
Comparison of the Electrophysiological Myoelectrical Activity
Evolution in Induction of Labor with Pharmacological and
Mechanical Methods
Alba Diaz-Martinez
1a
, Yiyao Ye-Lin
1b
, Rogelio Monfort-Ortiz
2c
, Javier Garcia-Casado
1d
,
Iria Rey-Ferreira
2
, Felix Nieto-del-Amor
1e
, Vicente Diago-Almela
2f
,
Jose Luis Martinez-de-Juan
1g
and Gema Prats-Boluda
1h
1
Centro de Investigación e Innovación en Bioingeniería, Universitat Politècnica de València, Valencia 46022, Spain
2
Servicio de Obstetricia, H.U.P. La Fe, Valencia 46026, Spain
Keywords: Electrohysterography, Induction of Labour, Foley, Dinoprostone, EHG-Biomarker.
Abstract: Induction of labour (IOL) refers to triggering the contractions onset, either by pharmacological (PIOL) or
mechanical methods (MIOL), and is indicated when maternal and foetal well-being is compromised. There is
great uncertainty regarding the success of IOL regardless of the method. In current clinical practice, it is based
on assessment of cervical status by Bishop's score and degree of uterine activity by tocography. However,
Bishop's score has been shown to be subjective and poorly reproducible and tocography requires constant
repositioning and is severely affected by obesity. Meanwhile, electrohysterography (EHG) has surpassed
traditional clinical measures in monitoring PIOL progress and predicting its outcome. Although there is no
evidence of uterine myoelectric activity response of MIOL. Therefore, this work aimed to identify EHG-
biomarkers to help to determine possible differences in myoelectric response between PIOL and MIOL
success. For this purpose, the uterine response during the first 5h after Dinoprostone (PIOL) administration
and Foley catheter (MIOL) insertion was compared by EHG. For PIOL, a significantly lower time to achieve
active phase of labor and delivery, together with faster myoelectric response was found: slightly higher
contraction force, significantly higher Mean Frequency and lower Spectral Entropy after 2.5h. Between-group
differences were especially marked in Spectral Entropy (90-150 and 210-300min). Overall, this pioneering
work has demonstrated the feasibility of EHG for the characterisation of evolution also in MIOL. Furthermore,
the results suggest that EHG biomarkers may be useful in the IOL method comparison, although they should
be cross-checked with expanded databases and further investigations.
1 INTRODUCTION
Induction of labour (IOL) refers to the process of
artificially stimulating the uterus to initiate labour by
pharmacological or mechanical agents when
continuation of gestation compromises maternal-fetal
well-being (Reshme, Samal, Padmaja, Shalini, &
Radhika, 2022). Indications for IOL include elective
induction at 40 weeks, prolonged pregnancy,
a
https://orcid.org/0000-0002-4605-6048
b
https://orcid.org/0000-0003-2929-181X
c
https://orcid.org/0000-0001-7931-8609
d
https://orcid.org/0000-0003-1410-2721
e
https://orcid.org/0000-0003-0050-9360
f
https://orcid.org/0000-0003-1882-1176
g
https://orcid.org/0000-0001-9133-3123
h
https://orcid.org/0000-0002-9362-5055
pregnancy-induced hypertension or diabetes,
oligohydramnios, intrauterine growth restriction and
Rh isoimmunisation (Liu et al., 2019; Reshme et al.,
2022).
The global incidence of IOL tripled between 1990
and 2019, going from 9.5% in 1990 to 29.4% in 2019
in the United States (Martin, Hamilton, Osterman, &
Driscoll, 2021), while the worldwide prevalence is
estimated by the WHO at 25% (World Health
66
Diaz-Martinez, A., Ye-Lin, Y., Monfort-Ortiz, R., Garcia-Casado, J., Rey-Ferreira, I., Nieto-del-Amor, F., Diago-Almela, V., Martinez-de-Juan, J. and Prats-Boluda, G.
Comparison of the Electrophysiological Myoelectrical Activity Evolution in Induction of Labor with Pharmacological and Mechanical Methods.
DOI: 10.5220/0011664700003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 4: BIOSIGNALS, pages 66-73
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

Organization, 2018). Out of the three and a half
million newborn registered in 2021 only in the USA,
between twenty thousand and forty thousand will end
the IOL process as failed, which in the broadest sense
is defined as the non-achievement of vaginal delivery
(Ayala & Rouse, 2022; Hamilton, Martin, &
Osterman, 2022). This entails an elevated risk of
maternal and perinatal complications, including higher
rates of obstetric intervention, cesarean delivery,
chorioamnionitis, admission to the neonatal intensive
care unit, and increased blood loss (Ayala & Rouse,
2022). If the IOL is unsuccessful, the protocol is to
perform a cesarean section which can cost up to $7,595
(1.3 times a standard cesarean section) in the USA
(Nicholson & Cyr, 2013). Not only does it have a major
impact on maternal and neonatal health, but IOL also
overburdens delivery rooms and affects health care
costs, costing more than $2 billion annually in the USA
(Kaimal et al., 2011). Given the volume of IOLs
performed each year, the development of a robust and
reliable system to aid in procedural decision making
would be a key factor in enabling clinicians to better
plan and manage deliveries, prevent maternal and fetal
complications, and optimize hospital resources.
