
Technical Realization and First Insights of the Multicenter
Integrative Breast Cancer Registry INTREST
Thomas Ostermann
1
, Sebastian Unger
1
, Michaela Warzecha
2
, Sebastian Appelbaum
1
,
Daniela Rodrigues Recchia
1
, Holger Cramer
3
and Heidemarie Haller
4
1
Department for Psychology and Psychotherapy, Witten/Herdecke University, Alfred Herrhausen-Straße 50,
58448 Witten, Germany
2
Medical Informatics, University of Applied Sciences Dortmund, Emil-Figge-Str. 42 44227 Dortmund, Germany
3
Institute for General Practice and Interprofessional Care, University Hospital Tuebingen and Bosch Health Campus,
Stuttgart, Auerbachstraße 110, 70376 Stuttgart, Germany
4
Department of Internal and Integrative Medicine, Evang. Kliniken Essen-Mitte, Faculty of Medicine,
University of Duisburg-Essen, Am Deimelsberg 34a,45276 Essen, Germany
Holger.Cramer@med.uni-tuebingen.de, Heidemarie.Haller@uk-essen.de
Keywords: Clinical Registry, Breast Cancer, Database, Baseline Characteristics.
Abstract: Cancer is one of leading causes of mortality worldwide. According to GLOBOCAN database, 19.3 million
new cancer cases and 10 million cancer deaths worldwide were counted in 2020. Thus, there is an absolute
necessity for statistical data on cancer incidence and treatments. This is mainly done by cancer registries,
which aim at collecting, managing, and analyzing health and demographic data on individuals diagnosed with
cancer. As more and more patients make use of integrative oncology to optimize their health and quality of
life during and after cancer treatment, it is important to gather clinical registry data of complementary as well
as conventional cancer care. The INTREST registry is the first approach that aims to identify predictors of
treatment-response in women undergoing individualized, integrative breast cancer treatment. This article
reports on the technical realization and representativity of the registry based on 3,341 eligible women and 885
cases included in interim statistical analysis. The analyses show that the INTREST sample of women suffering
from breast cancer does not significantly differ from population-based registries and pragmatic trial data of
breast cancer patients in Germany with respect to main sociodemographic and clinical cancer data. However,
completeness, particularly in tumor classification, currently is a major limitation.
1 INTRODUCTION
Cancer is still one of the leading causes of mortality
worldwide. According to the GLOBOCAN database,
19.3 million new cancer cases and 10 million cancer
deaths worldwide were counted in 2020 (Sung et al.,
2021; Ferley et al., 2021). Thus, there is an absolute
necessity for statistical data on cancer incidence and
treatments. This is mainly done by cancer registries,
which aim at collecting, managing, and analyzing
health and demographic data on persons diagnosed
with cancer (Jensen et al., 1991). Cancer registries
can be classified into three general types:
1. Hospital based registries, which maintain data on
all patients diagnosed and/or treated for cancer at
their facility and report cancer cases to the central
or state cancer registry as required by law.
2. Population-based central registries, which collect
data on all cancer patients within certain geogra-
phical areas.
3. Special purpose registries, providing data on a
particular type of cancer and/or treatment.
The INTREST cancer registry belongs to the third
class of registries and collects data of women diag-
nosed with breast cancer with a special focus on inte-
grative oncological treatment approaches. Integrative
Oncology has its origins in the United States and per
definition combines conventional cancer care with
evidenced-based complementary therapies (CM).
The main goal of Integrative Oncology is to reduce
side effects of oncological treatments and to improve
patient's quality of life with a first medical guideline
being published in 2007.
Common symptoms, accompanying with the
diagnosis and treatment of cancer, include fatigue,
298
Ostermann, T., Unger, S., Warzecha, M., Appelbaum, S., Recchia, D., Cramer, H. and Haller, H.
Technical Realization and First Insights of the Multicenter Integrative Breast Cancer Registry INTREST.
DOI: 10.5220/0011667800003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 5: HEALTHINF, pages 298-306
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)
sleep disturbances, pain, neuropathy, and affective
disorders (Cheng et al., 2013; Patrick et al., 2004;
Singer et al., 2021). In order to improve quality of life,
women with breast cancer frequently use CM
(Molassiotis et al., 2005; Boon et al., 2007). How-
ever, patients often do not mention the use of CM to
their physicians unless they are explicitly asked about
it (Koenig et al., 2015; Samuels et al., 2017). This
lack of communication can lead to undesired
interactions between conventional and CM therapies
that, at worst, negatively impacts quality and quantity
of life (Alsanad et al., 2014; Ben-Arye., 2015; Bode
& Dong, 2015; Zeller et al., 2013).
Asking patients and systematically exploring
their concurrent CM use is recommended by inter-
national clinical practice guidelines (Greenlee et al.,
2014; Leitlinienprogramm Onkologie, 2017; Lyman
et al., 2018) but implemented only gradually by
physicians (Paepke et al., 2020; Grimm et al., 2021)
and initial registries (Schad et al., 2013; Dusek et al.,
2016).
Standard clinical cancer registries, in contrast,
usually do not assess data beyond tumor characteris-
tics, conventional treatment algorithms, and patient
survival while other supportive treatments, streng-
thening the physical and psychosocial resilience of
cancer survivors, are not yet included.
This article reports on the technical realization
and first results of the data analysis of the INTREST
registry, which aims at assessing data on the influence
of conventional and CM treatments as well as
physical and psychosocial resilience using qualitative
and quantitative endpoints.
2 MATERIAL AND METHODS
2.1 Guidelines, Ethics and Partners
The INTREST registry uses an epidemiological,
multi-center cohort design according to the Trans-
parent Reporting of a multivariable prediction model
for Individual Prognosis or Diagnosis (TRIPOD) and
the Strengthening the Reporting of Observational
studies in Epidemiology (STROBE) statement
(Collins et al., 2015). The INTREST protocol is
approved by the respective ethics committees, regis-
tered at the World Health Organization (WHO) Inter-
national Clinical Trials Registry Platform / German
Clinical Trials Register (DRKS00014852), and pub-
lished in 2021 (Haller et al., 2021).
