
Analysis of a Simple Method to Change the Wettability of the PDMS
Surface for Biomicrofluidic Applications
Inês M. Gonçalves
1,2
, Diana Pinho
3
, Andrea Zille
4
, Hirokazu Kaji
5
, Graça Minas
6
, Rui Lima
1,7,8
,
Patrícia C. Sousa
3
and Ana Moita
2,9
1
METRICS, University of Minho, Guimarães, Portugal
2
IN+, Instituto Superior Técnico, Universidade de Lisboa, Lisboa, Portugal
3
Integrated Micro and Nanotechnologies, INL International Iberian Nanotechnology Laboratory, Braga, Portugal
4
2C2T - Centre for Textile Science and Technology, University of Minho, Guimarães, Portugal
5
Tokyo Medical and Dental University, Tokyo, Japan
6
Center for MicroElectromechanical Systems (CMEMS-UMinho), University of Minho, Guimarães, Portugal
7
CEFT, Faculty of Engineering of the University of Porto, Porto, Portugal
8
ALiCE, Faculty of Engineering, University of Porto, Porto, Portugal
9
Centro de Investigação Desenvolvimento e Inovação da Academia Militar, Academia Militar,
Instituto Universitário Militar, Rua Gomes Freire, 1169-203, Lisboa, Portugal
Keywords: Wettability, PDMS, Biomicrofluidic Applications, Microscopy, Optical Tensiometer, Spectroscopy.
Abstract: One of the most often utilized materials for making microfluidic devices is polydimethylsiloxane (PDMS).
Organs-on-a-chip (OoC) is a novel class of devices that blends cell culture with microfluidic technology.
These devices replicate the microphysiological characteristics of the human body to make it easier to research
both healthy and unhealthy conditions. Due to its mechanical and chemical characteristics, as well as the fact
that it is a biocompatible and inert substance, PDMS is one of the materials of choice to manufacture OoC.
However, PDMS has the tendency to promote the adsorption of non-specific molecules due to its hydrophobic
properties, which may impede cell culture adhesion and growth and reduce the specificity of several
biochemical tests. It is also necessary to use external sources for flow control, such as syringe pumps, due to
the hydrophobicity of the materials' potential effects on fluid flow within the microchannels of microfluidic
devices. Oxygen plasma treatment is one of the frequently used methods for enhancing the wettability of the
PDMS surface. This strategy is, however, only effective for a limited time. Another tactic is to add ingredients
like surfactants during manufacturing to change the bulk of PDMS. In this study, PDMS was mixed with a
variety of surfactants at a concentration of 1% wt. The wettability changes were examined on the day the
samples were collected and one week later. A week after manufacture, two surfactants continued to improve
the wettability of the PDMS surface to a hydrophilic behavior.
1 INTRODUCTION
The organic polymer polydimethylsiloxane (PDMS),
which has physicochemical and mechanical qualities
like optical transparency, gas permeability,
nontoxicity, and biocompatibility, is frequently used
in the biomedical sector. Additionally, its fabrication
is a quick and inexpensive procedure (Gokaltun et al.,
2017). The evaluation and research of blood
phenomena, such as the behavior of red blood cells or
the emergence of aneurysms, is one of its applications
(Miranda et al., 2021; Pinho et al., 2020).
Lab-on-a-chip (LoC) and organ-on-a-chip (OoC)
technology has also utilized PDMS for the
manufacture of the respective devices (Carvalho et
al., 2021; Gonçalves, Carvalho, et al., 2022;
Gonçalves, Rodrigues, et al., 2022; Miranda et al.,
2021; Vlassov et al., 2018). Although the
hydrophobicity of the polymer, due to a contact angle
with water of around 108⁰ ± 7⁰ (Gokaltun et al., 2017;
Klasner et al., 2009), helps the substance be removed
from molds, it can be detrimental for its use in
medicine because it promotes undesired, non-specific
protein and small molecule adsorption. This might
impact analyte mobility and lessen the sensitivity of
116
Gonçalves, I., Pinho, D., Zille, A., Kaji, H., Minas, G., Lima, R., Sousa, P. and Moita, A.