IOL methods can be broadly divided into
pharmacological (PIOL) and mechanical (MIOL)
(Liu et al., 2019). The former involve the
administration of prostaglandins, orally or vaginally,
to stimulate the onset of contractions. Among the
most commonly employed options, the use of E2
(PGE2-Dinoprostone) is distinguished by its slow-
release vaginal application, which allows clinicians to
respond quickly in case a complication arises
(Geethanjali & Palli, 2022; Reshme et al., 2022).
Whereas the latter consist of the use of balloon
devices and hygroscopic dilators that, applying
pressure to the internal face of the cervix so as to
increase endogenous prostaglandin secretion. c Of
these, the Foley catheter stands out for its low cost,
simplicity, reversibility and lack of serious side
effects. In comparison with PIOL, IOL with amniotic
balloons requires a subsequent oxytocin
augmentation procedure in many cases, which is
associated with a significant rate of dysfunctional
deliveries and caesarean sections (Geethanjali &
Palli, 2022; Salim et al., 2011). Despite of this, the
literature suggests that mechanical methods have
similar efficacy, incur fewer adverse events (such as
uterine tachycardia) and have lower costs compared
to pharmacological agents (Jozwiak et al., 2012).
On the other hand, in order to assess IOL
evolution in current clinical practice, the most
commonly used method consists of the assessment of
cervical status and uterine dynamics, as measured by
Bishop Score (BS) and tocography respectively
(Euliano et al., 2013; Geethanjali & Palli, 2022).
Despite being widely employed, BS has been shown
to be subjective and has poor reproducibility, making
it a poor predictor of IOL outcome (Marconi, 2019).
In terms of contraction detection, it should be noted
that electrohysterography (EHG) is a promising
research technique that has been shown to outperform
tocography in both pregnancy and childbirth (Mas-
Cabo et al., 2020; Song et al., 2021; J. Xu, Chen, Lou,
Shen, & Pumir, 2022), especially in the growing
population of obese patients (Krogh et al., 2022; Mas-
Cabo et al., 2020). EHG consists of recording uterine
myoelectric activity generated by billions of
myometrial cells on the abdominal surface. Its energy
is distributed over a bandwidth ranging from 0.1 to 4
Hz (Devedeux, Marque, Mansour, Germain, &
Duchêne, 1993). EHG-bursts are composed by the
slow wave (SW) -which has a period equal to the
duration of the contraction and whose bandwidth
overlaps with the baseline being difficult to analyse
and extract reliable information from it (Nieto-Del-
Amor et al., 2021)- and by the fast wave (FW), which
can be further divided into two components
(Devedeux et al., 1993; Terrien, Marque, & Karlsson,
2007): the Fast Wave Low (FWL) which ranges from
0.13 to 0.26 Hz and is associated with the propagation
of the contraction, and the Fast Wave High (FWH)
which ranges from 0.26 to 0.88 Hz and is related to
the excitability of the uterine cells (Benalcazar-Parra
et al., 2018; Mas-Cabo et al., 2020). Although the
frequency content of FWH is thought to extend up to
3-4 Hz (Fele-Žorž, Kavšek, Novak-Antolič, & Jager,
2008), a high proportion of studies focus down to 1Hz
perhaps as a consequence of maternal cardiac
interference (1.38-1.5 Hz) (J. Xu et al., 2022).
Considering the aforementioned, the aim of the
present work is therefore to characterize and compare
the uterine electrophysiological response to IOL by
Dinoprostone and by Foley catheter during the first 5
hours of induction by electrohysterography and its
associated EHG-Biomarkers of women who achieve
Active Period of Labour (APL). Given the large
increase in the rate of inductions in recent years,
improving the understanding of the myoelectric
response to pharmacological and mechanical
inductions is becoming increasingly relevant to
clinical practice. Not only to guide clinical practices,
but also to delve deeper into the underlying
physiological mechanism and thus promote a better
understanding of the optimal methods for IOL in each
case.