2.2 Technical Realization
In the initial phase, INTREST was developed for
local use. The basis was a Windows 10 machine with
the XAMPP package installed, an Apache distribu-
tion with a MySQL-Database and the scripting
language PHP (PHP: Hypertext Preprocessor) using
an architecture used in the medical learning context
(Ostermann et al.,
2018).
To make INTREST acces-
sible online, it was migrated to a server that was
already fully set up with a similar operating system
and software to those of the local machine, where
INTREST was previously running. Thus, design and
structure, which are briefly presented below, could be
retained during the migration without any
complications.
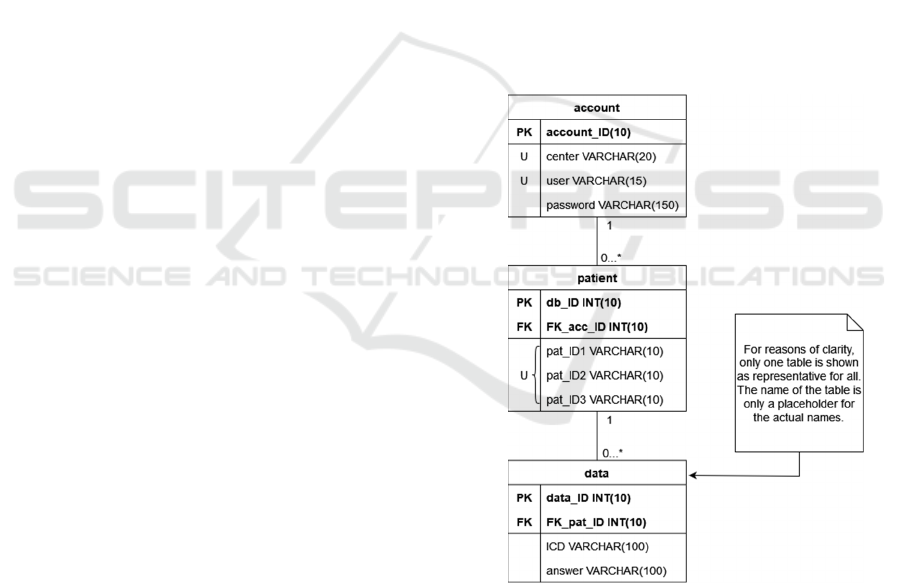
2.3 Data Model
All data are stored within a MySQL-Database. The
structure of the relational model is provided in
Figure 1.
Figure 1: Relational model of the data.
The structure of a table mostly follows the same
principle: The first column contains the primary key,
which consists of an integer value and is automa-
tically incremented when a new entry is created. This
is followed by the foreign key column, which is not
required only for the account table. Finally, there are
two columns for an alphanumeric input in the form of
a limited number of characters in the regular tables,
Technical Realization and First Insights of the Multicenter Integrative Breast Cancer Registry INTREST
299
while there are three such columns in the tables for
accounts and patients. In general, these columns
represent a survey item and the corresponding res-
ponse. The patient table uses these columns for
different types of IDs (identifiers), which are unique
in their three-way combination and consist of a
sequential center number, an individual five-digit
patient code, and a patient identification number of
the specific clinic. In the account table, on the other
hand, these columns are related to username,
password, and name of the study center. Another
characteristic of the account table is that two of its
columns are unique, since each study center receives
only one account with one user.
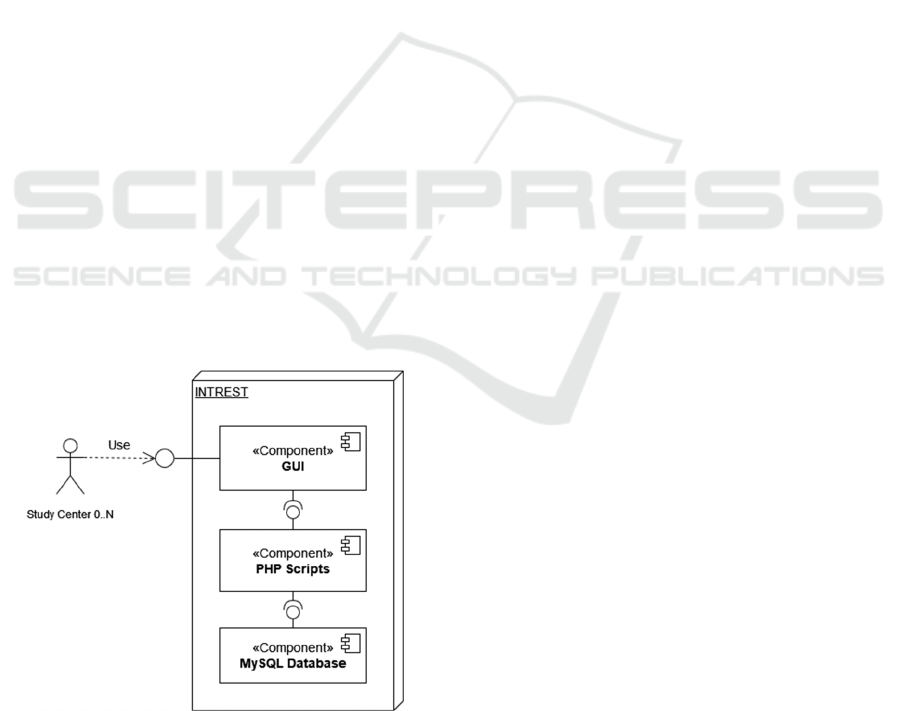
2.4 System Architecture
INTREST’s architecture can be summarized in three
main components (see Figure 2) that follow the
principle of a layer concept for smaller application
(Richards, 2015). On top, there is a GUI (Graphical
User Interface) that allows the communication
between client and server through internet. With this
study nurses of a center can access INTREST at any
time to create new patients or add their individual
Case Report Forms (CRFs). Each page of the GUI
uses a different script because a page refers to a
specific point in time when various items are
recorded. After the CRF inputs are transmitted to a
script, they are transformed to MySQL queries and
redirected to the database at the bottom of
INTREST’s architecture. If a page is accessed with
data already entered, a message appears stating that
the data already exists and can no longer be entered.