Analysis of a Simple Method to Change the Wettability of the PDMS Surface for Biomicrofluidic Applications.
DOI: 10.5220/0011651900003414
In Proceedings of the 16th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2023) - Volume 1: BIODEVICES, pages 116-120
ISBN: 978-989-758-631-6; ISSN: 2184-4305
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

the detection (Gokaltun et al., 2017; Han & Lee,
2018). Since it is challenging to move a fluid across
the surface of the polymer due to its hydrophobicity,
external pumping sources are necessary for
microfluidic research (Kim et al., 2010; Litwinowicz
et al., 2021).
Numerous techniques have been used to increase
the wettability of PDMS. These tactics could
comprise modifications to the surface and to the bulk
of the material. (Hu et al., 2020) The oxygen plasma
treatment is one method of surface modification that
is frequently employed (Han & Lee, 2018; Seo & Lee,
2006). The hydrophilicity produced by this method is
just momentary, whereas the PDMS quickly regains
its hydrophobicity (Hu et al., 2020; Kim et al., 2010).
The optical and mechanical properties of the polymer
may be better maintained with surface changes.
However, their processes are frequently challenging.
Adding hydrophilic moieties or surfactants to the
polymer surface is another option since surfactants
lower the substance's surface tension, promoting
aqueous solutions to scatter more broadly. (Hu et al.,
2020; Litwinowicz et al., 2021; Seo & Lee, 2006)
The composition of the material used in this study
was changed by combining PDMS with various
surfactants. The wettability of the surfaces was then
evaluated and compared over a week. The
transparency of the most promising samples was also
evaluated. Lastly, preliminary microfluidic studies
were performed using altered PDMS.
2 MATERIALS AND METHODS
Six distinct samples were prepared for this work. All
samples were produced starting from a 1:10 mixture
of the pre-polymer and PDMS curing agent. On five
of the samples, polyethylene glycol (PEG), PDMS-b-
ethylene oxide (PDMS-b-PEO), Triton X-100
(TX100), Leophen ML, or Leophen BN were added
at a concentration of 1% wt. The surfactants PEG,
PDMS-b-PEO and TX-100 were selected since
previous works showed that non-ionic surfactants can
present antimicrobial properties and have improved
PDMS wettability (Litwinowicz et al., 2021; Madadi
& Casals-Terré, 2013). Leophen ML and BN are non-
ionic emulsifiers used as wetting agents in the textile
field. Each sample was placed into a rectangular mold
and cured in an oven set to 80 °C for three hours.
After curing, the samples were taken out of the mold,
and the static contact angle between a 5 μL drop of
distilled water and the surface was measured to
evaluate the wettability of the surfaces.
Contact angles were determined on the optical
tensiometer THETA (Attention) by processing the
captured images of the profile of the droplet deposited
on the samples’ surface. 640 × 480 pixels were
evaluated during the image capture (corresponding to
240 images per test). The spatial resolution for the
system's optical configuration that was used is 15.6
μm/pixel. The images were post-processed using a
drop detection method based on the Young-Laplace
equation (One Attension software). According to
(Cheng et al., 1990), the algorithm's precision is in the
range of ±0.1°. After one week, the same approach
was used to evaluate the samples' contact angles once
more.
After the samples dried from the contact angle
readings, the transmittance spectrum was determined.
The UV-2600 spectrophotometer (Shimadzu, Japan)
and associated software were employed. A
wavelength between 200 and 800 nm was used for the
measurements.
In summary, the sample was introduced into the
apparatus after being placed in a holder. Using a
grating, the monochromator divides the light from the
emitting source into various wavelengths, allowing
only one beam at a single wavelength to pass through
the sample. The amount of light detected by the
detector on the other side then determines the
sample's transmittance. Depending on the user-
selected range, the beam's wavelength varies.