Comparison of the Electrophysiological Myoelectrical Activity Evolution in Induction of Labor with Pharmacological and Mechanical
Methods
67

2 MATERIALS AND METHODS
2.1 Study Design
A prospective observational cohort study was
conducted in pregnant women admitted for cervical
ripening at the Hospital Universitario y Politécnico
La Fe (Valencia, Spain). Either they were candidates
for pharmacological induction with Dinoprostone
(10mg, Propess, Ferring SAU) or mechanical
induction with a Foley catheter (Folysil, Coloplast).
Both methods were withdrawn after 12 hours. In case
the catheter fell out on its own during the MIOL
procedure it ended earlier. In this study only IOLs that
reached the active period of labor were considered.
Fetal macrosomia, multiple pregnancies, advanced
maternal age (>45 years), severe preeclampsia,
placenta previa, premature rupture of membranes,
vaginal bleeding during pregnancy and active cardiac,
renal, pulmonary or hepatic disease; were exclusion
factors for this study due to bias. This work adhered
to the guidelines of the Declaration of Helsinki and
was approved by the hospital's Institutional Review
Board (Registration Number 2018/0530). Patients
were informed of the nature of the study and gave
written informed consent.
The following clinical information was included:
maternal age, body mass index (BMI), number of
previous pregnancies, parity, gestational age at
delivery, initial BS, increase in BS during IOL (12 h
after insertion), time to achieve APL, time to delivery
and completion of vaginal delivery. The chi-square
test was used to detect statistically significant
differences in nominal variables between groups.
Ordinal variables were compared using the Wilcoxon
rank sum test. Continuous variables were compared
with Student's t test or the Wilcoxon rank sum test,
depending on whether they were considered normal
or not by the Shapiro-Wilk test.
2.2 Signal Acquisition
For EHG recording, the abdominal surface was
exfoliated using abrasive gel (Nuprep, Weaver and
Company, Aurora, CO, USA) and cleaned with
isopropyl alcohol to reduce skin-electrode
impedance. Four single-use Ag/AgCl electrodes (Red
Dot 2660-5, 3M, St. Paul, MN, USA) were then
placed as shown in Figure 1. Two electrodes (M1 and
M2) were placed symmetrically with respect to the
mid-axis at a distance of 6 cm from each other. The
other two electrodes were placed on each hip to
provide reference and ground biopotentials.
Figure 1: Electrodes positioning for uterine myoelectrical
recording. M1: monopolar electrode 1. M2: monopolar
electrode 2. REF: Reference electrode. GND: Ground
electrode.
Both monopolar signals were conditioned with a
custom-made wireless recording module, which
provided a gain of 2059 V/V in the 0.1-150 Hz
bandwidth and digitized with a 24-bit analog-to-
digital converter at 500 Hz (Ye-Lin, Bueno-
Barrachina, Prats-boluda, Rodriguez de Sanabria, &
Garcia-Casado, 2017). The recording starts 30
minutes before the start of the IOL and ends 5 hours
later. Unlike other research settings where up to 16
electrodes are placed (Muszynski et al., 2018; Y. Xu,
Hao, & Zheng, 2020), this simplified protocol was
chosen because it does not compromise routine
clinical practice or add additional complexity to the
highly stressful situation faced by women due to the
imminence of labor (Benalcazar-Parra et al., 2018).
Digitalized monopolar EHG signals were filtered
between 0.1-4 Hz (5th order zero-phase Butterworth
bandpass filter) and then downsampled to 20 Hz to
maintain a balance between temporal resolution and
computational cost (Diaz-Martinez et al., 2021; Mas-
Cabo et al., 2020). A bipolar signal was then
computed as its difference (M2-M1) to reduce
common-mode interference and increase signal
quality (Mas-Cabo et al., 2020; J. Xu et al., 2022).
Finally, two experts identified the onset and end of
the EHG bursts, which were related to uterine
contractions. They were associated with substantial
changes in amplitude and frequency with respect to
the reference tone with durations longer than 40
seconds and without respiratory interference or
motion artefacts (Diaz-Martinez et al., 2021; Mas-
Cabo et al., 2020; J. Xu et al., 2022).
2.3 EHG Parametrisation
In order to characterize uterine contractions, a set of
temporal, spectral and nonlinear parameters were
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
68

calculated from the EHG-Bursts. The Root Mean
Square (RMS) calculated at 0.1-4 Hz was included as
a measure of amplitude related to uterine contraction
intensity (Diaz-Martinez et al., 2021; Mas-Cabo et al.,
2020). As labor progresses, contractions are more
frequent and of greater intensity, which is equivalent
to a higher signal amplitude (Diaz-Martinez et al.,
2021; J. Xu et al., 2022). The RMS is therefore
expected to show an upward trend throughout the
IOL. In addition, the Mean Frequency (MNF) was
calculated in order to characterise the expected shift
in spectral content towards higher frequencies due to
enhanced cell excitability as parturition approaches.