Figure 2: Component model of INTREST, visualizing the
interactions between the main components and the study
centres.
2.5 Data Security and Validity
In any application that is connected to the Internet and
contain data, especially if it is medical or personal
data as with INTREST, certain security precautions
are necessary. Hoque et al. (2014) describe many
diverse network attacks and that these attacks often
target web sites or databases to gather information.
Therefore, approaches should be applied to reduce the
risk of exposing data in network applications, which
might have security issues or process medical data.
HTTPS (Hypertext Transfer Protocol Secure) is one
of the worldwide used approaches to encrypt the
communication between clients and servers. The
server, hosting INTREST, supports this protocol in
conjunction with an officially authorized certificate,
allowing secure data retrieval and transmission.
Since the data is transferred from collected
medical records in paper form, special attention is put
on this issue. Only medical personnel who have been
trained by the respective study center are authorized
for this. Their tasks are the formal monitoring for
completeness and the input of the paper CRFs into the
specially developed GUI. Therefore, they are suppor-
ted on the software side.
First, entered data is validated, using programmed
validation checks, e.g., checks for required values,
item types, and item ranges. And second, if a response
of an item is not recognizable, this item is stored in
the database with a discrepancy note. In regular data
review meetings, all such discrepancies are discussed
and clarified by comparing the entries in the database
with the source data.
Another aspect of data security concerns the
storage of data. Since it should not be possible to draw
conclusions about an individual participant, the data
are exclusively pseudonymized during transmission
to the registry. This even applies to the statistical
analysis, where pseudonymized data is transferred to
a CSV (Comma-Separated Values) file. At this stage,
the data is only checked for accuracy and comple-
teness by randomly comparing a set of items with the
original database.
2.6 Patients and Outcomes
Female patients diagnosed with primary breast cancer
stage I-III according to the pTNM (pathological
Tumor-Node-Metastasis) classification,
who received
individualized integrative cancer treatments in one of
the participating study centers, were included in the
registry. Cancer diagnosis and treatment data as well as
those on progression were retrieved from medical
records, while women were asked to complete
HEALTHINF 2023 - 16th International Conference on Health Informatics
300

sociodemographic data and the following Patient
Reported Outcomes (PROs);
• Cancer-related quality of life and fatigue,
assessed by the Functional Assessment of
Cancer Therapy General (FACT-G) (Brucker et
al., 2005) and the associated Fatigue Scale
(FACIT-F) (Yost & Eton, 2005),
• Distress assessed by the Questionnaire on
Distress in Cancer Patients (QSC) (Book et al.,
2011),
• Depression assessed by the Center for Epide-
miologic Studies Depression Scale (CESD)
(Stafford et al., 2014),
• Hopelessness assessed by the Brief Hopeless-
ness measure (BH) (Fraser et al., 2014),
• State anxiety assessed by the Patient-Reported
Outcomes Measurement Information System
Emotional Distress Anxiety Form (PROMIS-
EDA) (Schalet et al., 2016) and progression
anxiety assessed by the Fear of Relapse/Recur-
rence Scale (FRRS) (Thewes et al., 2012),
• Emotion regulation assessed by the Emotion
Regulation Questionnaire (ERQ) (Gross et al.,
2003),
• Sleep disturbance assessed by the Patient-
Reported Outcomes Measurement Information
System Sleep Disturbance Form (PROMIS-SD)
(Yu et al., 2011),
• Spiritual well-being assessed by the Functional
Assessment of Chronic Illness Spiritual Well-
Being Scale (FACIT-SP) (Bredle et al., 2011),
• Social support assessed by the perceived
Available Support subscale of the Berlin Social
Support Scales (BSSS) (Schulz et al., 2003),
• Physical activity assessed by International
Physical Activity Questionnaire (IPAQ) (Craig
et al., 2003),
• Healthy diet assessed by the Mediterranean Diet
Adherence Screener (MEDAS) (Schroder et al.,
2011),
• CM attitutes assessed by the CAM Health Belief
Questionnaire (CHBQ) (Lie et al., 2004),
• Interest in CM assessed by a numeric rating
scales (NRS),
• Use of CM assessed by an extended version of
the International Complementary and Alterna-
tive Medicine Questionnaire (I-CAM-Q) (Quant
et al., 2009),
• Adverse events assessed by the Memorial
Symptom Assessment Scale (MSAS) (Chang et
al., 2000) and
• Therapy satisfaction assessed by the Client
Satisfaction Questionnaire (CSQ) (Attkisson et
al., 1982).
2.7 Statistical Analysis
Statistical analysis included univariate analyses of
frequencies using Chi-Square statistics and analyses
of mean differences using a t-test with respect to
group differences. For all analyses, due to the high
sample size, a p-value of .01 was considered to be
significant.
3 RESULTS
Originally developed at the Department of Internal
and Integrative Medicine, KEM, University of
Duisburg-Essen and the KEM Breast Unit (Start in
September 2017), three additional German cancer
centers have joint into the INTREST-registry: the
Department of Gynecology at the Robert-Bosch-
Hospital (Stuttgart in January 2018), the Breast Unit
of the St. Franziskus-Hospital (Münster in September
2019), and the Breast Unit of Hall (Hall in November
2020).
The recruitment in the four study centers of the
INTREST project amounts to N = 1373 patients with
TNM I-III breast cancer of which N = 885 were
eligible for the present interim analysis at baseline.
For the individual study centers, patient recruit-
ment results are presented in Figure 3.
3.1 Sociodemographic Data
The mean age at baseline is 57.0 ± 11.6 years, with
the vast majority born in Germany (92 %). 62 % of
the patients are married and live with their spouse.