Preliminary microfluidic assays were performed
using unaltered PDMS and PDMS altered with
PDMS-b-PEO. Two types of microchannels were
used, as presented in Figure 3. One of the channels
presented a constriction in width while the other was
a linear channel, without any change in the geometry.
For the microfluidic assays, a blood analog fluid
was prepared as described in (Carneiro et al., 2021).
In brief, a PDMS pre-polymer mixed with a curing
agent in a ratio of 6:4 (Corning Sylgards 184 kit) and
added to an aqueous solution of sodium dodecyl
sulfate (SDS) 4% w/w. After vortex stirring the
solution was filtered using a filter with a hydrophilic
membrane, with 10 μm pore size (Versapors
Acrodiscs Syringe Filter, PALL). The filtered
solution was then left to cure in an oven at 80 ⁰C for
three hours. Lastly, a solution with 1% particle
concentration was prepared to be used for the
microfluidic assays.
Analysis of a Simple Method to Change the Wettability of the PDMS Surface for Biomicrofluidic Applications
117
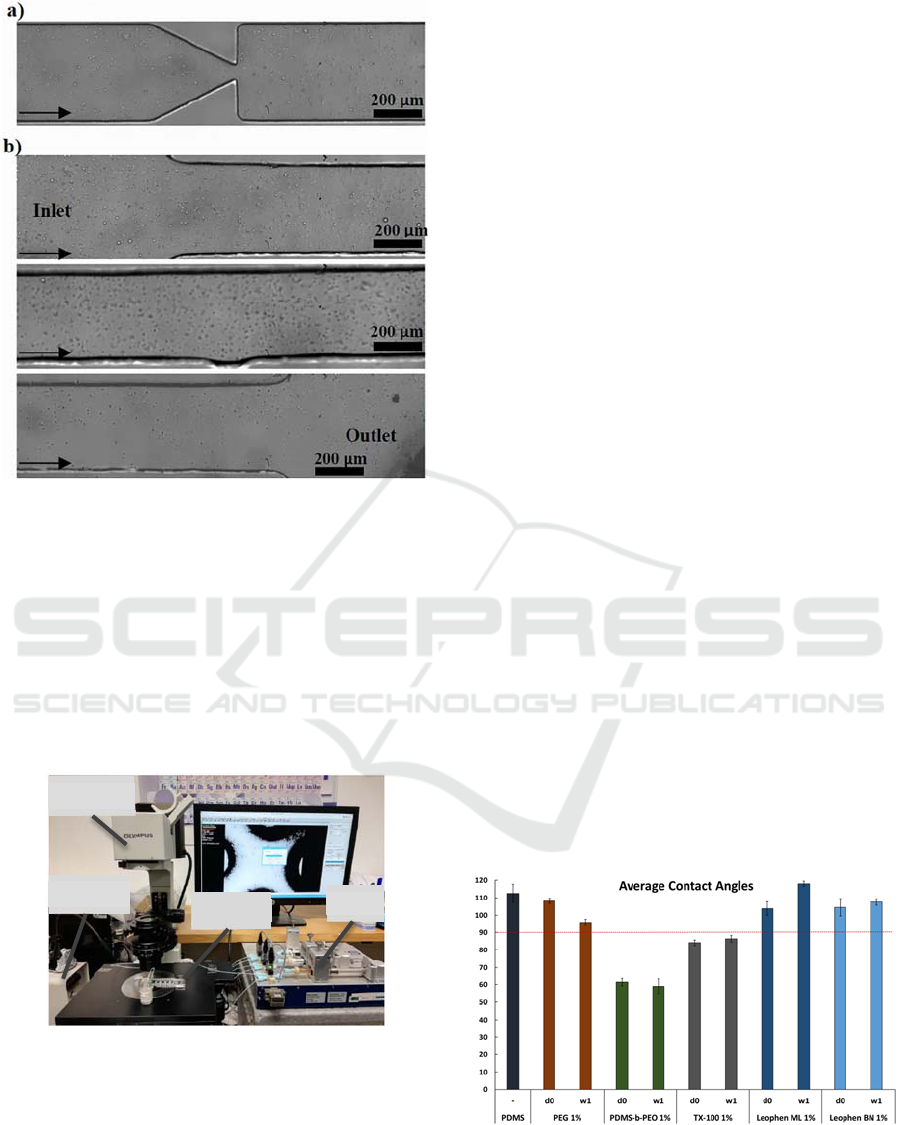
Figure 1: Microscopy images of the microchannels used for
preliminary microfluidic assays: a) constriction channel and
b) linear channel.