It was calculated at 0.2-1 Hz (Horoba et al., 2016;
Mas-Cabo et al., 2020) to minimise the influence of
cardiac interference and baseline fluctuation (J. Xu et
al., 2022). Successful IOL is associated with an
increase in MNF (Diaz-Martinez et al., 2021).
Finally, Spectral Entropy (SpEn) (Diaz-Martinez et
al., 2021; J. Xu et al., 2022) and Higuchi Fractal
Dimension (HFD) (Diaz-Martinez et al., 2021; Kesić
& Spasić, 2016) were computed as non-linearity
parameters. It was done in the FWH bandwidth to
provide a robust characterisation of the EHG (Mas-
Cabo et al., 2020; J. Xu et al., 2022). It is due to the
fact that as delivery approaches, myoelectric activity
also tends to become more organised and predictable,
resulting in a downward trend of non-linearity
parameters (Benalcazar-Parra et al., 2018; Diaz-
Martinez et al., 2021).
EHG parameters were calculated for each section
identified as contraction during the first five hours of
induction. Then, in order to reduce the effect of
intrinsic variability of uterine contractility, uterine
contractility was analysed at 30-minute time (from
now on, analysis window) (Benalcazar-Parra et al.,
2018; Diaz-Martinez et al., 2021). There were 11
windows per recording: 1 in the baseline condition
(before drug administration or probe placement) and
10 to assess the response during the first five hours of
IOL. Median values of the EHG-burst parameters
were calculated for each 30-minute window in order
to obtain a single representative value per analysis
window for each recording session. Then, the mean
of each parameter in each 30-minute window was
calculated for each group (MIOL and PIOL).
Finally, statistically significant differences in
uterine myoelectric response between MIOLs and
PIOLs were analysed. For this purpose, significant
changes from baseline activity of EHG parameters
throughout the recording session were determined for
each window of analysis and for each induction
method using the Wilcoxon signed-rank test
(α=0.05). The same statistical test was employed to
evaluate the differences between induction methods,
comparing each parameter in each window of
analysis between the methods.
3 RESULTS
A total of 73 patients were recruited, of which 52
were induced by pharmacological methods and the
remaining 21 by mechanical methods. Their obstetric
and delivery variables are summarised in
Table 1.
Significant differences have been found for parity,
gestational age at delivery, time to reach the active
period of labour and time to delivery between MIOL
and PIOL.
Table 1: Obstetric data and outcomes of labour induction of
women enrolled in the study, mean ± standard deviation or
number of cases. BMI: Body Mass Index. GAD:
Gestational Age at Delivery in weeks. BS: Bishop Score.
APL: Active Period of Labour. p: Wilcoxon Rank-sum or
t-student test p-value (in bold: statistically significant
difference, p<0.05).
Variable MIOL PIOL p
Mat. age
(y
ears
)
µ±σ 32.2±5.5 34.0±5.6 0.241
BMI (kg/m2) µ±σ 24.6±4.2 26.05±7.5 0.124
Gestations µ±σ 2.1±0.7 2.0±1.4 0.212
Parity µ±σ 0.1±0.4 0.6±0.7 0.009
GAD (weeks) µ±σ 39.4±1.5 40.7±0.5 <0.005
Initial BS µ±σ 3.1±1.2 2.6±2.6 0.427
ΔBS µ±σ 2.1±1.8 3.3±4.6 0.967
Time to
APL (h)
µ±σ 25.9±6.9 16.4±9.1 <0.005
Time to
Del. (h)
µ±σ 29.2±6.0 20.8±12.1 <0.005
Vag. Ending N 19/21 46/52 0.806
The uterine myoelectric activity parameters in
response to the IOL is represented in Figure 2 for the
MIOL (blue) and PIOL (red) groups. An increasing
trend is described for the RMS in both groups,
although it is slightly more accentuated for PIOL. No
differences were found with respect to baseline or
between IOL methods.
The MNF shows a more pronounced upward trend
again for the PIOL group, in which case differences
with respect to baseline are identified at 150 and
maintained from 210 to 300. By contrast, the MIOL
shows no significant evolution. Differences between
groups are found at 150 and 210-300.