The average weight and height are 72.2 ± 16.2 kg
(kilogram) and 167.3 ± 6.3 cm (centimeters), corres-
ponding to a Body Mass Index (BMI) of 25.8 ± 5.5.
More than half of the sample (61.2 %) is employed.
In addition, almost half of the sample had a high
school (18.1 %) or university degree (30.0 %).
3.2 Cancer Parameters
Table 1 provides cancer related baseline values com-
pared to similar cohort studies and representative
population data from a German/Saarland cancer
registry.
With respect to the age at first cancer diagnosis,
the INTREST data are significantly lower compared
to population data of the Saarland cancer registry (p
Technical Realization and First Insights of the Multicenter Integrative Breast Cancer Registry INTREST
301
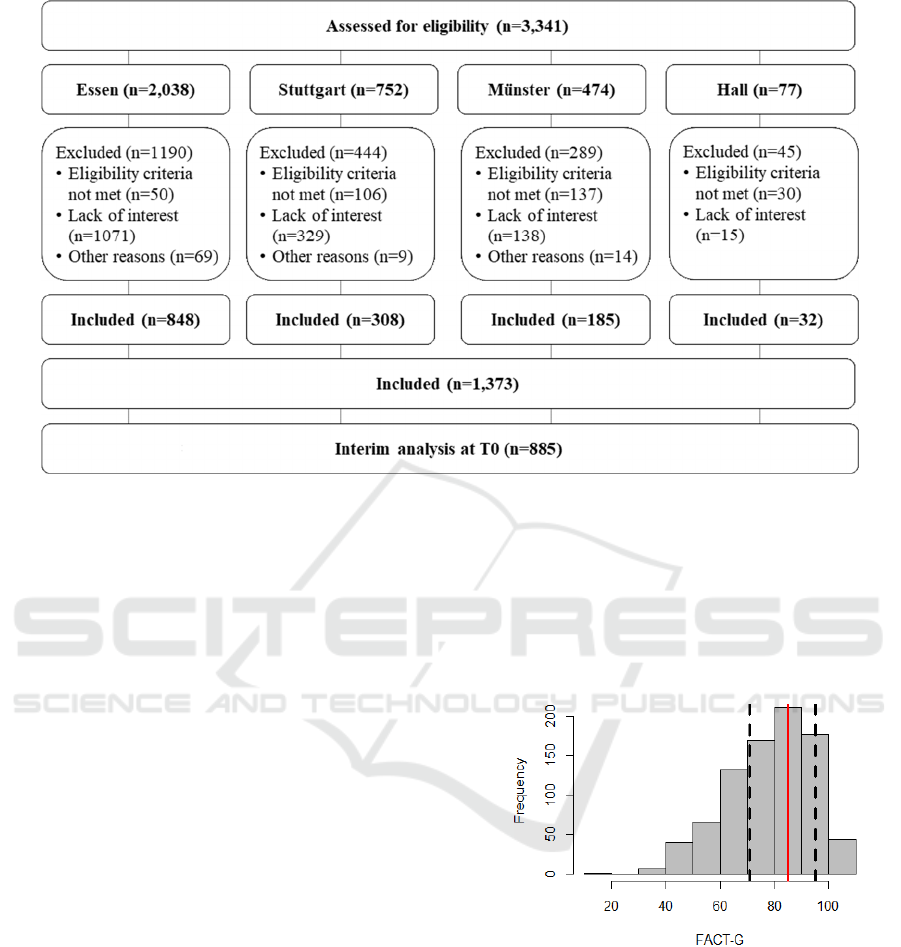
Figure 3: Patient flow chart of the INTREST registry.
< 0.001; Jansen et al., 2020), which, however,
includes not only breast cancer but mixed cancer
diagnoses. Tumor type shows similar percentages
compared with the Saarland registry (p = 0.017),
while the distribution of tumor receptor subtypes is
not comparable to the TMK cohort (p < 0.001)
(Marschner et al., 2019). However, it has to be noted
that the TMK cohort is significantly younger than the
INTERST sample (p < 0.001), as only women with
early breast cancer were included.
Tumor staging is comparable between INREST
and the CM trial (p = 0.11; Witt et al., 2015), while
INTREST shows significantly different percentages
compared to the Saarland registry and the TMK
cohort (p < 0.001, respectively). Tumor grading
significantly differ between the samples (p < 0.001,
respectively) except the amount of G2 grading.
Status of menopause in the INTREST registry
does not significantly differ from the TMK cohort (p
= 0.14) and the pragmatic CM trial (p = 0.15).
3.3 Quality of Life
Quality of life measured with the FACT-G and
FACIT-F showed comparable values with respect to
other studies.
Figure 4 displays the FACT-G total score distri-
bution together with the median and interquartile
range (IQR) of the female cancer norm (Brucker et
al., 2005).
With a mean value of 78.8 ± 15.7 and a median of
80.7 (IQR: [68.2; 91.0]) the FACT-G shows an
expected distribution. This value is underpinned
when comparing it to other, e.g., with the mean value
of 76.2 of the trial of Witt et al. (2015) or with the
mean of 75.7 ± 15.7 of the TMK cohort (Marschner
et al., 2019), both presented in Table 1.
Figure 4: FACT-G distribution of the sample with median
(red line) and 1
st
and 3
rd
Quartile (dashed lines).
Figure 5 displays the FACIT-F total score distri-
bution. In contrast to figure 4, there are no quartile
norm values for breast cancer. Thus, comparative
values were taken from a sample of non-fatigued and
fatigued breast cancer patients at baseline from
(Courtier et al., 2013).