The microfluidic devices used for the assays were
placed on an inverted microscope (IX71, Olympus,
Tokyo, Japan) connected to a high-speed camera
(Fastcam SA3, Photron, San Diego, CA, USA) for the
visualization and record of the particle flow. To
control the fluid flow in the devices a syringe pump
(CetoniNEMESYS Syringe Pump) was used. The set-
up for the microfluidic assays is shown in Figure 4.
Figure 2: Experimental apparatus used to perform the
preliminary microfluidic assays. In: Inverted microscope;
H: High-speed camera; M: Microfluidic device; S: syringe
pump.
The fluid flow values used for the constriction
channel were 10, 20, 30 and 50 μl/min while for the
linear channel the fluid flow was kept constant at 30
μl/min. The recording of the flow was performed at
3000 frames per second and using an objective with a
magnification of 10x and an aperture of 0.25. The
acquired videos were a
nalysed with an ImageJ plugin,
the MTrackJ.
3 RESULTS AND DISCUSSION
3.1 Contact Angle Measurements
Unaltered PDMS had an average contact angle of
112⁰ ± 5⁰, which is consistent with values reported in
the literature. Figure 5 illustrates how the samples
with a surfactant showed a reduction in the average
contact angle compared to PDMS alone. The
Leophen ML, Leophen BN and PEG modified
samples had average contact angles that were 108.23⁰
± 1.48⁰, 103.86⁰ ± 3.92⁰, and 104.55⁰ ± 5.00⁰,
respectively, immediately after manufacture. These
values were higher than 90⁰, even though they were
lower than the value found for unaltered PDMS,
demonstrating that the samples still exhibit
hydrophobic behavior. After manufacture, the
samples treated with PDMS-b-PEO and TX-100 had
average contact angles that were 61.35⁰ ± 2.17⁰ and
84.12⁰ ± 1.56⁰, respectively. These values are under
90⁰, demonstrating the hydrophilicity of the samples.
The samples continued to show a hydrophilic
behavior a week later, with an average contact angle
value that was similar to the value recorded on the day
of manufacturing. When PDMS-b-PEO was used, the
contact angle value reduction was more noticeable.
The presence of PDMS in the PDMS-b-PEO structure
enhanced the surfactant's compatibility with the
polymer. For the desired hydrophilic behavior using
the other substances, higher concentrations might be
needed.
Figure 3: Contact angle measurements of PDMS and
modified PDMS using distilled water. Bellow 90⁰ the
behavior is hydrophilic and above is hydrophobic.