Comparison of the Electrophysiological Myoelectrical Activity Evolution in Induction of Labor with Pharmacological and Mechanical
Methods
69
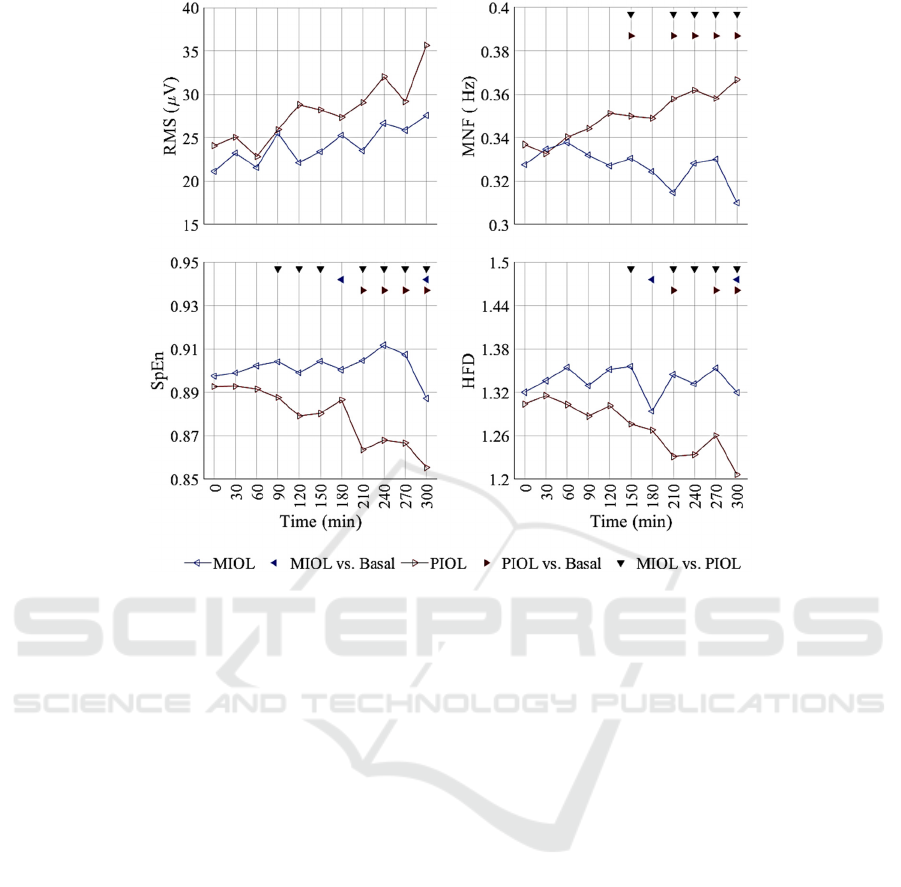
Figure 2: Temporal evolution of temporal, spectral and non-linear parameters for mechanical (MIOL) and Pharmacological
Induction of Labour (PIOL) groups. Statistical differences between groups are indicated by black downward-pointing
triangles and with respect to basal activity by blue leftward (MIOL) and red rightward (PIOL) triangles.
As for the non-linearity parameters, the trends are
also more noticeable for PIOL. For both parameters,
difference with respect to baseline were identified for
PIOL from 210 and for MIOL only at 180 and 300.
By contrast, MIOL did not show any trend during the
first 5 hours. When comparing MIOL and PIOL
groups, significant differences were found for SpEn
from 90 except for 180, and for HFD from 150.
4 DISCUSSION
In this work we have analysed and compared the
difference in uterine myoelectric response between
the induction methods of Dinoprostone and Foley
catheter. As far as we are concerned, this is the first
study to report this type of EHG-biomarker in the
comparison between IOL methods. These initial
results in this line of research will need to be
corroborated with expanded databases and future
studies, such as the comparison of MIOL successes
and failures. We believe that the EHG-biomarker
information proposed could lead to the design of
robust and generalizable systems for predicting the
success of labour induction.
Regarding obstetric variables, differences were
found in parity and gestational age at delivery. This
can be explained because after a previous caesarean
section, IOL is associated with a higher risk of uterine
dehiscence, uterine rupture and repeat caesarean
section compared to women with spontaneous onset
of labour. Mechanical methods are suggested to this
group because they are associated to lower risk of
hyperstimulation and uterine rupture (Kruit,
Wilkman, Tekay, & Rahkonen, 2017). Thus, at equal
gestations, there are fewer vaginal deliveries in the
MIOL group. Despite this, we believe that the
differences found in myoelectric activity are not due
to the difference in gestational age, as it is considered
that in both groups the uterus are sufficiently mature,
in addition to the fact that no significant differences
are found at the basal analysis window. We therefore
attribute the differences found to the IOL method
It should be added that previous studies in the
PIOL field have suggested that the differences that
might be due to the number of previous deliveries are
minimal, especially in RMS, MNF, SpEn and HFD
(Diaz-Martinez et al., 2021). On the other hand, the
lower gestational age in the MIOL group is again due
to protocol indications, as the use of the Foley
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
70

catheter was used in case of the fetal growth
restrictions, which requires induction at 37 weeks,
with a favourable safety profile compared to
Dinoprostone (Villalain et al., 2019).