With a mean value of 37.8 ± 10.5 and a median of
41.0 (IQR: [30.0; 46.0]) the FACIT-F shows a distri-
bution between non-fatigued and fatigued patients (M
HEALTHINF 2023 - 16th International Conference on Health Informatics
302
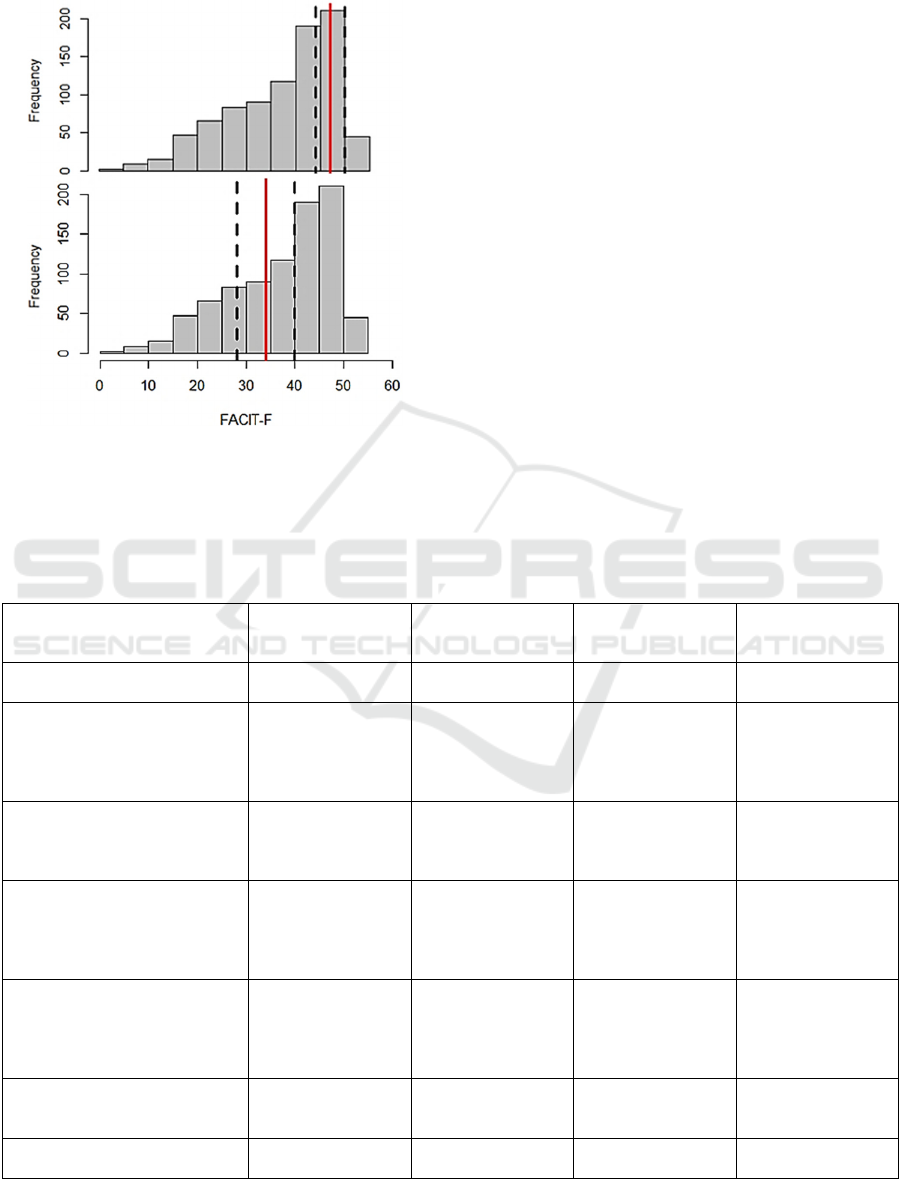
± SD: 36.4 ± 11.1; see Table 1) similar to the trial of
Witt et al. (2015).
Figure 5: FACIT-F distribution of the sample with median
(red line) and 1
st
and 3
rd
Quartile (dashed lines).
3.4 Interest in and Prior Use of CM
Finally, the interest in integrative cancer treatment
was remarkably high. On an NRS from 0 = no interest
to 10 = high interest, patients rated 7.7 ± 3.0.
However, their decision to be treated in an inte-
grative hospital was not driven by their interest: 73.4
% reported that integrative medicine was not relevant
for choosing the respective clinical center. 12.5 %
reported a slight moderating effect and only 14.0 %
based their decision for the hospital on the offer of
integrative therapies.
This is somehow in accordance with the fact that
only half of the patients (51.1 %) previously did not
use integrative therapies.
4 DISCUSSION
This paper presents the technical realization and first
results on representativity of the INTREST data, a
cancer registry for breast cancer patients treated with
integrative oncology.
Table 1: Baseline characteristics and medical history. Abbreviations: FAC(I)T-G/F = Functional Assessment of Cancer
Therapy-General/Fatigue Scale; N/A = Not applicable; pTNM = Classification of Malignant Tumors by histopathologic
examination. Missing data is not displayed. Metrical Values are displayed as means and standard deviations if not otherwise
described.
INTREST registry Saarland
registry
TMK
cohort
CM
trial
(N = 858) (N = 93,721) (N = 729) (N = 275)
Age at baseline 57.0 (11.6) N/A N/A 56.1 (11.0)
Age at first cancer diagnosis 56.9 (11.5) 63.7 (13.9) 26.8 (5.4) 52.9 ( N/A)
Tumor type
Invasive ductal carcinoma 78.9 % 74.0 % N/A 75.6 %
Invasive lobular carcinoma 15.8 % 12.8 % N/A 15.6 %
Inflammatory breast cance
r
0 % 0 % N/A 0 %
Other BCs 5.3 % 3.3 % N/A N/A
Tumor stage (pTNM)
Stage I 31.3 % 39.3 % 26.6 % 39.3 %
Stage II 24.3 % 39.4 % 46.2 % 38.5 %
Stage III 4.3 % 13.7 % 15.9 % 9.1 %
Tumor grading
GX 0.1 % 4.7 % N/A N/A
G1 13.4 % 3.9 % N/A 10.9 %
G2 55.2 % 53.3 % N/A 45.1 %
G3 31.2 % 28.2 % N/A 44.4 %
Tumor receptor subtype
Luminal A 69.3 % N/A 59.9 % N/A
Luminal B 12.5 % N/A 15.5 % N/A
HER2-
p
ositive 3.5 % N/A 6.7 % N/A
Triple-negative 14.7 % N/A 16.2 % N/A
Menopause
Pre-/perimenopausal 38.1 % N/A 34.4 % 40.4 %
Postmenopausal 61.9 % N/A 65.6 % 52.7 %
FACT-G at baseline 78.8 (15.7) N/A 75.7 (15.7) 76.2 ( N/A)
FACIT-F subscale at baseline 37.8 (10.5) N/A N/A 36.4 (11.1)
Technical Realization and First Insights of the Multicenter Integrative Breast Cancer Registry INTREST
303

Our analyses show that our sample of women
suffering on breast cancer does not significantly differ
from other registry and pragmatic trial data of breast
cancer patients in Germany with respect to main
sociodemographic and clinical cancer data.