In
H
S
M
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
118
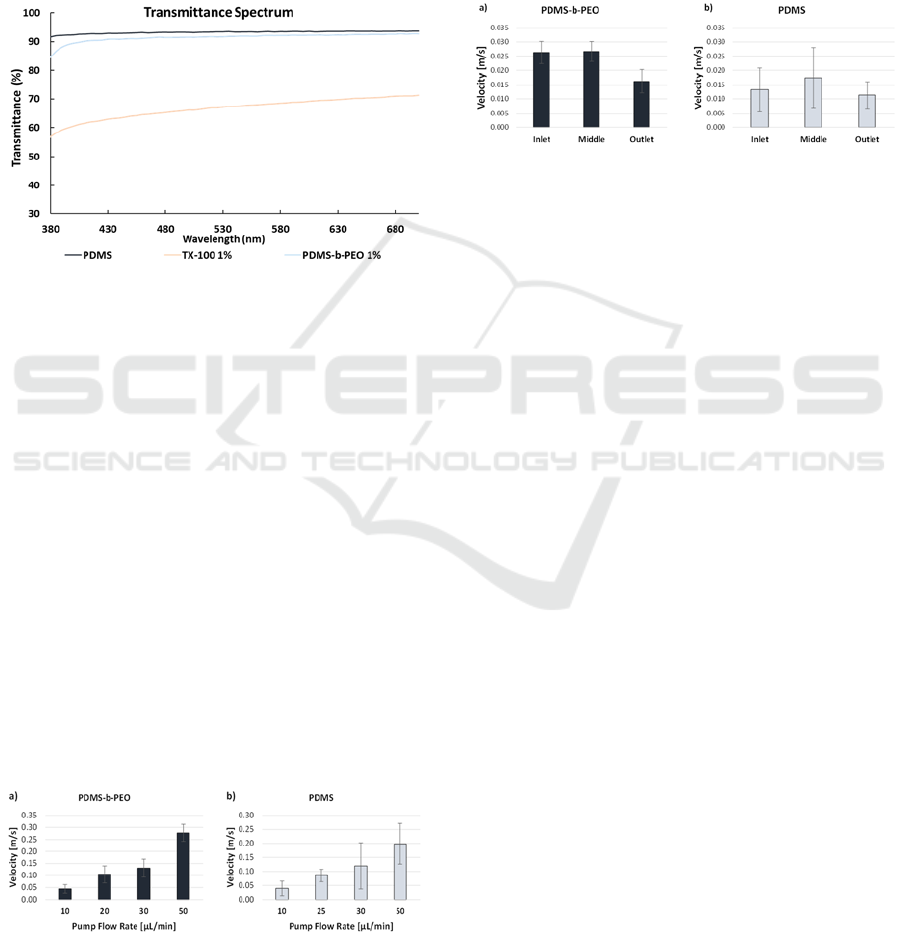
3.2 Transmittance Spectrum
Since the modified PDMS has potential to be used on
microfluidic assays that require optical analysis, the
transparency of the samples is a relevant parameter to
consider. The impact of the bulk PDMS alteration on
the material's transparency was one challenge that
was confirmed. As shown in Figure 6, the unaltered
PDMS sample exhibits a transmittance of
approximately 93% in the visible light spectrum.
Figure 4: Transmittance spectrum of unaltered and
modified PDMS.
While the sample containing 1% wt. of PDMS-b-
PEO kept the value at 91%, the addition of TX-100
caused the transparency to drop to about 67%. The
addition of TX-100 can drastically change the optical
characteristics of PDMS despite the improvement in
wettability. As PDMS-b-PEO only slightly reduced
the material's optical characteristics, it demonstrated
superior compatibility with the PDMS pre-polymer.
3.3 Microfluidic Assays
The maximum velocity of different particles passing
through the constriction channel was measured and
the average for each flow rate is presented in Figure
7. The velocity values increase with the increase of
the flow rate, as expected, and are slightly higher on
the microchannels with the altered PDMS. Also, the
variation between the values is smaller on the altered
PDMS channels.
Figure 5: Maximum particle velocity for different flow rates
on microchannels made using a) altered PDMS and b)
unaltered PDMS.
On the linear channels, the average velocity of
several particles was measured on three different
regions of the microchannel: near the inlet, on the
middle and near the outlet. The average of all the
velocities on each region is presented in Figure 8.
Higher velocities were registered on the
microchannel with altered PDMS, and the variation
between the values was also smaller than the ones
presented on the microchannels with unaltered
PDMS.
