
given in 0. 0 summarizes graphically the amplitude
averages of the five non-overlapping frequency bands
(δ, ϑ, α, β, and γ) in the five evaluation instants (T0-
T4), and the VFS unbalance (as a reference) for the
two cases being considered, their relative differences
concerning T0, and the same differences weighted by
the in-between-evaluation time intervals, following
expression (4).
Table 2: LLR scores according to expression (2) on the β-
band of post-stimulus evaluations (T1-T4) relative to pre-
stimulus (T0).
PwP code T1 T2 T3 T4
1400 0.675 0.607 0.469 0.593
1900 0.192 -0.005 0.083 -0.015
Table 3: p-values on the β-band from MW U-tests of post-
stimulus evaluations (T1-T4) relative to pre-stimulus (T0)
according to expression (3).
PwP code T1 T2 T3 T4
1400 <0.001 <0.001 <0.001 <0.001
1900 <0.001 <0.001 <0.001 0.036
Table 4: Improvement scores according to expression (4)
summarizing the β-band of post-stimulus evaluations
(T1-T4) relative to pre-stimulus (T0).
PwP code T1 T2 T3 T4
1400 -0.135 -0.418 -0.615 -0.878
1900 -0.058 -0.074 -0.233 -0.105
5 DISCUSSION
The aim of this study was focused on exploring the
capability of EEG-related frequency bands to explain
the activity on the neuromotor pathways related to
phonation using acoustic signals analyzing sustained
Figure 5: Normalized average values of each EEG-band (δ-γ) compared with the vocal fold stiffness unbalance from an active
stimulation case (1400) and a sham case (1900): a-b) absolute values; c-d) relative post-stimulus values (T1-T4) compared
with the pre-stimulus evaluation (T0); e-f) relative results weighted accordingly to the time interval between the pre-stimulus
and each post-stimulus interval, as defined in expression (4).
Description of PD Phonation in Terms of EEG-Related Frequency Bands
231

vowel vocalizations from PwPs submitted to active
and sham rTMS.
The comparison among pre- and post-stimulus
estimations in terms of LLRs given in 0 confirms the
observations on the β-band, pointing to strong
improvements in the active case (λ>0), whereas the
sham case shows mixed behavior and moderate
improvements in T1 and T3 which might be due to
circumstantial or confounding factors. The p-values
which are shown in 0 avail the estimations given in 0
for a significance level of 0.05 on the null hypothesis
of equal medians.
After examining the global improvement scores
on all the non-overlapping frequency bands given in
0, it may be concluded that taking the time interval
between the pre-stimulus and each post-stimulus
evaluation into account, the progress in the process
induced by rTMS seems steady, at least for the
observation time intervals considered. These findings
may be better examined on the evolution templates
given in 0.a and b, where the normalized amplitude
average values of the frequency-band components are
given, as well as the VFS unbalance regarding
expression (5) which is added as a reference. The
improvements of the phonation instability conditions
for the active case 1400 are evident (0.a), whereas the
evaluations from the sham case (0.b) do not show a
clear tendency. When considering the difference
between the pre-stimulus and each post-stimulus
estimations by bands given in 0.c and d, the droppings
observed in the active case (1400) become more
evident when compared with the random behavior of
the sham case (1900). This comparison is even more
meaningful when comparing the same differences in
all frequency bands weighted by the time intervals
between each pre- and post-stimulus pair, as seen in
0.e and f. The monotonous descent observed in 0.e is
indicative of the almost-permanent improvements
observed in the active case during the period
considered, contrasting with the quasi-erratic
behavior of the sham case.
The character of this study is very specific,
exploratory, and limited to the observations from the
two cases considered, and further efforts would be
required to generalize its potential application on a
large database.
6 CONCLUSIONS
The present paper is intended to explore the
possibilities of predicting the interactions on the
EEG-related β-γ frequency bands of the NMA from
the phonation acoustical signal. Albeit the specificity
of the cases studied is a limit to the findings observed,
the methodology proposed to extract neuromotor
activity from acoustical information to characterize
PwP vocalization may provide new meaningful
insights into the neuromotor activity related to
phonation stability. The three scores used in the
assessment of potential improvement behavior of
PwP phonation after active rTMS are in full
agreement, and can be used alternatively or
combined. These facts may open new applications of
signal processing in the field of speech neuromotor
understanding, and neurodegenerative disease
monitoring.