However, completeness particularly in tumor
classification currently is a major limitation, which
has also been reported in other registries (Ording et
al., 2012; Ramos et al., 2015). Whether technical so-
lutions in the sense of machine learning algorithms,
e.g., to predict missing TNM-staging (Appelbaum et
al., 2023), might be a helpful tool will be discussed
when analyzing the missing data more deeply.
In the next step of the analysis, which is planned
when the data of the respective follow-up assessment
points have been entered into the database and mis-
sing data have been imputed according to the strate-
gies described in Haller et al. (2021), logistic regres-
sion analyses and other predictive models will be run
to identify potential responders and non-responders.
ACKNOWLEDGEMENTS
We would like to thank all the scientific assistants
who actively supported us in entering the patient data.
REFERENCES
Appelbaum, S., Krüerke, K., Baumgartner, S., Schenker,
M., Ostermann, T. (2023). Development,
implementation and validation of a stochastic
prediction model of the TNM classification for missing
values in large data sets in a hospital cancer registry. In
Proceedings of the 16th International Joint Conference
on Biomedical Engineering Systems and Technologies
- HEALTHINF.
Alsanad, S. M., Williamson, E. M., & Howard, R. L.
(2014). Cancer patients at risk of herb/food
supplement-drug interactions: A systematic review.
Phytotherapy Research : PTR, 28(12), 1749–1755.
https://doi.org/10.1002/ptr.5213
Attkisson, C. C., & Zwick, R. (1982). The Client
Satisfaction Questionnaire: Psychometric properties
and correlations with service utilization and
psychotherapy outcome. Evaluation and program
planning, 5(3), 233-237.
Ben-Arye, E., Samuels, N., Goldstein, L. H., Mutafoglu, K.,
Omran, S., Schiff, E., Charalambous, H., Dweikat, T.,
Ghrayeb, I., Bar-Sela, G., Turker, I., Hassan, A.,
Hassan, E., Saad, B., Nimri, O., Kebudi, R., &
Silbermann, M. (2016). Potential risks associated with
traditional herbal medicine use in cancer care: A study
of middle eastern oncology health care professionals.
Cancer, 122(4), 598–610. https://doi.org/10.1002/
cncr.29796
Bode, A. M., & Dong, Z. (2015). Toxic phytochemicals and
their potential risks for human cancer. Cancer
Prevention Research, 8(1), 1–8. https://doi.org/
10.1158/1940-6207.CAPR-14-0160
Book, K., Marten‐Mittag, B., Henrich, G., Dinkel, A.,
Scheddel, P., Sehlen, S., ... & Herschbach, P. (2011).
Distress screening in oncology—evaluation of the
Questionnaire on Distress in Cancer Patients—short
form (QSC‐R10) in a German sample. Psycho‐
Oncology, 20(3), 287-293.
Boon, H. S., Olatunde, F., & Zick, S. M. (2007). Trends in
complementary/alternative medicine use by breast
cancer survivors: Comparing survey data from 1998
and 2005. BMC Women's Health, 7(1), 4.
https://doi.org/10.1186/1472-6874-7-4
Bredle, J. M., Salsman, J. M., Debb, S. M., Arnold, B. J., &
Cella, D. (2011). Spiritual well-being as a component
of health-related quality of life: the functional
assessment of chronic illness therapy—spiritual well-
being scale (FACIT-Sp). Religions, 2(1), 77-94.
Brucker, P. S., Yost, K., Cashy, J., Webster, K., & Cella, D.
(2005). General population and cancer patient norms
for the Functional Assessment of Cancer Therapy-
General (FACT-G). Evaluation & the health
professions, 28(2), 192-211.
Chang, V. T., Hwang, S. S., Feuerman, M., Kasimis, B. S., &
Thaler, H. T. (2000). The memorial symptom assessment
scale short form (MSAS‐SF) validity and reliability.
Cancer: Interdisciplinary International Journal of the
American Cancer Society, 89(5), 1162-1171.
Cheng, K. K. F., & Yeung, R. M. W. (2013). Impact of
mood disturbance, sleep disturbance, fatigue and pain
among patients receiving cancer therapy. European
Journal of Cancer Care, 22(1), 70–78.
https://doi.org/10.1111/j.1365-2354.2012.01372.x
Collins, G. S., Reitsma, J. B., Altman, D. G., & Moons, K.
G. (2015). Transparent reporting of a multivariable
prediction model for individual prognosis or diagnosis
(TRIPOD): the TRIPOD statement. Journal of British
Surgery, 102(3), 148-158.
Courtier, N., Gambling, T., Enright, S., Barrett-Lee, P.,
Abraham, J., & Mason, M. D. (2013). Psychological
and immunological characteristics of fatigued women
undergoing radiotherapy for early-stage breast cancer.
Supportive Care in Cancer, 21(1), 173-181.
Craig, C. L., Marshall, A. L., Sjöström, M., Bauman, A. E.,
Booth, M. L., Ainsworth, B. E., ... & Oja, P. (2003).
International physical activity questionnaire: 12-
country reliability and validity. Medicine and science in
sports and exercise, 35(8), 1381-1395.
Dusek, J. A., Abrams, D. I., Roberts, R., Griffin, K. H.,
Trebesch, D., Dolor, R. J., Wolever, R. Q., McKee, M.