Finally, the time from the induction onset to the
active period of labor was significantly shorter for
PIOL. Our results are consistent with Wang's meta-
analysis (Wang, Hong, Liu, Duan, & Yin, 2015) of up
to 731 women induced with controlled-release
Dinoprostone and 722 with Foley, which suggests
that PIOL results in a reduction in time to delivery
and oxytocin use. Lastly, our results seem consistent
with other studies in terms of BS modification, as in
Pennell's study (Pennell et al., 2009), we found no
significant changes between induction methods.
Of note, while the uterine electrophysiological
response to PIOL has been researched previously
(Benalcazar-Parra et al., 2018; Diaz-Martinez et al.,
2021), studies on myoelectric uterine response to
MIOL are limited and primarily focused on obstetric
output. MIOL is known to be associated with a
decreased risk of uterine hyperstimulation. This could
be consistent with the lower contraction strength
obtained in the present work, although no differences
were found in the first 5 hours of IOL. In addition,
MIOL has been associated with a higher oxytocin
requirement than PIOL (Reshme et al., 2022; Wang
et al., 2015), suggesting that cells are less excitable
after this procedure. This is consistent with our
results, as MIOL MNF shows no difference from
baseline, while PIOL MNF does.
On the other hand, non-linearity results obtained
suggest lower complexity in the case of PIOL uterine
activity, a trait associated with shorter time to
delivery. The literature points out that the use of
prostaglandins is associated with an acceleration of
gap junction formation, which in turn leads to more
coordinated uterine contractions (Rayburn, 2002). In
addition, Pennell (Pennell et al., 2009) described by
Kaplan-Meier curves a higher proportion of
deliveries in PIOL compared to MIOL before 10
hours from the start of the IOL process, which is
consistent with our results.
5 CONCLUSIONS
In this work, the EHG technique and its associated
EHG-biomarkers have demonstrated their feasibility
to characterise the evolution of uterine dynamics also
during the MIOL process. They have been shown to
provide new relevant information on cellular
excitability and the coordination of contractile
activity that could not be perceived with the
traditional tocography technique. Our results suggest
that PIOL triggers a faster uterine myoelectric
response than MIOL, with contractions of higher
amplitude, a significantly higher MNF after 2.5h of
IOL onset and a higher degree of regularity and less
complexity, with also statistically significant
differences for both groups also after about 2.5h from
the start of induction.
Therefore, this accurate and quantitative
assessment of the IOL process based on EHG could
lead to more reliable IOL success prediction systems
and help to improve maternal-fetal wellbeing. In
addition to helping to better understand the
electrophysiological response in the IOL
environment.
ACKNOWLEDGEMENTS
This work was supported by the Spanish Ministry of
Economy and Competitiveness and the European
Regional Development Fund (MCIU/AEI/FEDER,
UE RTI2018-094449-A-I00-AR and PID2021-
124038OB-I00).
REFERENCES
Ayala, N. K., & Rouse, D. J. (2022). Failed induction of
labor. American Journal of Obstetrics and Gynecology,
1–6. https://doi.org/10.1016/J.AJOG.2021.06.103
Benalcazar-Parra, C., Ye-Lin, Y., Garcia-Casado, J.,
Monfort-Orti, R., Alberola-Rubio, J., Perales, A., &
Prats-Boluda, G. (2018). Electrohysterographic
characterization of the uterine myoelectrical response
to labor induction drugs. Medical Engineering and
Physics, 56, 27–35. https://doi.org/10.1016/j.meden
gphy.2018.04.002
Devedeux, D., Marque, C., Mansour, S., Germain, G., &
Duchêne, J. (1993). Uterine electromyography: A
critical review. American Journal of Obstetrics and
Gynecology, 169(6), 1636–1653. https://doi.org/10.10
16/0002-9378(93)90456-S
Diaz-Martinez, A., Monfort-Ortiz, R., Ye-Lin, Y., Garcia-
Casado, J., Nieto-Del-Amor, F., Diago-Almela, V. J.,
… Prats-Boluda, G. (2021). Comparative Study of
Uterine Myoelectrical Response to Labour Induction
Drugs in Nulliparous and Parous Women with Different
EHG Analysis Techniques. 2021 International
Conference on E-Health and Bioengineering (EHB), 1–
4. https://doi.org/10.1109/EHB52898.2021.9657548
Euliano, T. Y., Nguyen, M. T., Darmanjian, S., McGorray,
S. P., Euliano, N., Onkala, A., & Gregg, A. R. (2013).
Monitoring uterine activity during labor: a comparison
of 3 methods. American Journal of Obstetrics and
Gynecology, 208(1), 1–15.
Comparison of the Electrophysiological Myoelectrical Activity Evolution in Induction of Labor with Pharmacological and Mechanical
Methods
71

Fele-Žorž, G., Kavšek, G., Novak-Antolič, Ž., & Jager, F.