ACKNOWLEDGEMENTS
This research received funding from European
Union’s Horizon 2020 research and innovation
program under the Marie Skłodowska-Curie grant
agreement no. 734718 (CoBeN), a grant from the
Czech Ministry of Health, 16-30805A, a grant from
EU – Next Generation EU (project no.
LX22NPO5107 (MEYS)), and grants TEC2016-
77791-C4-4-R (Ministry of Economic Affairs and
Competitiveness of Spain), and Teca-Park-
MonParLoc FGCSIC-CENIE 0348-CIE-6-E
(InterReg Programme). Andrés Gómez-Rodellar
holds a scholarship from the Medical Research
Council Doctoral Training Programme in the Usher’s
Institute (University of Edinburgh Medical School).
REFERENCES
Alku, P., et al. (2019). OPENGLOT-An open environment
for the evaluation of glottal inverse filtering, Speech
Communication 107 (2019) 38-47. https://doi.org/
10.1016/j.specom.2019.01.005.
Brabenec, L. et al. (2021) Non-invasive brain stimulation
for speech in Parkinson’s disease: A randomized
controlled trial, Brain Stimulation, 14, 571-578.
https://doi.org/10.1016/j.brs.2021.03.010.
Brambilla, C. et al., (2021). Combined Use of EMG and
EEG Techniques for Neuromotor Assessment in
Rehabilitative Applications: A Systematic Review,
Sensors, 21 7014. https://doi.org/10.3390/s21217014.
Deller, J. R., Proakis, J. G., and Hansen, J. H. L. (1993)
Discrete-Time Processing of Speech Signals,
NewYork, Macmillan.
Dorsey, E. R., et al (2007). Projected number of people with
Parkinson's disease in the most populous nations, 2005
through 2030, Neurology 68(5) 384-386.
https://doi.org/10.1212/01.wnl.0000247740.47667.03.
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
232

Duffy, J. R. (2013). Motor Speech Disorders: Substrates,
Differential Diagnosis, and Management, 3rd Ed.,
Elsevier.
Gómez, P. et al. (2009). Glottal Source biometrical
signature for voice pathology detection, Speech
Communication 51(9) 759-781. https://doi.org/10.10
16/j.specom.2008.09.005.
Gómez, A., et al. (2021). Acoustic to kinematic projection
in Parkinson’s disease dysarthria. Biomedical Signal
Processing and Control 66 Art. 102422. https://doi.org/
10.1016/j.bspc.2021.102422.
Ibarra-Lecue, I., Haegens, S., and Harris, A. Z. (2022)
Breaking Down a Rhythm: Dissecting the Mechanisms
Underlying Task-Related Neural Oscillations, Front.
Neural Circuits, 16 846905. https://doi: 10.3389/
fncir.2022.846905.
McKeown, M. J., et al. (2006). Cortical muscle coupling in
Parkinson’s disease (PD) bradykinesia. Parkinson’s
Disease and Related Disorders, P. Reiderer et al. (eds.),
Springer, Vienna (2006) 31-40. https://doi.org/
10.1007/978-3-211-45295-0_7.
Mekyska, J., et al. (2015) Robust and complex approach of
pathological speech signal analysis, Neurocomputing
167 94-111. https://doi.org/10.1016/j.neucom.2015.02.
085
Rektorova, I., et al. (2012). Functional neuroanatomy of
vocalization in patients with Parkinson’s disease, J.
Neu. Sci. 313 7-12. https://doi.org/ 10.1093/cercor/bhi
06.
Schulz, G. M. et al. (2005). Functional neuroanatomy of
human vocalization: an H215O PET study, Cereb.
Cortex 15 1835–47. https://doi.org/10.1016/j.jns.2011.
10.020.
Description of PD Phonation in Terms of EEG-Related Frequency Bands
233