D., & Kligler, B. (2016). Patients receiving integrative
medicine effectiveness registry (primier) of the
bravenet practice-based research network: Study
protocol. BMC Complementary and Alternative
Medicine, 16(1), 53. https://doi.org/10.1186/s12906-
016-1025-0
HEALTHINF 2023 - 16th International Conference on Health Informatics
304

Ferlay, J., Colombet, M., Soerjomataram, I., Parkin, D. M.,
Piñeros, M., Znaor, A., & Bray, F. (2021). Cancer
statistics for the year 2020: An overview. International
Journal of Cancer, 149(4), 778-789.
Fraser, L., Burnell, M., Salter, L. C., Fourkala, E. O., Kalsi,
J., Ryan, A., ... & Menon, U. (2014). Identifying
hopelessness in population research: a validation study
of two brief measures of hopelessness. BMJ open, 4(5),
e005093.
Greenlee, H., Balneaves, L. G., Carlson, L. E., Cohen, M.,
Deng, G., Hershman, D., Mumber, M., Perlmutter, J.,
Seely, D., Sen, A., Zick, S. M., & Tripathy, D. (2014).
Clinical practice guidelines on the use of integrative
therapies as supportive care in patients treated for breast
cancer. JNCI Monographs, 2014(50), 346–358.
https://doi.org/10.1093/jncimonographs/lgu041
Grimm, D., Voiss, P., Paepke, D., Dietmaier, J., Cramer,
H., Kümmel, S., Beckmann, M. W., Woelber, L.,
Schmalfeldt, B., Freitag, U., Kalder, M., Wallwiener,
M., Theuser, A.‑K., & Hack, C. C. (2021).
Gynecologists' attitudes toward and use of
complementary and integrative medicine approaches:
Results of a national survey in Germany. Archives of
Gynecology and Obstetrics, 303(4), 967–980.
https://doi.org/10.1007/s00404-020-05869-9
Gross, J. J., & John, O. P. (2003). Individual differences in
two emotion regulation processes: implications for
affect, relationships, and well-being. Journal of
personality and social psychology, 85(2), 348.
Haller, H., Voiß, P., Cramer, H., Paul, A., Reinisch, M.,
Appelbaum, S., Dobos, G., Sauer, G., Kümmel, S., &
Ostermann, T. (2021). The intrest registry: Protocol of
a multicenter prospective cohort study of predictors of
women's response to integrative breast cancer
treatment. BMC Cancer, 21(1), 724. https://doi.
org/10.1186/s12885-021-08468-2
Hoque, N., Bhuyan, M. H., Baishya, R. C., Bhattacharyya,
D. K., & Kalita, J. K. (2014). Network attacks:
Taxonomy, tools and systems. Journal of Network and
Computer Applications, 40, 307–324. https://doi.org/
10.1016/j.jnca.2013.08.001
Jansen, L., Holleczek, B., Kraywinkel, K., Weberpals, J.,
Schröder, C. C., Eberle, A., et.al. (2020). Divergent
patterns and trends in breast cancer incidence, mortality
and survival among older women in Germany and the
United States. Cancers, 12(9), 2419.
Jensen, O. M., Whelan, S., Jensen, O., Parkin, D.,
MacLennan, R., Muir, C., & Skeet, R. (1991). Planning
a cancer registry. IARC Sci. Publ, 95, 22-28.
Koenig, C. J., Ho, E. Y., Trupin, L., & Dohan, D. (2015).
An exploratory typology of provider responses that
encourage and discourage conversation about
complementary and integrative medicine during routine
oncology visits. Patient Education and Counseling,
98(7), 857–863. https://doi.org/10.1016/j.pec.2015.
02.018
Leitlinienprogramm Onkologie. (2017). S3-Leitlinie
Früherkennung, Diagnose, Therapie und Nachsorge
des Mammakarzinoms (Version 4.0, AWMF
Registernummer: 032-045OL). Retrieved from
http://www.leitlinienprogramm-onkologie.de/leitlinien/
mammakarzinom/
Lie D, Boker J. Development and validation of the CAM
health belief questionnaire (CHBQ) and CAM use and
attitudes amongst medical students. BMC Med Educ.
2004;4(1):1–9.
Lyman, G. H., Greenlee, H., Bohlke, K., Bao, T.,
DeMichele, A. M., Deng, G. E., Fouladbakhsh, J. M.,
Gil, B., Hershman, D. L., Mansfield, S., Mussallem, D.
M., Mustian, K. M., Price, E., Rafte, S., & Cohen, L.
(2018). Integrative therapies during and after breast
cancer treatment: Asco endorsement of the SIO clinical
practice guideline. Journal of Clinical Oncology,
36(25), 2647–2655. https://doi.org/10.1200/JCO.2018.
79.2721
Marschner, N., Trarbach, T., Rauh, J., Meyer, D., Müller-
Hagen, S., Harde, J., et al. (2019). Quality of life in pre-
and postmenopausal patients with early breast cancer: a
comprehensive analysis from the prospective MaLife
project. Breast cancer research and treatment, 175(3),
701-712.
Molassiotis, A., Fernández-Ortega, P., Pud, D., Ozden, G.,
Scott, J. A., Panteli, V., Margulies, A., Browall, M.,
Magri, M., Selvekerova, S., Madsen, E., Milovics, L.,
Bruyns, I., Gudmundsdottir, G., Hummerston, S.,
Ahmad, A. M.‑A., Platin, N., Kearney, N., & Patiraki,
E. (2005). Use of complementary and alternative
medicine in cancer patients: A European survey. Annals
of Oncology, 16(4), 655–663. https://doi.org/10.1093/
annonc/mdi110
Ording, A. G., Nielsson, M. S., Frøslev, T., Friis, S., Garne,
J. P., & Søgaard, M. (2012). Completeness of breast
cancer staging in the Danish Cancer Registry, 2004–
2009. Clinical epidemiology, 4(Suppl 2), 11.
Ostermann, T., Ehlers, J. P., Warzecha, M., Hohenberg, G.,
& Zupanic, M. (2018). Development of an Online-
System for Assessing the Progress of Knowledge
Acquisition in Psychology Students. Data 2018:
Proceedings of the 7th International Conference on
Data Science, Technology and Applications: 77–82.