(2008). A comparison of various linear and non-linear
signal processing techniques to separate uterine EMG
records of term and pre-term delivery groups. Medical
and Biological Engineering and Computing, 46(9),
911–922. https://doi.org/10.1007/s11517-008-0350-y
Geethanjali, S., & Palli, S. (2022). Comparative study of
induction of labour with Dinoprostone gel versus
mechanical dilatation in unfavorable cervix (low
Bishop Score). Journal of Cardiovascular Disease
Research, 13(5), 1946–1954.
Hamilton, B. E., Martin, J. A., & Osterman, M. J. K. (2022).
Births: Provisional Data for 2021. Vital Statistics Rapid
Release, 20, 1–11. Retrieved from https://www.cdc.
gov/nchs/products/index.htm.
Horoba, K., Jezewski, J., Matonia, A., Wrobel, J.,
Czabanski, R., & Jezewski, M. (2016). Early predicting
a risk of preterm labour by analysis of antepartum
electrohysterograhic signals. Biocybernetics and
Biomedical Engineering, 36(4), 574–583.
https://doi.org/10.1016/J.BBE.2016.06.004
Jozwiak, M., Bloemenkamp, K. W., Kelly, A. J., Mol, B.
W., Irion, O., & Boulavain, M. (2012). Mechanical
methods for induction of labour. The Cochrane
Database of Systematic Reviews, 42(3), 674–680.
https://doi.org/10.1002/14651858.CD001233.PUB2
Kaimal, A. J., Little, S. E., Odibo, A. O., Stamilio, D. M.,
Grobman, W. A., Long, E. F., Caughey, A. B. (2011).
Cost-effectiveness of elective induction of labor at 41
weeks in nulliparous women. American Journal of
Obstetrics and Gynecology, 204(2), 137.e1-9.
https://doi.org/10.1016/j.ajog.2010.08.012
Kesić, S., & Spasić, S. Z. (2016). Application of Higuchi’s
fractal dimension from basic to clinical
neurophysiology: A review. Computer Methods and
Programs in Biomedicine, 133, 55–70. https://doi.org/
10.1016/J.CMPB.2016.05.014
Krogh, L. Q., Boie, S., Henriksen, T. B., Thornton, J.,
Fuglsang, J., & Glavind, J. (2022). Induction of labour
at 39 weeks versus expectant management in low-risk
obese women: study protocol for a randomised
controlled study. BMJ Open, 12(4), e057688.
https://doi.org/10.1136/BMJOPEN-2021-057688
Kruit, H., Wilkman, H., Tekay, A., & Rahkonen, L. (2017).
Induction of labor by Foley catheter compared with
spontaneous onset of labor after previous cesarean
section: a cohort study. Journal of Perinatology 2017
37:7, 37(7), 787–792. https://doi.org/10.1038/jp.20
17.50
Liu, X., Wang, Y., Zhang, F., Zhong, X., Ou, R., Luo, X.,
& Qi, H. (2019). Double- versus single-balloon
catheters for labour induction and cervical ripening: A
meta-analysis. BMC Pregnancy and Childbirth, 19(1),
1–13. https://doi.org/10.1186/s12884-019-2491-4
Marconi, A. M. (2019). Open Peer Review Recent advances
in the induction of labor. F1000 Research, 8(F1000
Faculty Revision), 1829. https://doi.org/10.12688/f100
0research.17587.1
Martin, J. A., Hamilton, B. E., Osterman, M. J. K., &
Driscoll, A. K. (2021). Births: Final Data for 2019. In
National Vital Statistics Reports (Vol. 70).
https://doi.org/10.15620/cdc:100472
Mas-Cabo, J., Ye-Lin, Y., Garcia-Casado, J., Díaz-
Martinez, A., Perales-Marin, A., Monfort-Ortiz, R., …
Prats-Boluda, G. (2020). Robust Characterization of the
Uterine Myoelectrical Activity in Different Obstetric
Scenarios. Entropy, 22(7), 743. https://doi.org/10.33
90/e22070743
Muszynski, C., Happillon, T., Azudin, K., Tylcz, J. B.,
Istrate, D., & Marque, C. (2018). Automated
electrohysterographic detection of uterine contractions
for monitoring of pregnancy: Feasibility and prospects.