Figure 6: Average particle velocity in three different
regions of the microchannel using a) altered PDMS and b)
unaltered PDMS.
The results on both channels indicate an
improvement regarding the flow on microchannels
when using altered PDMS. However, the results are
still insufficient to draw major conclusions regarding
the benefits of changing the PDMS wettability
through the proposed technique.
4 CONCLUSIONS
To improve the PDMS wettability, our current focus
is on undertaking quantitative study with various
surfactants and hydrophilic solutions. Using PDMS-
b-PEO was the more effective technique to boost
hydrophilicity while keeping transparency. By
applying a small percentage of the surfactant, a
hydrophilic behavior could be kept for over a week.
Preliminary microfluidic studies showed a potential
improvement of the fluid flow when using
microchannels with altered PDMS. The modified
PDMS will be optimized in the future to promote the
manufacture of self-driven microfluidic devices. LoC
and OoC devices could also be improved by such
technology. Future studies will examine the
wettability behavior of the modified PDMS in
prolonged fluid contact, as well as the release of the
surfactant into the fluid and its effects on cell culture.
Biocompatibility and protein adsorption will also be
analysed in future works to determine which type of
PDMS will be better for each part of an OoC device.
Analysis of a Simple Method to Change the Wettability of the PDMS Surface for Biomicrofluidic Applications
119

ACKNOWLEDGEMENTS
I. M. Gonçalves acknowledges FCT for the grant
SFRH/BD/08646/2020, supported by national funds
from Ministérios da Ciência, Tecnologia e Ensino
Superior. This work has been also supported by the
projects, EXPL/EMD-EMD/0650/2021 and
PTDC/EEI-EEE/2846/2021 through the
COMPETE2020, under the PORTUGAL 2020
Partnership Agreement through the European
Regional Development Fund (FEDER) and by
Fundação para a Ciência e Tecnologia (FCT). The
authors acknowledge the partial financial support
within the R&D Units Project Scope:
UIDB/00690/2020, UIDB/04077/2020, UIDB/
04436/2020, UIDB/00532/2020. The authors also
acknowledge the financial support (4004) from the
Research Center for Biomedical Engineering from
Tokyo Medical and Dental University, Japan.
REFERENCES
Carneiro, J., Lima, R., Campos, J. B. L. M., & Miranda, J.
M. (2021). A microparticle blood analogue suspension
matching blood rheology. Soft Matter. https://doi.org/
10.1039/D1SM00106J
Carvalho, V., Gonçalves, I., Lage, T., Rodrigues, R. O.,
Minas, G., Teixeira, S. F. C. F., Moita, A. S., Hori, T.,
Kaji, H., & Lima, R. A. (2021). 3D Printing Techniques
and Their Applications to Organ-on-a-Chip Platforms:
A Systematic Review. Sensors, 21(9), 3304.
https://doi.org/10.3390/s21093304
Cheng, P., Li, D., Boruvka, L., Rotenberg, Y., & Neumann,
A. W. (1990). Automation of axisymmetric drop shape
analysis for measurements of interfacial tensions and
contact angles. Colloids and Surfaces, 43(2), 151–167.
https://doi.org/https://doi.org/10.1016/0166-6622(90)8
0286-D
Gokaltun, A., Yarmush, M. L., Asatekin, A., & Usta, O. B.
(2017). Recent advances in nonbiofouling PDMS
surface modification strategies applicable to
microfluidic technology. Technology, 05(01), 1–12.
https://doi.org/10.1142/s2339547817300013
Gonçalves, I. M., Carvalho, V., Rodrigues, R. O., Pinho, D.,
Teixeira, S. F. C. F., Moita, A., Hori, T., Kaji, H., Lima,
R., & Minas, G. (2022). Organ-on-a-Chip Platforms for
Drug Screening and Delivery in Tumor Cells: A
Systematic Review. Cancers, 14(4), 935. https://doi.
org/10.3390/cancers14040935
Gonçalves, I. M., Rodrigues, R. O., Moita, A. S., Hori, T.,
Kaji, H., Lima, R. A., & Minas, G. (2022). Recent
trends of biomaterials and biosensors for organ-on-chip
platforms. Bioprinting, 26, e00202. https://doi.org/
10.1016/j.bprint.2022.e00202
Han, C. M., & Lee, B. K. (2018). Effect of hydrophilicity
of polydimethylsiloxane stamp in capillary force
lithography process of thermoplastic polyurethane.