https://doi.org/10.5220/0006892500770082
Paepke, D., Wiedeck, C., Hapfelmeier, A., Karmazin, K.,
Kiechle, M., & Brambs, C. (2020). Prevalence and
predictors for nonuse of complementary medicine
among breast and gynecological cancer patients. Breast
Care (Basel, Switzerland), 15(4), 380–385.
https://doi.org/10.1159/000502942
Patrick, D. L., Ferketich, S. L., Frame, P. S., Harris, J. J.,
Hendricks, C. B., Levin, B., Link, M. P., Lustig, C.,
McLaughlin, J., Ried, L. D., Turrisi, A. T., Unützer, J.,
& Vernon, S. W. (2003). National institutes of health
state-of-the-science conference statement: Symptom
management in cancer: Pain, depression, and fatigue,
July 15-17, 2002. Journal of the National Cancer
Institute, 95(15), 1110–1117. https://doi.org/10.1093/
jnci/djg014
Quandt, S. A., Verhoef, M. J., Arcury, T. A., Lewith, G. T.,
Steinsbekk, A., Kristoffersen, A. E., ... & Fønnebø, V.
(2009). Development of an international questionnaire
to measure use of complementary and alternative
Technical Realization and First Insights of the Multicenter Integrative Breast Cancer Registry INTREST
305

medicine (I-CAM-Q). The Journal of Alternative and
Complementary Medicine, 15(4), 331-339.
Ramos, M., Franch, P., Zaforteza, M., Artero, J., & Durán,
M. (2015). Completeness of T, N, M and stage grouping
for all cancers in the Mallorca Cancer Registry. BMC
cancer, 15(1), 1-6.
Richards, M. (2015). Software architecture patterns,
O'Reilly Media, Inc. Sebastopol, 1
st
edition.
Samuels, N., Ben-Arye, E., Maimon, Y., & Berger, R.
(2017). Unmonitored use of herbal medicine by patients
with breast cancer: Reframing expectations. Journal of
Cancer Research and Clinical Oncology, 143(11), 2267–
2273. https://doi.org/10.1007/s00432-017-2471-x
Schad, F., Axtner, J., Happe, A., Breitkreuz, T., Paxino, C.,
Gutsch, J., Matthes, B., Debus, M., Kröz, M., Spahn,
G., Riess, H., Laue, H.‑B. von, & Matthes, H. (2013).
Network oncology (no)--a clinical cancer register for
health services research and the evaluation of
integrative therapeutic interventions in anthroposophic
medicine. Forschende Komplementarmedizin (2006),
20(5), 353–360. https://doi.org/10.1159/000356204
Schalet, B. D., Pilkonis, P. A., Yu, L., Dodds, N., Johnston,
K. L., Yount, S.,et al. (2016). Clinical validity of
PROMIS depression, anxiety, and anger across diverse
clinical samples. Journal of clinical epidemiology, 73,
119-127.
Schröder, H., Fitó, M., Estruch, R., Martínez‐González, M.
A., Corella, D., Salas‐Salvadó, J., et al. (2011). A short
screener is valid for assessing Mediterranean diet
adherence among older Spanish men and women. The
Journal of nutrition, 141(6), 1140-1145.
Schulz, U., & Schwarzer, R. (2003). Social support in
coping with illness: the Berlin Social Support Scales
(BSSS). Diagnostica, 49(2), 73-82.
Stafford, L., Judd, F., Gibson, P., Komiti, A., Quinn, M., &
Mann, G. B. (2014). Comparison of the Hospital
Anxiety and Depression Scale and the Center for
Epidemiological Studies Depression Scale for detecting
depression in women with breast or gynecologic
cancer. General hospital psychiatry, 36(1), 74-80.
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M.,
Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global
cancer statistics 2020: GLOBOCAN estimates of
incidence and mortality worldwide for 36 cancers in
185 countries. CA: a cancer journal for clinicians,
71(3), 209-249.
Singer, S., Das-Munshi, J., & Brähler, E. (2010).
Prevalence of mental health conditions in cancer
patients in acute care--a meta-analysis. Annals of
Oncology : Official Journal of the European Society for
Medical Oncology, 21(5), 925–930. https://doi.org/
10.1093/annonc/mdp515
Thewes, B., Butow, P., Zachariae, R., Christensen, S.,
Simard, S., & Gotay, C. (2012). Fear of cancer
recurrence: a systematic literature review of self‐report
measures. Psycho‐oncology, 21(6), 571-587.
Witt, C. M., Außerer, O., Baier, S., Heidegger, H., Icke, K.,
Mayr, O., et al. (2015). Effectiveness of an additional
individualized multi-component complementary
medicine treatment on health-related quality of life in
breast cancer patients: a pragmatic randomized trial.
Breast cancer research and treatment, 149(2), 449-460.
Yost, K. J., & Eton, D. T. (2005). Combining distribution-
and anchor-based approaches to determine minimally
important differences: the FACIT experience.
Evaluation & the health professions, 28(2), 172-191.
Yu, L., Buysse, D. J., Germain, A., Moul, D. E., Stover, A.,
Dodds, N. E.,et.al.. (2012). Development of short forms
from the PROMIS™ sleep disturbance and sleep-
related impairment item banks. Behavioral sleep
medicine, 10(1), 6-24.
Zeller, T., Muenstedt, K., Stoll, C., Schweder, J., Senf, B.,
Ruckhaeberle, E., Becker, S., Serve, H., & Huebner, J.
(2013). Potential interactions of complementary and
alternative medicine with cancer therapy in outpatients
with gynecological cancer in a comprehensive cancer
center. Journal of Cancer Research and Clinical
Oncology, 139(3), 357–365.
HEALTHINF 2023 - 16th International Conference on Health Informatics
306