BMC Pregnancy and Childbirth, 18(1), 1–8. https://
doi.org/10.1186/S12884-018-1778-1/TABLES/2
Nicholson, G., & Cyr, P. L. (2013). Cost Of Failed Labor
Induction: A Us Hospital Perspective. Value in Health,
16(3), A75. https://doi.org/10.1016/J.JVAL.2013.03.3
39
Nieto-Del-Amor, F., Ye-Lin, Y., Garcia-Casado, J., Diaz-
Martinez, A., Martínez, M. G., Monfort-Ortiz, R., &
Prats-Boluda, G. (2021). Dispersion entropy: A
measure of electrohysterographic complexity for
preterm labor discrimination. Proceedings of the 14th
International Joint Conference on Biomedical
Engineering Systems and Technologies (BIOSTEC
2021), 4, 260–267. https://doi.org/10.5220/001031660
2600267
Pennell, C. E., Henderson, J. J., O’Neill, M. J., McCleery,
S., Doherty, D. A., & Dickinson, J. E. (2009). Induction
of labour in nulliparous women with an unfavourable
cervix: A randomised controlled trial comparing double
and single balloon catheters and PGE2 gel. BJOG: An
International Journal of Obstetrics and Gynaecology,
116(11), 1443–1452. https://doi.org/10.1111/j.1471-
0528.2009.02279.x
Rayburn, W. F. (2002). Preinduction cervical ripening:
Basis and methods of current practice. Obstetrical and
Gynecological Survey, 57(10), 683–692.
https://doi.org/10.1097/00006254-200210000-00022
Reshme, N., Samal, R., Padmaja, P., Shalini, S., & Radhika,
K. (2022). Induction of Labour –Foleys Catheter Vs
Dinoprostone Gel: A Randomized Controlled Trial. In
Current Practice in Medical Science (1st ed., Vol. 3,
pp. 58–67). https://doi.org/10.9734/bpi/cpms/v3/1648
8D
Salim, R., Zafran, N., Nachum, Z., Garmi, G., Kraiem, N.,
& Shalev, E. (2011). Single-balloon compared with
double-balloon catheters for induction of labor: A
randomized controlled trial. Obstetrics and
Gynecology, 118(1), 79–86. https://doi.org/10.1097/
AOG.0b013e318220e4b7
Song, X., Qiao, X., Hao, D., Yang, L., Zhou, X., Xu, Y., &
Zheng, D. (2021). Automatic recognition of uterine
contractions with electrohysterogram signals based on
the zero-crossing rate. Scientific Reports, 11(1), 1–10.
https://doi.org/10.1038/s41598-021-81492-1
Terrien, J., Marque, C., & Karlsson, B. (2007). Spectral
characterization of human EHG frequency components
based on the extraction and reconstruction of the ridges
in the scalogram. Annual International Conference of
BIOSIGNALS 2023 - 16th International Conference on Bio-inspired Systems and Signal Processing
72

the IEEE Engineering in Medicine and Biology -
Proceedings, 54(3), 1872–1875. https://doi.org/10.11
09/IEMBS.2007.4352680
Villalain, C., Herraiz, I., Quezada, M. S., Gómez Arriaga,
P., Simón, E., Gómez-Montes, E., & Galindo, A.
(2019). Labor Induction in Late-Onset Fetal Growth
Restriction: Foley Balloon versus Vaginal
Dinoprostone. Fetal Diagnosis and Therapy, 46(1), 67–
74. https://doi.org/10.1159/000491784
Wang, H., Hong, S., Liu, Y., Duan, Y., & Yin, H. (2015).
Controlled-release dinoprostone insert versus Foley
catheter for labor induction: a meta-analysis. Maternal-
Fetal & Neonaral Medicine, 29(14), 2382–2388.
https://doi.org/10.3109/14767058.2015.1086331
World Health Organization. (2018). WHO
recommendations: Induction of labour at or beyond
term. Retrieved from https://apps.who.int/iris/bit
stream/handle/10665/277233/9789241550413-eng.pdf
Xu, J., Chen, Z., Lou, H., Shen, G., & Pumir, A. (2022).
Review on EHG signal analysis and its application in
preterm diagnosis. Biomedical Signal Processing and
Control, 71, 103231. https://doi.org/10.1016/J.BSPC.
2021.103231
Xu, Y., Hao, D., & Zheng, Di. (2020). Analysis of
Electrohysterographic Signal Propagation Direction
during Uterine Contraction: the Application of Directed
Information. 2020 42nd Annual International
Conference of the IEEE Engineering in Medicine &
Biology Society (EMBC), 21–25. https://doi.org/
10.1109/EMBC44109.2020.9175423
Ye-Lin, Y., Bueno-Barrachina, J. M., Prats-boluda, G.,
Rodriguez de Sanabria, R., & Garcia-Casado, J. (2017).
Wireless sensor node for non-invasive high precision
electrocardiographic signal acquisition based on a
multi-ring electrode. Measurement, 97, 195–202.
https://doi.org/10.1016/J.MEASUREMENT.2016.11.0
09
Comparison of the Electrophysiological Myoelectrical Activity Evolution in Induction of Labor with Pharmacological and Mechanical
Methods
73