Microelectronic Engineering, 190, 38–43. https://
doi.org/10.1016/j.mee.2018.01.001
Hu, H., Li, S., Ying, C., Zhang, R., Li, Y., Qian, W., Zheng,
L., Fu, X., Liu, Q., Hu, S., & Wong, C. P. (2020).
Hydrophilic PDMS with a sandwich-like structure and
no loss of mechanical properties and optical
transparency. Applied Surface Science, 503(September
2019), 144126. https://doi.org/10.1016/j.apsusc.
2019.144126
Kim, Y. C., Kim, S. H., Kim, D., Park, S. J., & Park, J. K.
(2010). Plasma extraction in a capillary-driven
microfluidic device using surfactant-added
poly(dimethylsiloxane). Sensors and Actuators, B:
Chemical, 145(2), 861–868. https://doi.org/
10.1016/j.snb.2010.01.017
Klasner, S. A., Metto, E. C., Roman, G. T., & Culbertson,
C. T. (2009). Synthesis and characterization of a
poly(dimethylsiloxane)-poly (ethylene oxide) block
copolymer for fabrication of amphiphilic surfaces on
microfluidic devices. Langmuir, 25(17), 10390–10396.
https://doi.org/10.1021/la900920q
Litwinowicz, M., Rogers, S., Caruana, A., Kinane, C.,
Tellam, J., & Thompson, R. (2021). Tuning the Bulk
and Surface Properties of PDMS Networks through
Cross-Linker and Surfactant Concentration.
Macromolecules, 54(20), 9636–9648. https://doi.org/
10.1021/acs.macromol.1c01600
Madadi, H., & Casals-Terré, J. (2013). Long-term behavior
of nonionic surfactant-added PDMS for self-driven
microchips. Microsystem Technologies, 19(1), 143–
150. https://doi.org/10.1007/s00542-012-1641-7
Miranda, I., Souza, A., Sousa, P., Ribeiro, J., Castanheira,
E. M. S., Lima, R., & Minas, G. (2021). Properties and
Applications of PDMS for Biomedical Engineering: A
Review. Journal of Functional Biomaterials, 13(1), 2.
https://doi.org/10.3390/jfb13010002
Pinho, D., Carvalho, V., Gonçalves, I. M., Teixeira, S., &
Lima, R. (2020). Visualization and Measurements of
Blood Cells Flowing in Microfluidic Systems and
Blood Rheology: A Personalized Medicine Perspective.
Journal of Personalized Medicine, 10(4), 249.
https://doi.org/10.3390/jpm10040249
Seo, J., & Lee, L. P. (2006). Effects on wettability
by surfactant accumulation/depletion in bulk
polydimethylsiloxane (PDMS). Sensors and Actuators,
B: Chemical, 119(1), 192–198. https://doi.org/
10.1016/j.snb.2005.12.019
Vlassov, S., Oras, S., Antsov, M., Sosnin, I., Polyakov, B.,
Shutka, A., Krauchanka, M. Y., & Dorogin, L. M.
(2018). Adhesion and Mechanical Properties of PDMS-
Based Materials Probed with AFM: A Review.
REVIEWS ON ADVANCED MATERIALS SCIENCE,
56(1), 62–78. https://doi.org/10.1515/rams-2018-0038.
BIODEVICES 2023 - 16th International Conference on Biomedical Electronics and Devices
120
